Abstract
A lentivirus pseudotyped with vesicular stomatitis virus G protein (VSV-G) encoding rat erythropoietin (EPO) complementary DNA was administered to rat skeletal muscle and red blood cell production was serially monitored. After a single intramuscular injection hematocrit values increased and reached a plateau at about 35 days and were sustained for at least 14 months. Virus doses of 6 × 107infectious units and 6 × 106 infectious units produced significantly increased mean hematocrit values of 68.5% ± 2.1% (P < .001, n = 4) and 52.7% ± 1.3% (P < .001, n = 3), respectively, over values of control animals receiving normal saline (46.2% ± 1.5%, n = 2). A polymerase chain reaction (PCR) assay for vector sequences in genomic DNA showed muscle tissue at the site of injection was positive and undetectable in liver, spleen, kidney, and lung. The intramuscular administration of lentivirus provided a dose-responsive, highly efficient and sustained EPO gene delivery, suggesting these vectors may be applied generally to the systemic delivery of proteins such as hormones and clotting factors.
Introduction
The recently described lentiviral vectors have the advantage over murine leukemia retroviral vectors of enabling provirus integration into nondividing cells.1-7 These vectors are constructed by incorporating elements from human immunodeficiency virus 1 (HIV-1) that interact with the nuclear import system and mediate transport via the nucleopore into the cell's nucleus.1-3Significantly, this vector system has achieved transduction of terminally differentiated brain tissue,1,8 liver, muscle,3 and a variety of human cells.5,7,9-11 In most reports lentiviral vectors were pseudotyped with envelope glycoproteins from amphotropic murine leukemia virus (MLV) or vesicular stomatitis virus G protein (VSV-G).1-3,8 12-14 Two major benefits conferred by VSV-G pseudotyping are a more robust virus that can be easily concentrated by centrifugation and a broad tropism.
Erythropoietin (EPO) is a 30-kd glycoprotein hormone that is the regulator of red blood cell production and maintenance in mammals.15,16 The administration of recombinant EPO (rEPO) is now widely used for long-term treatment of anemia associated with chronic renal failure, cancer chemotherapy, and HIV infections.16 Delivery of this hormone by gene therapy rather than by repeated injections may provide clinical and economic benefits and would serve as a model for the expression of other therapeutic proteins. We investigated whether a self-inactivating lentiviral vector encoding rat EPO was able to mediate long-term gene expression after a single administration to rat muscle.
Materials and methods
Vector construction and production
The lentiviral expression vector pHR′CMV-rEPO-SIN was created by excising β gal transgene from pHR′CMV-LACZ-SIN4and inserting a rat EPO complementary DNA (cDNA) from LrEPSN.17 Virus was produced by transient calcium phosphate cotransfection in 293T cells of the HIV Gag/Pol/Tat/Rev packaging construct, pchelpΔvifΔvprΔvpu,18 VSV-G expression vector, pLTR-VSV-G,18 and the lentiviral transfer vector pHR′CMV-rEPO-SIN. Control virus pRRLCMV-eGFP-SIN was prepared as detailed.11 Viral supernatants were filtered through 0.45-μm pore filters, concentrated by ultracentrifugation at 20 000g for 2 hours, resuspended in Tris-buffered saline (TBS), pH 7.0, overnight, and stored at −80°C.
Virus titer
Virus titer for the eGFP lentivirus was determined by infection of HeLa cells in the presence of 10 μg/mL diethylaminoethyl dextran (DEAE-D) followed 3 days later by fluorescence activated cell sorting (FACS) analysis.10,11 Viral p24 Gag protein was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Coulter, Miami, FL) and comparing with titers of GFP virus obtained by FACS analysis.4,18 19 This assay showed a functional titer of 2.5 × 107 infectious units (IU)/mL for eGFP virus and this was equivalent to 1100 ng p24 protein/IU. Virus preparations were screened for replication competent virus by serial passaging transduced HeLa cells and monitoring supernate for p24 Gag protein. Such assays were negative.
Lentiviral infection and analysis of EPO production in vitro
HeLa cells were plated at a density of 105/6-cm diameter dish and infected the next day for 4 hours in the presence of 10 μg/μL DEAE-D and after 48 hours conditioned supernatants assayed for EPO by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Animal experiments
Virus was injected into 4 to 6 sites in the hind legs of anesthetized male Fisher 344 rats weighing 100 to 150 g, each rat receiving a total volume of 0.5 to 0.7 mL, to deliver a virus dose of 6 × 106 to 6 × 107 IU. DEAE-D was added to viral preparations at a final concentration of 20 μg/mL. Serial blood analyses were performed on EDTA-anticoagulated blood samples (300 μL) obtained from tail vein.17 Hematocrit (%), hemoglobin, platelet, and white blood cell (WBC) numbers were measured using a Coulter counter.17
Genomic DNA isolation and polymerase chain reaction analysis of vector tissue distribution
Genomic DNA was isolated from fresh or frozen tissue samples using a Nucleospin Tissue Kit (Clontech, Palo Alto, CA) following the manufacturer's instructions. Muscle from the injection site and liver, spleen, kidney, and lung tissues were harvested from treated and control animals. Polymerase chain reaction (PCR) was carried out using Taq DNA polymerase under the following cycling conditions: 94°C for 4 minutes followed by 40 cycles of 94°C for 30 seconds, 54°C for 30 seconds, 72°C for 30 seconds followed by 72°C for 5 minutes. Genomic DNA (50 ng) was used as template per each 25-μL PCR reaction. PCR primers to rEPO were as follows: sense 5′-AGGCGCGGAGATGGGGGTGC-3′, antisense 5′-GCCTCCTTGGCCTCCAAGA-3′. Because the genomic sequence of rat EPO is not available, we based the primers on the human genomic structure. The rEPO sense primer was located at the end of the first exon and the rEPO antisense primer was located at the end of the fifth exon. We estimated the amount of vector DNA present in the genomic DNA samples by adding increasing molar ratios of 0.1% to 1000% of vector DNA to genomic DNA to perform a competitive titration. PCR products were isolated on a 2% agarose gel and stained with ethidium bromide; stain intensity assessed using Gel Doc software (Biorad, Hercules, CA).
Results and discussion
HeLa cells infected with pHR′CMV-rEPO-SIN at a multiplicity of infection (MOI) of about 1 were cultured and conditioned medium assayed for EPO. The mean value of duplicate experiments assayed in triplicate were 16 500 ± 13.0 mU/106 cells per 24 hours. Bioactivity of secreted EPO was confirmed by showing proliferation of HCD57 cells, an EPO-responsive cell line20 (data not shown). Cells infected with pHR′CMV-rEPO-SIN in the presence of the HIV reverse transcriptase inhibitor zidovudine (AZT) secreted EPO at a level of 122.3 ± 6.1 mU/mL per 106 cells per 24 hours, indicating that cytokine expression was lentivirus mediated and not a result of pseudotransduction due to plasmid or protein transfer.
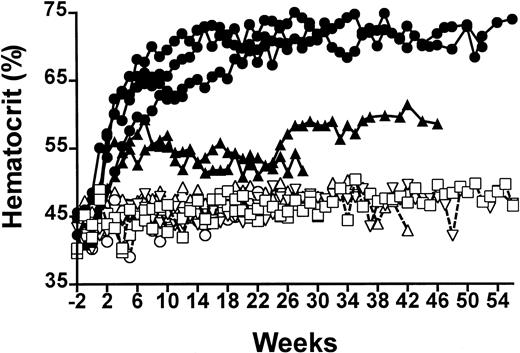
Rats injected intramuscularly with the pHR′CMV-rEPO-SIN lentivirus exhibited elevated hematocrit values for at least 14 months and showed a dose response to virus particle number (Figure1). The highest level of virus administration was 6 × 107 IU and induced peak sustained hematocrit levels of 68.5% ± 2.1%, which were significantly different from the 46.4% ± 1.6% hematocrit values of control animals receiving pHR′CMV-rEPO-SIN virus (P < .001, n = 4). Rats that received a 10-fold lower dose of virus showed mean elevated hematocrit values of 52.7% ± 1.3% (n = 3), which were significantly different from both control animals and those receiving the higher virus dose (P < .001). When 100 μg of the pHR′CMV-rEPO-SIN plasmid was injected into muscle of control rats, hematocrit levels of 46.3% ± 0.9% (n = 3) were observed. These levels were not significantly different from those in control animals that received injections of saline (46.2% ± 11.5%; P > .09). Animals injected with pRRLCMV-eGFP-SIN, a control lentiviral vector capable of conferring GFP expression, had hematocrit levels of 46.4% ± 1.6% (n = 2), which were not significantly different from saline control values (P > .12; Figure 1). Mean hemoglobin levels measured in rats receiving high-dose virus were 21.6 ± 2.6 g/dL and in animals receiving low-dose virus were 18.0 ± 0.8 g/dL; these levels were significantly elevated over values obtained from control rats (P < .005 andP < .02, respectively; Table1). Platelet numbers and WBC counts were not significantly different between any of the rats (P > .2; Table 1). These data suggest that the elevated hematocrit values we observed were due to sustained EPO expression from lentivirus transduction and were not caused by either viral proteins or pseudotransduction by plasmid DNA contained in the virus preparations.
Serial analysis of percent hematocrit of EPO-lentivirus–treated and control rats.
Closed symbols are rats receiving EPO-lentivirus 6 × 107IU (●) or 6 × 106 IU (▴). Open symbols are control rats receiving saline (○), eGFP lentivirus 5 × 106 IU (■) or 100 μg lentivirus plasmid (▵).
Serial analysis of percent hematocrit of EPO-lentivirus–treated and control rats.
Closed symbols are rats receiving EPO-lentivirus 6 × 107IU (●) or 6 × 106 IU (▴). Open symbols are control rats receiving saline (○), eGFP lentivirus 5 × 106 IU (■) or 100 μg lentivirus plasmid (▵).
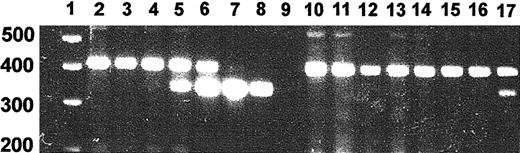
Because of the potential for virus spread beyond the muscle injection sites, we developed a PCR assay of genomic DNA that yielded a 350-bp product from the lentiviral integrant and a genomic product of 450 bp (Figure 2). The presence of 1% vector per haploid genome could be detected by this method. The predicted genomic product was 780 bp and this difference to the observed band size may be attributable to a difference between the rat and human genomic structure. We assayed the muscle injection sites and tissues obtained from spleen, lung, kidney, and liver of rats treated with pHR′CMV-rEPO-SIN and control rats injected with pRRLCMV-eGFP-SIN and pHR′CMV-rEPO-SIN expression plasmid. Four DNA samples obtained from muscle of a rat killed 14 months after EPO lentivirus injection were assayed and one was strongly positive for provirus (Figure 2, lane 17). DNA samples from spleen, lung, kidney, and liver from the same rat were all negative (Figure2). We observed that 20% of muscle samples from injection sites of 3 rats receiving EPO lentivirus were positive for vector DNA. This may reflect the difficulty of locating the exact injection sites more than 12 months after injection. All control tissues, including plasmid DNA-injected muscle, were negative for vector PCR.
PCR assay of provirus.
Genomic DNA was isolated from tissue samples and subjected to PCR using EPO-specific primers. PCR products were separated on a 2% agarose gel and stained with ethidium bromide. Shown is a PCR on negative control rat DNA in which increasing amounts of vector plasmid DNA were added. We established that the presence of 1% vector DNA could be detected by this method. Shown are assays of representative tissue DNA samples from a rat with an hematocrit of 74% killed 14 months after injection of EPO virus. Lane 1, molecular weight marker; lane 2, genomic DNA; lane 3, genomic DNA + 0.1% vector; lane 4, genomic DNA + 1% vector; lane 5, genomic DNA + 10% vector; lane 6, genomic DNA + 100% vector; lane 7, genomic DNA + 1000% vector; lane 8, vector; lane 9, water; lane 10, lung; lane 11, kidney; lane 12, liver; lane 13, spleen; lanes 14 to 17, muscle samples.
PCR assay of provirus.
Genomic DNA was isolated from tissue samples and subjected to PCR using EPO-specific primers. PCR products were separated on a 2% agarose gel and stained with ethidium bromide. Shown is a PCR on negative control rat DNA in which increasing amounts of vector plasmid DNA were added. We established that the presence of 1% vector DNA could be detected by this method. Shown are assays of representative tissue DNA samples from a rat with an hematocrit of 74% killed 14 months after injection of EPO virus. Lane 1, molecular weight marker; lane 2, genomic DNA; lane 3, genomic DNA + 0.1% vector; lane 4, genomic DNA + 1% vector; lane 5, genomic DNA + 10% vector; lane 6, genomic DNA + 100% vector; lane 7, genomic DNA + 1000% vector; lane 8, vector; lane 9, water; lane 10, lung; lane 11, kidney; lane 12, liver; lane 13, spleen; lanes 14 to 17, muscle samples.
These data are encouraging and demonstrate that a single administration of a lentivirus encoding EPO will permit sustained elevations of hematocrit and suggest this approach to the delivery of clotting factors and cytokines such as granulocyte colony-stimulating factor that do not require finely regulated expression. Our data showing increases in hematocrit values related to virus dose suggest the potential to control gene expression by controlling virus administration.
We thank Dr D. C. Dale for much helpful advice and Bryan Grogan for technical assistance, Drs J.-P. R. Biossel and H. F. Bunn for kindly supplying the rat EPO cDNA, and Drs R. Zufferey and D. Trono for supplying the lentivirus backbone plasmids.
Supported by grants DK50686, DK43727, and DK47754 from the National Institutes of Health and 902-23-248 from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William R. A. Osborne, Department of Pediatrics, RR244, MS 356320, University of Washington School of Medicine, Seattle, WA 98195; e-mail: wosborne@u.washington.edu.