Abstract
Standard allogeneic stem cell transplant (allo-SCT) regimens have been associated with a high transplant-related mortality (TRM) in multiple myeloma (MM). Nonmyeloablative therapy can establish stable engraftment after allo-SCT and maintain the antitumor effect with less toxicity, which is important in heavily pretreated and elderly patients. We report on 16 poor-risk MM patients receiving allo-SCT from an HLA-matched (n = 14) or mismatched (n = 2) sibling following conditioning with melphalan 100 mg/m2 (MEL-100). Ten patients had refractory relapse, 4 responsive relapse, and 2 patients were in near complete remission (nCR) with poor-prognosis disease. Patients had received 1 (n = 9) or 2 (n = 7) prior autotransplants. Donor lymphocyte infusions (DLIs) were given to 14 patients with no clinical evidence of graft versus host disease (GVHD) either to attain full donor chimerism (n = 4) or to eradicate residual disease (n = 10). Fifteen patients showed myeloid engraftment, and 12 patients were full donor chimeras at day +21. No TRM was observed during the first 100 days. Acute GVHD developed in 10 patients; 1 had fatal grade IV GVHD. Seven progressed to chronic GVHD, limited in 3 and extensive in 4 patients. At a median follow-up of 1 year, 5 patients achieved and sustained CR, 3 nCR, and 4 partial remission. Of 4 patients progressing after transplantation, 3 achieved a remission following further chemotherapy and DLI. Remarkable graft versus myeloma responses were seen in chemotherapy-refractory patients. Two patients died of progressive disease, and 3 died of GVHD complications without active disease. GVHD remains a major problem with this procedure.
Introduction
Multiple myeloma (MM) is a chemotherapy-refractory hematologic malignancy. High-dose therapy with melphalan 200 mg/m2 (MEL) has affected complete remission (CR) rates in 50% of newly diagnosed patients, which can be durable in the absence of chromosome 13 abnormalities and in patients presenting with low β2-microglobulin.1 The role of standard allogeneic stem cell transplantation (allo-SCT) in MM patients remains controversial. Allo-SCT has usually been evaluated in the setting of advanced and refractory disease, often after failure of high-dose therapy and auto-SCT. In such setting, transplant-related mortality (TRM) in the first 100 days was 30% to 50% in most published series.2-7 However, 30% to 50% of patients who survive the first year remain disease-free at 3 to 6 years, with well-documented cases of sustained molecular remissions.8,9 Such durable remissions have been attributed to a graft versus myeloma (GVM) effect. Further support for a GVM effect comes from donor lymphocyte infusion (DLI)-induced remissions following allo-SCT relapses.10-13
Recently, several groups have reported successful stable engraftment following immunosuppressive nonmyeloablative conditioning regimens and peripheral blood stem cell (PBSC) infusions.14-21 In this report, we evaluate the effect of a nonmyeloablative preparative regimen of MEL 100 mg/m2 (MEL-100) with cyclosporine A on engraftment in patients who had received at least one auto-SCT previously. Preemptive DLIs were scheduled on day 21, 42, and 112 to maximize GVM and to establish full donor chimerism. This approach aims at expanding the applications of allo-SCT to patients who otherwise would not have been eligible for standard allo-SCT.
Patients and methods
Study design
Patients and donors in the study provided written informed consent that had been approved by the Institutional Review Board of the University of Arkansas for Medical Sciences. The conditioning regimen consisted of intravenous infusion of MEL-100 over 20 minutes on day −1. Cyclosporine A was given as intravenous infusion at 3 mg/kg/day starting on day −1, with a target level of 300 ng/mL. Patients were switched to oral cylosporine when tolerated.
On day 0, patients received granulocyte colony-stimulating factor (G-CSF)–mobilized unmanipulated allogeneic PBSC grafts from their HLA-matched siblings. Donors were mobilized with 10 μg/kg/day G-CSF. On day 4, large-volume leukapheresis (> 15 L) was performed. Mobilized PBSCs were also collected on days 5, 6, and 7, if necessary, to attain 10 × 106 CD34 cells per kilogram of the patient's weight. Targeted stem cell infusion was 5 × 106 CD34 per kilogram (or the first collection); the rest was stored for DLI. Stem cells were cryopreserved unmanipulated.
In the absence of graft versus host disease (GVHD), cyclosporine A was tapered over 1 month starting on day 60 after allo-SCT, and additional DLIs with escalating doses of donor CD3 lymphocytes were scheduled on days 21, 42, and 112 to establish full chimeric engraftment in patients with no GVHD. Initially all patients received DLIs on the scheduled days. Later in the study, decisions regarding DLI were based on the chimeric status of the patients. Those with mixed chimerism received additional donor cells after 3 to 4 weeks of cylosporine withdrawal in the absence of acute GVHD. Patients who had residual or progressive disease after cyclosporine A withdrawal and who had no evidence of GVHD received additional DLIs with no GVHD prophylaxis. Patients who relapsed or had no response to DLI received chemotherapy followed by additional DLIs. In the absence of GVHD, some relapses were treated with subcutaneous interferon alfa at a low dose of 1 × 106 to 2 × 106 IU given every other day in an attempt to enhance a GVM effect.
Antimicrobial prophylaxis during the transplantation period consisted of acyclovir, 400 mg/day orally from day 0 to 6 months, and itraconazole, 400 mg/day until the CD4 count exceeded 400/μL. Patients received levofloxacin, 500 mg/day from day 0 to 2 years after transplantation, and cotrimoxazole from neutrophil recovery to 6 months. Patients at high risk of fungal infection, those unable to tolerate oral itraconazole, and those with severe diarrhea received AmBisome, 1 mg/kg/day. Blood samples were checked each week for cytomegalovirus (CMV) antigenemia; those who tested positive were treated with ganciclovir. Patients received intravenous immunoglobulin, 0.5 g/kg, if the immunoglobulin G level was below 500 mg/dL.
Study end points
The primary end points of the study were to evaluate engraftment, chimerism, toxicity, incidence of acute GVHD, and day 100 TRM following MEL-100. Engraftment was defined as neutrophil recovery to more than 0.5 × 109/L for 3 consecutive days and an untransfused platelet count above 20 × 109/L. The degree of donor-recipient chimerism was assessed by polymerase chain reaction assay of short tandem repeat loci according to the published methods.22 Chimeric studies were assessed at day 14, 21, 42, 100, and monthly thereafter for 1 year. Chimerism was quantitated by calculating the percentage of donor DNA in comparison to the pretransplant donor and recipient genotype. The assay is able to detect chimerism if more than 5% donor DNA is present. Mixed chimerism was defined as the presence of more than 5% donor- and host-derived cells on more than one occasion in the whole blood. Acute GVHD was assessed according to the criteria of the International Bone Marrow Transplant Registry. Other toxicities were assessed using National Cancer Institute criteria.
Secondary end points included myeloma response, incidence of chronic GVHD, and immune reconstitution. Chronic GVHD was defined as GVHD occurring 100 days or more after bone marrow transplantation and was graded as none, limited, or extensive. Immune reconstitution was studied at 1, 2, 3, 6, 9, 12, and 15 months by analysis of peripheral blood T cells using fluorescein isothiocyanate–phycoerythrin-labeled antibodies against CD3, CD4, CD8, and immunoglobulin levels.
CR required the disappearance of monoclonal gammopathy in serum and urine on immunofixation analysis and normal bone marrow aspirate and biopsy on 2 successive occasions 2 months apart. Near complete remission (nCR) is defined as a CR with only positive immunofixation. Partial remission (PR) is 75% tumor mass reduction. Very good PR (VGPR) required more than 90% tumor mass reduction. Early TRM included any death within 100 days following allo-SCT. Late TRM included any death after day 100 related to complications of GVHD, immunodeficiency, or organ failure not caused by disease progression. Relapse was defined as recurrence of monoclonal protein or bone marrow plasmacytosis in cases of CR. Progression for non-CR patients implied at least a 25% increase in tumor mass or the development of new extramedullary disease.
Results
Patients
Patient characteristics before allo-SCT are shown in Table1. The study population included 16 MM patients who were ineligible for conventional myeloablative conditioning because of prior auto-SCT or comorbidity. Of the 16 patients, 14 received a fully HLA-compatible graft as determined serologically for class I (A, B, C loci) and molecularly for class II (DRB1, DQB1 loci). Two patients received a graft from a family donor mismatched at a single class II HLA locus. The patients ranged in age from 42 to 70 years (median 57). All patients had received one (n = 9) or 2 (n = 7) prior auto-SCT. Eleven patients had been treated with salvage thalidomide following relapse. At the time of allograft, 10 patients had progressive and refractory disease following relapse, whereas 6 patients were chemosensitive (4 patients were in PR after therapy for relapse and 2 were recently diagnosed and in nCR after auto-SCT). All of these patients had one or more prognostic factors associated with poor outcome, such as complex cytogenetic profile (n = 16), deletion of chromosome 13 (n = 13), or cytogenetic and clinical evidence of myelodysplastic syndromes (MDS) in addition to active myeloma (n = 2). Before conditioning, the median number of CD4 lymphocytes was 151/μL (range 55-420) and CD8 lymphocytes was 82/μL (range 42-676).
Engraftment, chimerism, and immune reconstitution
Patients received a median of 5.5 × 106CD34+ cells per kilogram (range 3.1 × 106 to 12.5 × 106) and 2 × 108 CD3 cells per kilogram (range 1.2 × 108 to 2.2 × 108). None received G-CSF after transplantation. All patients became neutropenic (< 0.5 × 109/L) for a median of 6 days (range 4-30). Neutrophil recovery to more than 1 × 109/L was reached at median of 15 days after SCT (range 10 to 46); the median time to an unsupported platelet count above 20 × 109/L was 14 days (range 0 to 50). The median number of red blood cell transfusions was 9 (range 2-29); the median number of platelet transfusions was 8 (range 0-70), and the median time from transplantation to last platelet transfusion was 30 days (range 0-160+). Five patients did not require any platelet support.
At 21 to 30 days after transplantation, 12 of 16 patients had engrafted with all cells of donor origin; 10 of 11 evaluable patients have maintained full donor hematopoiesis. One patient undergoing transplantation in PR failed to show myeloid engraftment even after an additional allo-PBSC infusion on day 21. She eventually received autologous stem cell rescue and remains in nCR at last follow-up. The other 3 patients (patients 1, 5, and 15) remained mixed chimeras until additional DLIs were given; 2 of them (patients 5 and 15) had a pre–allo-SCT CD4 count above 400/μL (Table2). Four patients had major ABO incompatibilities. There was no significant clinical hemolysis in these patients and no increase in the numbers of red cell and platelet transfusions in the first 100 days.
All evaluable patients have experienced delayed recovery of T-cell numbers, which appeared to be caused mainly by the development and treatment of GVHD. The median CD4 count was 148/μL (range 70-351) and 299/μL (range 107-599) at day 60 and 120, respectively. In patients with no or grade I GVHD (n = 7), the median CD4 count was 201/μL and 338/μL at day 60 and 120, respectively, compared with 95/μL and 231/μL for those with higher than grade II GVHD (n = 9). In general, CD8 recovery preceded CD4 recovery with a median CD8 count of 210/μL (range 57-988) at day 60 and 396/μL (range 116-1671) at day 120. Seven of 11 surviving patients have achieved CD4 counts of more than 300/μL. The 4 patients with low persistent CD4 counts are still on immunosuppressive therapy for chronic GVHD.
Clinical response
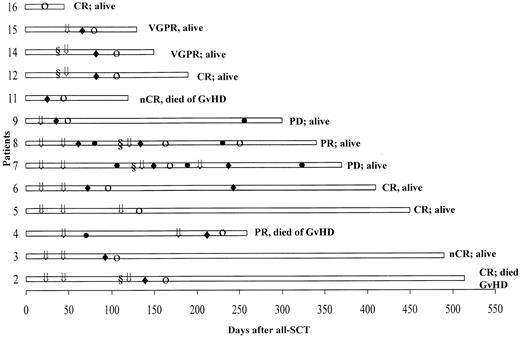
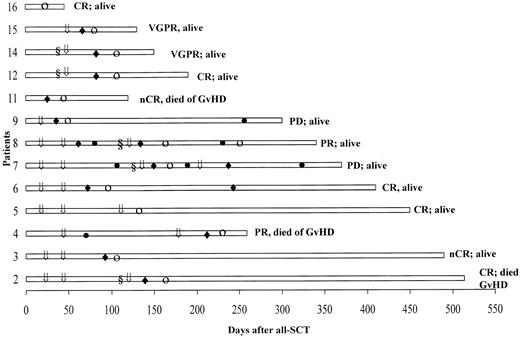
Table 3 lists the data on monoclonal protein and bone marrow plasmacytosis before and after allo-SCT (best response). In these 16 refractory and poor-prognosis patients, 12 (75%) had an excellent response following the allograft, with a stringently defined CR achieved in 5 patients, nCR in 3, VGPR in 2, and PR in 2 patients. The onset of response was typically delayed, as illustrated in Figure 1. The time to response following development of GVHD was brief, with a median of 14 days (range 7-42). Patients 3 and 6 with cytogenetic evidence of MDS prior to allo-SCT had normal karyotypes at last follow-up. GVHD developed in 6 patients following discontinuation of cyclosporine A at a median of 24 days (range 11-40). Seven patients developed GVHD following additional DLIs. Patient 11 had severe GVHD, which resulted in her demise by day 120. Myeloma responses in refractory patients were only seen following the development of acute GVHD.
Posttransplantation course in 13 patients who achieved a response.
The onset of response (○) typically followed the onset of GVHD (♦). Patients received DLI (⇓) alone or following chemotherapy (§); ● denotes progressive disease.
Posttransplantation course in 13 patients who achieved a response.
The onset of response (○) typically followed the onset of GVHD (♦). Patients received DLI (⇓) alone or following chemotherapy (§); ● denotes progressive disease.
Donor lymphocyte infusions and subsequent therapy
Three patients received all planned DLIs at days 21, 42, and 112. Five patients received only one DLI (2 at day 21, 3 at day 42). Four patients received infusions only on day 21 and day 42. Because of a high incidence of GVHD and because stable grafts were established following allo-PBSC infusion, additional DLIs were only given to establish full chimerism if it was not reached by day +30 or to patients with evidence of extensive residual or progressive disease. Patients 12 and 14 had a persistent large tumor burden on day 45 with no response to discontinuation of cyclosporine A; they received DCEP (dexamethasone, cyclophosphamide, etoposide, and cisplatin) chemotherapy before DLI on day +60. Both patients developed GVHD of the liver (grade II) at day 90; one attained a CR and one a PR 2 weeks later.
Toxicity and mortality
In this cohort of heavily pretreated high-risk MM patients, allo-SCT with this nonmyeloablative protocol was extremely well tolerated. No TRM occurred during the first 100 days. The median duration of hospitalization was 14 days (range 12-67). The major regimen-related toxicity observed was GVHD (Table4). Eight patients developed grade II acute GVHD and one, who received an HLA-mismatched graft, had grade IV GVHD. Seven patients developed GVHD following additional DLIs (Table2). Grade II GVHD responded well to prednisolone and cylcosporine. Aspergillosis developed in patients 3 and 11 following corticosteroid therapy for acute GVHD. Three patients developed limited chronic GVHD. Four patients had extensive chronic GVHD, 2 of whom died of infectious complications. One patient had a generalized seizure on day 18. Magnetic resonance imaging revealed limbic encephalopathy, which resolved after stopping cyclosporine A. Idiopathic pneumonitis developed in 2 patients, one of whom progressed to bronchiolitis obliterans and is currently maintained on corticosteroid inhalers. Venoocclusive disease was not observed. All patients had more than one episode of fever. Six patients had coagulase-negative staphylococcus bacteremia at a median of 120 days (range 60-300). CMV antigenemia was detected in 12 patients at a median of 38 days (range 18-160) after allo-SCT; all were CMV antibody-positive prior to allo-SCT with a CMV+ donor. This complication was successfully treated with ganciclovir in 11 or foscarnet in 1 patient. None developed CMV disease. Overall, 3 patients died of late transplant-related complications (18%); 2 patients died of sepsis and 1 of disseminated aspergillosis as a complication of immune suppressive therapy for GVHD.
Progression-free and overall survival
As of September 15, 2000, 11 patients are alive 45 to 490 days after transplantation (median follow-up 1 year) with a Karnofsky performance status of 90 to 100. Five patients have died; the cause of death was progressive disease in 2 and late TRM in 3 patients. Of the 8 patients in CR/nCR, 2 died of GVHD complications and 6 remain in stable remissions, 1 after infusion of autologous stem cells. Two patients (patients 14 and 15) achieved a VGPR following GVHD. Three patients (patients 2, 7, and 8) had evidence of progressive disease after allo-SCT. They received salvage therapy with DT-PACE (dexamethasone, thalidomide, cisplatin, adriamycin, cyclophosphamide, and etoposide) and DLI. Patient 2, who had an extramedullary relapse with a large renal mass and central nervous sytem involvement, achieved subsequently a CR; he eventually died of GVHD complications. Patient 8 achieved a stable PR. Patient 7 had a brief response and is currently alive with stable on interferon alfa.
Discussion
Our study suggests that MEL-100 without fludarabine or total body irradiation can establish a durable and stable alloengraftment in heavily treated MM patients. None of these patients were candidates for conventional myeloablative conditioning, because of age, comorbid conditions, or extent of prior therapy. It is conceivable that low-dose MEL-100 is not adequately immunosuppressive for newly diagnosed patients with an intact immune system. In our center, 2 relapsed lymphoma patients with no preceding auto-SCT rejected their allograft following MEL-100 (unpublished data, 2000).
Mel-100 resulted in mild toxicity and brief hospitalization. TRM was much lower than in historical patients receiving myeloablative conditioning therapy (18% vs 30%-50%). None of the patients died in the immediate 100 days following allo-SCT. The incidence of infectious complications, especially CMV reactivation after engraftment, was high, as reported previously with nonmyeloablative transplants.23 This is probably related to the immunocompromised state caused by the allo-SCT, acute and chronic GVHD, and its treatment. There are minimal data concerning the kinetics of immune reconstitution in MM patients receiving nonmyeloablative conditioning regimens. Nevertheless, the pace of recovery of T-cell numbers and function in the absence of chronic GVHD and continuing immunosuppressive therapy appears adequate. Sixty percent of evaluable patients achieved normal CD3 and CD4 counts at a median of 6 months. Recovery of lymphocyte counts appeared to parallel immune function recovery; these patients were now able to clear viral infections (eg, CMV antigenemia) following a brief therapy with ganciclovir. Longer follow-up will be necessary to confirm that adequate T-cell and B-cell functions can be reestablished.
Chimeric data showed that most patients (12 of 16) engrafted with full donor hematopoiesis at day 21. Three patients remained mixed chimeras until additional allo-PBSCs were infused. Because of the high incidence of GVHD, the most serious complication of our regimen, the planned DLIs were subsequently limited to those with either residual disease or mixed chimerism. The incidence of acute GVHD was comparable to that seen with conventional SCT and occurred mostly after DLI or after early withdrawal of cyclosporine A. No acute GVHD was seen in the first 45 days. The absence of early GVHD is likely a reflection of the minimal tissue damage and the absence of a severe cytokine storm associated with MEL-100. The incidence of debilitating chronic GVHD resulting from this approach remains to be determined. However, the fact that this complication developed in 6 of 11 long-term survivors is concerning.
We found that despite the advanced refractory disease of most of our patients at the time of allo-SCT, 75% had a remarkable response (at least PR) and 30% achieved a stringently defined CR. A GVM effect must be responsible for these responses because most patients were refractory to or had relapsed after high-dose chemotherapy with MEL conditioning. Moreover, all the responses in these refractory and relapsed patients occurred shortly after development of GVHD. The median time to response was therefore delayed, which means that patients with rapidly growing disease may not benefit from this therapy as seen in patients 1 and 10.
In summary, despite the small number of patients in this study and the relatively short follow-up, we have shown that MEL-100 is sufficiently immunosuppressive to establish full donor engraftment in high-risk MM patients after auto-SCT. The regimen was well tolerated even in heavily pretreated elderly patients with no early TRM. The preemptive DLI, while maximizing GVM, exposed patients to significant risk of GVHD. GVM effect consistently followed the development of GVHD, which remained the most significant complication. Future studies will focus on minimizing the risk of GVHD by escalating the T-cell doses in the graft and subsequent DLIs. We are now applying this nonmyeloablative allo-SCT to newly diagnosed patients with high-risk disease after an initial auto-SCT, preferentially at a stage of minimal residual disease.
We dedicate our work to all our patients, in the hope that one day this disease will be cured. We are indebted to the 7A nursing staff for their dedication and care.
Supported in part by National Institutes of Health grant PO1-CA55819-06.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ashraf Badros, 4301 West Markham, Slot 776, Little Rock, AR 72205; e-mail: badrosashrafz@exchange.uams.edu.