Rituximab (IDEC-C2B8) is a chimeric antibody that binds to the B-cell surface antigen CD20. Rituximab has significant activity in follicular non-Hodgkin lymphomas. Much less is known about the effects in chronic lymphocytic leukemia (CLL). We have initiated a phase II trial to evaluate the efficacy and safety of rituximab in patients with CD20+ pretreated CLL. To avoid the rituximab-associated toxicity, we restricted the tumor cell load, as measured by the number of circulating lymphocytes and the spleen size, in the first 2 cohorts of patients included in the study. Patients received 4 intravenous infusions of 375 mg/m2 once a week over a period of 1 month. Of the 28 patients evaluable for response, 7 patients showed a partial remission (National Cancer Institute criteria) lasting for a median of 20 weeks, with 1 patient still in remission after 71 weeks. Based on lymphocyte counts only, we found at least a 50% reduction of lymphocyte counts lasting for at least 4 weeks in 13 (45%) of 29 patients. Fifteen patients from 3 institutions were monitored for the immunophenotype profile of lymphocyte subsets. The number of CD5+CD20+ cells decreased significantly and remained low until day 28 after therapy. T-cell counts were not affected. With the exception of one rituximab-related death, adverse events in the remaining patients were mild. The results suggest that rituximab has clinical activity in pretreated patients with B-CLL. Toxicity is tolerable. Response duration after withdrawal of rituximab is rather short. Therefore, other modes of application and the combination with other agents need to be tested.
Introduction
Rituximab (IDEC-C2B8) is a chimeric antibody that binds to the B-cell surface antigen CD20. It has been approved for use in relapsed or refractory low-grade follicular lymphoma. Phase II trials in patients with low-grade non-Hodgkin lymphoma (NHL) have demonstrated response rates ranging from 48% to 62%.1-3The overall response rate rose to 95% in 38 of 40 patients when rituximab was administered in combination with chemotherapy. Among patients with mantle cell lymphoma, the response rate was only 30%.4 5
In most trials, rituximab was administered as 4 weekly infusions of 375 mg/m2. For the treatment of patients with relapsing or refractory aggressive lymphoma (diffuse large B-cell lymphoma and mantle cell lymphoma), 8 weekly infusions at a dose of 375 mg/m2 in arm A or 1 infusion of 375 mg/m2followed by 7 weekly infusions of 500 mg/m2 in arm B have been compared. A total of 5 complete responses and 12 partial responses were observed among the 54 enrolled patients, with no difference between the 2 schedules.6
The CD20 antigen expression is restricted to the B-cell lineage, including most B-cell neoplasias. The immunophenotype of B-cell chronic lymphocytic leukemia (B-CLL) shows the expression of B-cell–associated antigens (CD19, CD20, CD79a) as well as CD5 and CD23.7However, the levels of CD20 expressed in B-CLL are lower than in other NHLs. This may explain why clinical trials with rituximab in CLL are rare.8 9 To test the safety and efficacy of rituximab as a single agent in CLL, a phase II study was initiated by the German CLL Study Group.
High tumor cell load in peripheral blood was associated with severe side effects upon administration of rituximab. This phenomenon may be induced by rapid tumor lysis and a release of large amounts of cytokines.10-13 To reduce the potential toxicity of rituximab, we excluded patients with a very high tumor cell load, as measured by lymphocyte numbers in the blood and by spleen size, in the first 2 cohorts of 5 patients.
The clinical observations were complemented by monitoring the immunophenotypic profile of lymphocytes in a subset of patients.
Patients, materials, and methods
Patients
Patients between 18 and 75 years of age with relapsed and/or treatment-refractory B-CLL as confirmed by cytology and immunophenotyping or prolymphocytic leukemia (REAL classification), Binet stage B or C, with confirmed expression of CD20 were eligible. Any previous treatment had to be completed at least 3 weeks before recruitment to the study. ECOG (Eastern Cooperative Oncology Group) performance status was 0-3 (Karnofsky index ≥ 40%). All patients gave their written informed consent prior to the study. The exclusion criteria included pretreatment with a murine antibody; known allergic reactions; active infections; serious concurrent cardiovascular, lung, psychiatric, or metabolic diseases; inadequate renal or hepatic function; as well as previous organ transplantation.
Patients were divided into 3 cohorts according to tumor mass as defined by spleen size and mass of the circulating tumor cells. Group 1 (patients 1-5) had a lymphocyte count below 20.0 × 109/L (20.0/nL) and spleen less than 5 cm below the costal margin; group 2 (patients 6-10) had fewer than 50.0 × 109/L (50.0/nL) lymphocytes with no limitation for spleen size; and group 3 (patients 11-30) had no limitations. After the occurrence of severe complications that resulted in death of patient no.16 (see below), a protocol amendment was released re-establishing the limitations of group 2 for further patient recruitment.
Treatment plan
Anti-CD20 monoclonal antibody (Mabthera, rituximab) was supplied by Hoffmann-La Roche AG (Grenzach-Wyhlen, Germany). The antibody was diluted in normal saline up to a maximum concentration of 1 mg/mL. Patients were scheduled to receive 4 intravenous infusions of 375 mg/m2 once a week over a period of 1 month. Premedication with acetaminophen and diphenhydramine as well as sufficient hydration was recommended. At the initiation of the trial, the infusion rate was started at 50 mg/hour during the first hour and increased to final 400 mg/hour if well tolerated, and the use of concomitant corticosteroids was limited to the treatment of severe allergic reactions. After the occurrence of lethal complications in patient no. 16, an amendment to the protocol was introduced and the dosing schedule of rituximab was modified (starting with patient no. 17): infusion of 50 mg rituximab on day 1, 150 mg on day 2, and the remainder of the dose on day 3 of the first infusion cycle. Each dose was dissolved in 1000 mL saline solution, and the infusion time for the first 50 mg was extended to at least 4 hours. Premedication with acetaminophen, diphenhydramine, and prednisone was recommended.13
Evaluation
Baseline evaluation included disease history and previous medication; current stage of disease, including signs and symptoms as well as measurement of lesions by physical examination; and radiographic studies (chest x-ray, computed tomography, ultrasound). Bone marrow biopsy and aspiration were performed. Laboratory testing included complete and differential blood counts, immunophenotyping of mononuclear cells in the peripheral blood, clinical chemistry, testing of serum immunoglobulins, testing of β2-microglobulin, urinalysis, and Coombs test.
Physical examination, laboratory values, and adverse events were recorded weekly throughout the treatment period. Hematologic and physical examinations were performed monthly thereafter until progression.
Exact quantitation of the phenotypic CD20 expression of CLL cells has not been performed. As a substitute, we have determined the ratio of the mean fluorescence intensities of the CD20+ cells and of the CD20− cells in the samples stained with anti-CD20 monoclonal antibody coupled to fluorescein isothiocyanate. This ratio, in all participating laboratories, is typically below 40:1 in B-CLL. In B-CLL with CD20dim expression, this ratio is less than 10:1.
Lymphocyte subgroups according to phenotype as defined by flow cytometry during and up to 1 month after therapy were investigated in 15 patients from 3 participating centers.
Definition of end points
The objective remission rate was the primary efficacy end point of the study. All responses as well as the time point of subsequent progression were discussed by an expert group in an extramural review session. The National Cancer Institute (NCI)-sponsored Working Group guidelines14 were applied for the definition of response and time to progression (with 2 minor modifications: To be considered a complete remission (CR) or partial remission (PR), the patient must maintain the response criteria for at least 1 month, and down-staging of a Binet stage must occur to define a remission).
Complete remission.
Criteria for CR include no evidence of disease; absence of lymphadenopathy, hepatomegaly, splenomegaly, or constitutional symptoms; and normal blood count: neutrophils more than 1.5 × 109/L (1.5/nL), platelets more than 100 × 109/L (100/nL), hemoglobin more than 110 g/L (11 g/dL), lymphocytes less than 4.0 × 109/L (4.0/nL), and bone marrow biopsy with normal cellularity and lymphocytes less than 30% (NCI criteria).
Partial remission.
Criteria for PR include a change from stage C to stage A or B or a change from stage B to A (Binet); or at least a 50% reduction in blood lymphocytes and at least a 50% reduction in lymphadenopathy and/or at least a 50% reduction in splenomegaly and/or hepatomegaly, plus at least one of the following features: neutrophils more than 1.5 × 109/L (1.5/nL) or at least a 50% improvement over baseline, platelets more than 100 × 109/L (100/nL) or at least a 50% improvement over baseline, hemoglobin more than 110 g/L (11.0 g/dL) (not supported by transfusion) or at least a 50% improvement over baseline (NCI criteria).
Stable disease.
In stable disease, there is no change in the stage of the disease (Binet) and no CR, PR, or progression.
Progression.
Criteria for progression include a change from stage A disease to stage B or C or from stage B to stage C. At least one of the following occurs: at least a 50% increase in the largest diameter of at least 2 lymph nodes or new palpable lymph nodes; at least a 50% increase of splenomegaly or hepatomegaly or transformation to a more aggressive histology, Richter syndrome, or prolymphocytic leukemia; and at least a 50% increase in the absolute number of circulating lymphocytes (all percentages in relation to “nadir” findings). Time to progression is defined as time from the first application of rituximab to detection of progression as defined here.
All adverse events were categorized and graded according to the NCI-CTC (Common Toxicity Criteria) system.
Hematologic response.
In addition to the remission rate, the hematologic response was evaluated as defined by the at least 50% reduction of lymphocytes lasting for at least 4 weeks from the end of rituximab treatment.
Statistics
Descriptive biostatistical methods were used throughout. Time to progression and duration of response were analyzed by the product limit method of Kaplan and Meier.15 Comparisons of response data between prognostic subgroups were performed using the Fisher exact test. The Wilcoxon-Pratt test was applied for within-group comparison of laboratory data.
Results
Patient characteristics
Between September 1998 and July 1999, 31 patients were enrolled into the study by 8 German clinical institutions. One patient withdrew his consent after inclusion and did not receive any trial medication. The remaining 30 cases received rituximab and form the basis of the following analyses. One patient (no. 16) died within 24 hours after start of rituximab therapy and was excluded from several analyses.
The demographic and pretreatment disease characteristics of the study population are presented in Table 1. For most patients, pretreatment included chlorambucil. Twenty-three (77%) of 30 were also treated with fludarabine prior to the study. Most patients showed lymphadenopathy, whereas isolated splenomegaly was seen in one patient. Table 2 summarizes the baseline clinical findings.
Treatment
Twenty-nine of 30 patients received 4 courses of 375 mg/m2 rituximab once a week over a period of 1 month. Interruptions or delays in the drug infusion were reported in 6 cases (5% of infusions). Three of these episodes were due to the “allergy type” reactions that occurred during the first cycle. Three others were caused by diarrhea or acute infections.
Response to therapy
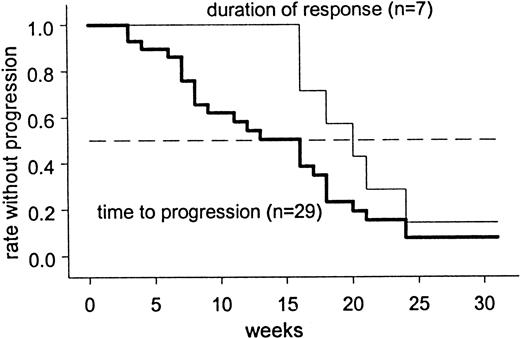
Based on the criteria of the NCI Working Group, 7 of 28 patients showed an objective response, corresponding to a response rate of 25% (95% confidence interval, 11%-45%). One case was not evaluable due to insufficient data. All 7 patients fulfilled the criteria for PR. PR was defined in patients 0, 4, 5, and 10 by the decrease of lymphocyte counts and of enlarged lymph nodes (and spleen, if enlarged before therapy) by more than 50%. In patient no. 27, PR was defined by the decrease of enlarged lymph nodes only, because lymphocyte count was normal before the start of treatment. As in patients 9 and 24, the lymphocyte count was reduced by more than 50%, lymph nodes remaining stable after treatment, and the change from Binet stage C to B was considered to be a sufficient additional criterion for PR. The change of Binet stage was caused by the increase of thrombocyte counts from 44 (82) before treatment up to 130 (135) after treatment, respectively. Twelve (43%) patients fulfilled criteria for transient disease stabilization. Nine (32%) patients were refractory to treatment. Intention-to-treat analysis (including the patients with early death and insufficient response data, respectively) showed an overall PR rate of 7 (23%) of 30 (95% confidence interval, 10%-42%). The overall median response duration was 20 weeks, with one patient still in remission after 71 weeks (Figure 1).
Response duration and time to disease progression (Kaplan-Meier estimation).
Table 3 shows overall response according to potential prognostic factors. The individual groups were too small for the statistical analysis. No responses were detected in a subgroup of 8 cases with a Karnofsky index 80% or less and increased lactate dehydrogenase (LDH) values.
Based on the analysis of lymphocyte counts (hematologic response), 13 (45%) of 29 patients showed a 50% or greater reduction or normalization during or shortly after therapy, lasting for at least 4 weeks from the end of therapy. In most cases with initially elevated lymphocyte counts, an increase in lymphocyte numbers 1 to 2 months after the treatment was observed (Figure2). Neutrophil, hemoglobin, and platelet levels remained stable during and up to 2 months after therapy (Table4). LDH values decreased significantly from a median value of 240 U/L pretreatment to 200 U/L on day 15, 190 U/L on day 22, and 200 U/L on day 29. Of 13 evaluable patients suffering from B symptoms at study entry, 5 (38%) showed a resolution of this condition at the time of the first posttreatment follow-up.
Individual lymphocyte counts of responders and nonresponders.
Individual lymphocyte counts before, during, and 1 to 3 months after the end of treatment for the 7 patients with partial response (NCI criteria) (A) and the 22 nonresponders (B). (Patient no. 16, who died on the first day of treatment with a pretreatment lymphocyte count of 274.0 × 109/L [274.0/nL], is not included.)
Individual lymphocyte counts of responders and nonresponders.
Individual lymphocyte counts before, during, and 1 to 3 months after the end of treatment for the 7 patients with partial response (NCI criteria) (A) and the 22 nonresponders (B). (Patient no. 16, who died on the first day of treatment with a pretreatment lymphocyte count of 274.0 × 109/L [274.0/nL], is not included.)
Disease progression occurred after a median of 16 weeks (Figure 1). Currently, there are 21 survivors.
Immunophenotyping
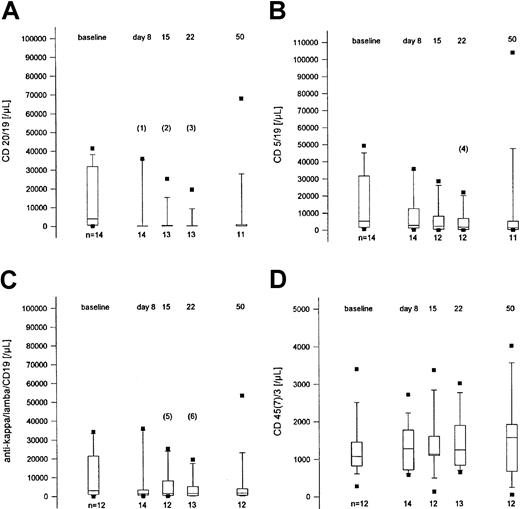
Rituximab treatment reduces the number of peripheral blood CLL cells expressing the CD20 antigen. Fifteen patients from 3 institutions were monitored for the immunophenotypical profile of lymphocyte subsets during and up to 4 weeks after treatment (Figure3). The number of CD5+CD19+ cells and CD19+CD20+ cells decreased distinctly against the baseline values and remained low until day 28 after therapy (50 days from the beginning of treatment). Similarly, the monoclonal lymphocyte population as defined by light chain expression showed parallel behavior. T-cell counts (CD3+CD45+CD7+) were not affected by rituximab.
Lymphocyte subsets at baseline, during treatment, and 4 weeks after the end of treatment.
Box plot representation of counts of lymphocyte subpopulations before, during, and after treatment of 15 patients from 3 institutions. (A) CD20+/CD19+ lymphocytes. (B) CD5+/CD19+ lymphocytes. (C) The κ+ or λ+/CD19+ lymphocytes. (D) CD45+/CD7+ or CD3+lymphocytes. Indicated P values (Wilcoxon-Pratt test, 2-sided): (1) .022, (2) .0015, (3) .0005, (4) .083, (5) .065, (6) .014.
Lymphocyte subsets at baseline, during treatment, and 4 weeks after the end of treatment.
Box plot representation of counts of lymphocyte subpopulations before, during, and after treatment of 15 patients from 3 institutions. (A) CD20+/CD19+ lymphocytes. (B) CD5+/CD19+ lymphocytes. (C) The κ+ or λ+/CD19+ lymphocytes. (D) CD45+/CD7+ or CD3+lymphocytes. Indicated P values (Wilcoxon-Pratt test, 2-sided): (1) .022, (2) .0015, (3) .0005, (4) .083, (5) .065, (6) .014.
Adverse events
Rituximab may cause severe side effects. One treatment-related death occurred during this study in a 65-year-old patient (no.16) with leukocyte counts at treatment initiation of 274.0 × 109/L (274.0/nL). Ten hours after the first infusion of rituximab (42.5 mg in 50 mL over 6.5 hours) the patient developed a syndrome of multiorgan failure with renal failure, elevation of serum potassium to 6.6 mM, acidosis, and, finally, cardiac arrest. Autopsy revealed prominent leukostasis, especially in the lungs, and a transition to Richter syndrome in all lymph nodes investigated. Subsequent to this adverse event, further patient selection was restricted to a maximum baseline lymphocyte count of 50.0 × 109/L (50.0/nL) (Volker Kunzmann et al, manuscript submitted, June 2000).
The following toxicity analyses are based on studies of 29 patients who received the full protocol treatment. A total of 86 events were reported. Because some events of identical characteristics were repeatedly reported in individual patients, the number of CTC event types “per patient” was reduced to 78. The most frequent adverse reactions were related to the typical syndrome arising during the first infusion of rituximab. This included flulike symptoms (52% of the patients), fever (31%), rigors and/or chills (34%), tachycardia (17%), hypotension (10%), vomiting (10%), and headache (7%). Infections of all CTC grades occurred in 7 (24%) of 29 patients. Table5 shows the frequency of grade 3 or 4 adverse events by organ sites. Five cases of CTC grade 3-4 infections consisted of sinusitis and/or laryngitis, pneumonia, generalized herpes zoster infection, and phlegmon of the arm. One infection occurred 1 month after the initiation of rituximab with septicemia resistant to antibiotic treatment and, later, a lethal pulmonary aspergillosis. In 21 patients without infection, neutrophil counts at baseline were higher (mean 3.0 × 109/L [3.0/nL], median 2.7 × 109/L, range 0.0-8.8 × 109/L) when compared with 7 patients with infections (mean 1.7 × 109/L, median 1.8 × 109/L, range 0.4-3.2 × 109/L). In one patient, cancer of the bladder was diagnosed some weeks after start of rituximab treatment. Patients 17 to 30 were premedicated with prednisone after lethal toxicity occurred in patient no. 16. The frequency of flulike symptoms in patients 1 to 15 was 11 (73.3%), in comparison to 4 (28.6%) in patients 17 to 30.
Discussion
Rituximab has exhibited significant antilymphoma activity in more than 300 patients with low-grade NHL, but its role in CLL patients has been controversial. In a study focusing on the safety of rituximab in patients with high numbers of circulating CD20+ cells, 9 patients with heavily pretreated CLL were treated with 375 mg/m2 rituximab weekly for 4 weeks. Rapid reduction of peripheral malignant cells was observed in all patients.13In our trial PR was observed in 7 of 28 patients evaluable for response, and a reduction specifically of lymphocyte counts was detected more frequently, with 13 of 29 patients showing at least a 50% reduction or normalization of lymphocytes lasting for at least 4 weeks. These remission rates in CLL are comparable to those obtained in mantle cell lymphoma (13 of 35 pretreated patients),4 in small B-cell lymphocytic lymphoma (4 of 28 patients and 8 of 14 patients),4,16 and in Waldenström macroglobulinemia (3 of 7 patients)3 using rituximab.
Higher response rates have been observed when circulating B-CLL cells rather than lymph nodes and spleen size were used as parameters. This finding may be explained by different antibody penetration into various tumor compartments.17
Increased infusion-related side effects and rapid tumor clearance have been seen in patients with high numbers of circulating blood tumor cells.10,12 13 Therefore, in our trial patients were entered in 3 subsequent cohorts according to the tumor mass: group 1 (5 patients) with lymphocytes below 20.0 × 109/L (20.0/nL) and spleen less than 5 cm below costal margin; group 2 (5 patients) with lymphocytes below 50.0 × 109/L (50.0/nL). In the first 2 cohorts, no serious side effects were observed. Therefore, after toxicity evaluation of cohorts 1 and 2, the subsequent patients were included without limitation of lymphocyte counts, but a modified schedule with administration of 50 mg rituximab on day 1, 150 mg on day 2, and 400 to 500 mg (rest of 375 mg/m2) on day 3 of the first infusion cycle was applied. Severe toxicity was observed in a patient with a very high tumor load when a 42.5 mg total dose of rituximab was given for 6.5 hours. After this severe complication, the protocol was amended to exclude patients with lymphocytes numbering more than 50.0 × 109/L (50.0/nL). No severe toxicity has been observed in the following 15 patients enrolled.
The syndrome observed in patients with high tumor cell load and treated with rituximab is different from the tumor lysis syndrome observed in patients with high-grade lymphoma, in which severe electrolyte disturbances and renal failure were characteristic, and thrombocytopenia was frequent.11 Instead, symptoms of cytokine release were observed, followed by gradual tumor cell destruction and tumor cell agglutination in the lung, liver, heart, and spleen. Indeed, cytokine release (tumor necrosis factor-α, interferon-γ, interleukin-6) has been described in context of side effects of rituximab13 and other monoclonal antibodies.18 19
Apart from toxicity in patients with very high cell counts, rituximab was generally well tolerated, with most toxicity being directly related to the infusion (most commonly the first infusion). All patients (excluding patient no. 16) received the full protocol treatment. The hematologic toxicity also was similar to previous reports and was usually mild and transient. Hemoglobin, thrombocytes, T-lymphocyte subsets, and immunoglobulins were not significantly altered. Bacterial infections were found in 6 of 29 patients during rituximab therapy.
Lymphocyte subgroups were investigated in 15 patients. The most pronounced decline in lymphocyte subsets was demonstrated in CD19+/CD20+ lymphocytes. T lymphocytes were not affected. The rituximab-induced decrease in lymphocyte subpopulations lasted for at least 4 weeks after the end of rituximab application. These findings suggest that (1) decline of B lymphocytes is not only simulated by modulation of CD20 expression or by blockade of the cell surface epitope by rituximab; (2) a subgroup of CD20+and/or monoclonal B lymphocytes escape from the effect of rituximab; and (3) T lymphocytes are not affected.
Single-agent rituximab as given in this trial did not result in a higher response rate than reported with alkylating agents. Therefore, rituximab as a single agent might play a role in patients with poor bone marrow reserve for whom other options have failed. In addition, other modes of application and combination with other agents could produce more encouraging results. Response duration in our patients was short. The role of maintenance therapy therefore needs to be investigated. Extended rituximab therapy (8 consecutive infusions of 375 mg/m2) in patients with recurrent or relapsed low-grade or follicular NHL has been reported to yield a favorable 60% response rate.20 Using rituximab for the maintenance therapy might also be a promising approach. Stable disease has been achieved in B-CLL patients with progression after an initial response to rituximab when 100 mg rituximab per patient was given monthly in an ongoing trial (unpublished data, S.S., June 2000). Thrice-weekly application has been investigated in CLL patients in a phase I/II study and proved to be a feasible and active regimen.8 Dose escalation was tested in different subtypes of NHL, including CLL. All patients received a first dose of 375 mg/m2, and almost no toxicity was seen on subsequent escalated doses until 2250 mg/m2 was reached.9
To analyze the effect of combination immunotherapy, patients were treated with rituximab in combination with interferon-α. Thirty-one patients with relapsed or refractory follicular lymphoma were treated with 5 × 106 units IFN-α subcutaneously 3 times weekly for 3 months and with rituximab 375 mg/m2 weekly on weeks 5 to 8. In 26 evaluable patients the response rate was 58%.21
The combination of rituximab and chemotherapy was investigated in 40 patients with low-grade, mostly follicular B-cell NHL receiving 6 infusions of rituximab (375 mg/m2) together with 6 doses of cyclophosphamide, vincristine, adriamycin, and prednisone (CHOP) chemotherapy. The overall response rate was 95% with a response duration of 46-plus months.5 The combination of fludarabine and rituximab will be tested in untreated CLL patients in an ongoing trial of the German CLL Study Group.
In conclusion, rituximab as a single agent shows efficacy in the treatment of B-CLL. Side effects are usually mild, but in patients with high blood tumor cell counts toxicity may be lethal and therapy should be initiated with caution. A reduction of the numbers of circulating tumor cells below 50.0 × 109/L (50.0/nL) before using rituximab by conventional chemotherapy seems reasonable. To improve the therapeutic potential, prolonged application of rituximab and maintenance therapy as well as the combination with other therapeutic principles like chemotherapy, response modifiers, high-dose chemotherapy, or radioimmunoconjugates22 should be investigated.
Supported by funding from Hoffmann-La Roche AG (D.H., M.H., S.S., C.P., and B.E.).
U.R. is employed by Hoffman-La Roche AG, whose product was studied in the present work.
Preliminary results presented as a poster at the 41st Annual Meeting of the American Society of Hematology in New Orleans, December 3-7, 1999.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dieter Huhn, Dept of Medicine/Hematology and Oncology, Charité Campus Virchow-Klinikum, Augustenburger Platz 1, D-13353 Berlin, Germany; e-mail: dieter.parczany@charite.de.

![Fig. 2. Individual lymphocyte counts of responders and nonresponders. / Individual lymphocyte counts before, during, and 1 to 3 months after the end of treatment for the 7 patients with partial response (NCI criteria) (A) and the 22 nonresponders (B). (Patient no. 16, who died on the first day of treatment with a pretreatment lymphocyte count of 274.0 × 109/L [274.0/nL], is not included.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/5/10.1182_blood.v98.5.1326/5/m_h81711467002.jpeg?Expires=1768142433&Signature=VZc02ewZxtdho6jB~u6AeoTGEuPMmuxebZBBUPWcvtyDqrIClxTRUKHjVedYhPw~zI86~J-mdFntnfbvO3jUngfOJ315vNfaJdnNigBoD81BHT3FSn8rbcqyoE15q8wIBVUybGS8hvcN-g6Z9T1tyZj9dj6bhJipPh0ZpNu17bBKkCa5kBBKBpD9TduvYRZorD46HOwKnyAbodP0OVMVIruPn3SP2Blnsv74Hp0Hz8RtxCXGJlId69wgCtofKd6Fp-5oEVcDjqOXC7iDQgcvibk2q2iIwNFxdEy-e9Hc2i1485MfGFp1997XPEpnlPEKulvlUKS7LVqMyZu7jsoCgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)