Abstract
Erythrodermic cutaneous T-cell lymphoma (CTCL) includes patients with erythrodermic mycosis fungoides who may or may not exhibit blood involvement and Sézary syndrome and in whom hematological involvement is, by definition, present at diagnosis. These patients were stratified into 5 hematologic stages (H0-H4) by measuring blood tumor burden, and these data were correlated with survival. The study identified 57 patients: 3 had no evidence of hematologic involvement (H0), 8 had a peripheral blood T-cell clone detected by polymerase chain reaction (PCR) analysis of the T-cell receptor gene and less than 5% Sézary cells on peripheral blood smear (H1), and 14 had either a T-cell clone detected by Southern blot analysis or PCR positivity with more than 5% circulating Sézary cells (H2). Twenty-four patients had absolute Sézary counts of more than 1 × 109 cells per liter (H3), and 8 patients had counts in excess of 10 × 109 cells per liter (H4). The disease-specific death rate was higher with increasing hematologic stage, after correcting for age at diagnosis. A univariate analysis of 30 patients with defined lymph node stage found hematologic stage (P = .045) and lymph node stage (P = .013) but not age (P = .136) to be poor prognostic indicators of survival. Multivariate analysis identified only lymph node stage to be prognostically important, although likelihood ratio tests indicated that hematologic stage provides additional information (P = .035). Increasing tumor burden in blood and lymph nodes of patients with erythrodermic CTCL was associated with a worse prognosis.The data imply that a hematologic staging system could complement existing tumor-node-metastasis staging criteria in erythrodermic CTCL.
Introduction
Mycosis fungoides is the most common form of cutaneous lymphoma and represents 70% of all cases of cutaneous T-cell lymphoma (CTCL).1 Patients with mycosis fungoides normally present with cutaneous patches and plaques and pursue an indolent clinical course. However some patients may progress to erythrodermic disease, which may be associated with morphological changes in the peripheral blood. By contrast, Sézary syndrome is a leukemic variant of CTCL that is characterized clinically by erythroderma, pruritus, and peripheral lymphadenopathy. It is an aggressive disease associated with a poor prognosis and median survival of 3 years.
Mycosis fungoides may be classified by the tumor-node-metastasis (TNM) staging system devised by the Mycosis Fungoides Co-operative Group and the National Cancer Institute (NCI), Bethesda, MD, in 1979.2 It is based upon the type of skin lesion, extent of skin involvement, and the presence of lymph node or visceral disease. This staging system has been shown to stratify patients with mycosis fungoides into useful prognostic categories.3,4 A review of the reported 5-year survival rates of patients with different stages of mycosis fungoides found survival rates of 80% to 90% for patients with stage I; 60% to 70%, stage II; 40% to 50%, stage III; and 25% to 35%, stage IV.5
The NCI also defined a B1 category for hematological involvement representing more than 5% Sézary cells, as a percentage of the total lymphocyte count. However, the prognostic significance of B1 was uncertain at that time and was not therefore included in the overall staging system. In 1988, the NCI revised its definition of B1 from 5% to 20% on the grounds that this figure carried greater prognostic significance,6 although in multivariate analysis the most important variables for survival were skin stage and visceral involvement.6
Circulating Sézary cells were originally identified by Sézary et al7 in 1938 as large atypical mononuclear cells (“cellules monstreuses”). Some 30 years later, the distinctive grooved nucleus was described and became the morphologic hallmark of the Sézary cell.8 Later that decade the Sézary cell nucleus was further characterized by electron microscopy.9 Large Sézary cells greater than 14 μm are specific to Sézary syndrome, but smaller cells with Sézary-type morphology may be present in 20% to 25% of patients with mycosis fungoides,10 in certain inflammatory dermatoses including eczema and psoriasis,11 and even in some healthy controls.12 Furthermore, Sézary cells may be produced in vitro by stimulating normal T cells.13
A peripheral blood smear is used to count the number of Sézary cells per 100 lymphocytes and/or leukocytes, but there has been no consensus on the percentage of Sézary cells required for the diagnosis of Sézary syndrome. Prior to the introduction of T-cell receptor gene analysis, the main concern was to exclude patients with benign inflammatory disorders. Vonderheid et al14 reported that a Sézary count of greater than 15% was seldom found in benign diseases, and on examination of a peripheral blood smear, it was the criterion that best correlated with the demonstration of a chromosomally abnormal clone. The presence of large cells (15 to 20 μm) was also predictive of a malignant clone.14Schechter et al found that patients with more than 20% large Sézary cells (greater than 11 μm) had a poorer survival rate than those with a predominately small cell variant.10However, the skin stage and age were the most important pretreatment risk factors for survival.10 Of note, peripheral blood involvement was not found to be an independent risk factor for T4 stage disease. However, a more recent study by Kim et al15 reported that in erythrodermic CTCL, the presence of peripheral blood Sézary cells representing more than 5% of the total lymphocyte count was found to be an independent prognostic factor influencing survival. Other important variables affecting survival included stage III versus stage IV and age more than 65 years.15 Russell-Jones and Whittaker16therefore proposed that 5% may be used as a minimum definition of Sézary syndrome provided that a peripheral blood T-cell clone can be demonstrated by other means. By contrast, the EORTC cutaneous lymphoma group suggested that a peripheral blood T-cell clone plus a CD4:CD8 ratio greater than 10 were useful criteria in distinguishing Sézary syndrome from other forms of erythroderma.1Finally, Winkelman and Peters17 proposed an absolute Sézary cell count of 1 × 109 cells per liter for the diagnosis of Sézary syndrome. This has recently been adopted by the International Society for Cutaneous Lymphoma (ISCL) in their consensus conference on erythrodermic CTCL (Vonderheid et al, manuscript submitted, 2000).
Our group has recently shown that single-strand conformational polymorphism/polymerase chain reaction (PCR) analysis of the T-cell receptor γ gene is a useful method for detecting early peripheral blood involvement in patients with mycosis fungoides. The presence of a peripheral blood T-cell clone was found to have prognostic significance, which was independent of skin stage and age.18 Single-strand conformational polymorphism/PCR analysis has a detection sensitivity between 0.1% and 1% compared with Southern blot T-cell receptor gene analysis, which has a sensitivity closer to 5%. We therefore stratified our patients according to the different methods for detection of hematologic involvement in erythrodermic CTCL. Patients with no evidence of a peripheral blood T-cell clone by both PCR and Southern blot T-cell receptor gene analysis were assigned H0 stage. Patients who were PCR-positive but Southern blot–negative and had less than 5% circulating Sézary cells on a peripheral blood smear were assigned H1 stage disease. Patients with H2 stage disease were either PCR- or Southern blot–positive and had more than 5% circulating Sézary cells. The H3 stage comprised patients with more than 1 × 109 circulating Sézary cells per liter; H4 stage included patients with more than 10 × 109circulating Sézary cells per liter. We undertook a retrospective analysis of 57 patients with erythrodermic CTCL, stratified the patients into one of these 5 groups according to results at initial presentation, and correlated this hematologic stage with outcome.
Patients, materials, and methods
Patient selection
We initially selected 84 patients with erythrodermic CTCL who were seen at the Skin Tumour Unit, St John's Institute of Dermatology, London, England, between 1980-1999. All patients had erythroderma (defined as skin involvement of more than 90%). Those with mycosis fungoides had shown diagnostic skin histology and a cutaneous T-cell clone, as demonstrated by T-cell receptor gene rearrangement studies. Patients with Sézary syndrome had erythroderma with compatible skin histology, atypical circulating cells, and a peripheral blood T-cell clone. For the purposes of this study, patients were not divided into those with features of erythrodermic mycosis fungoides and those with Sézary syndrome, as this distinction is made on hematological criteria, which is the subject of the study.
Only patients with at least a 3-year follow-up (diagnosed in 1997 or earlier) or patients who were diagnosed after 1997 but subsequently died from a CTCL-related disease were included in the study. Patients diagnosed elsewhere and referred to St John's within 6 months, providing no systemic treatment had been initiated, were also included in the study. Using these criteria, 57 of the 84 patients with erythrodermic CTCL were eligible for the study.
From the hospital records, the automated total white cell count, lymphocyte count, CD4:CD8 ratio, Sézary count, and serum lactate dehydrogenase (LDH) level were recorded from the time of diagnosis of erythrodermic CTCL. Peripheral blood smears were examined for the presence of Sézary cells (A.D., Norfolk and Norwich Hospital, Norwich, England) as previously described.14 The absolute Sézary count was calculated from the percentage of atypical cells on a smear and the absolute lymphocyte count. These data were used to stage the extent of hematologic involvement in each patient. The staging criteria H0-H4, which was discussed in the “Introduction,” is shown in Table 1. Each patient was staged from initial diagnosis at the Skin Tumour Unit or, in the case of those referred from elsewhere, within the first 6 months after diagnosis.
In addition, we recorded the age at diagnosis of erythrodermic CTCL, presence of clinical lymphadenopathy, and results of lymph node biopsies performed at diagnosis. A lymph node stage was assigned to each patient according to TNM criteria (Table2).2 In our institute, only patients with palpable lymphadenopathy have lymph node biopsies, and therefore patients with N2 could not be determined. The clinical outcome of each patient was recorded in years survived since diagnosis. In cases where patients died, cause of death was divided into either CTCL-related or CTCL-unrelated deaths. This information was obtained from the death certificate.
Statistical analysis
H0 and H1 stages were considered together when comparing survival data due to the small numbers in each group. The Cox proportional hazard regression model was first used to correct for age at diagnosis, as increasing age is known to be an independent poor prognostic factor in CTCL. We corrected for age as a continuous variable. Each increasing decade in age at diagnosis was found to be associated with a hazard ratio of 1.6 (confidence interval (CI) = 1.12-2.29; P = .009).
The number of observed and expected deaths from disease during follow-up was compared, and any differences between each group were tested for statistical significance using the log-rank test. The disease-specific death rate in patients with H0-H1 stage was compared to those with H2 stage; H0-H2 stage was compared with H-3 stage; and H0-H3 stage was compared with H4 stage disease. We tested the disease-specific death rate using the Cox model. Actuarial survival curves were calculated from the date of diagnosis using the Kaplan-Meier method for both death from CTCL-related disease and death from any cause.
Patients in whom a lymph node biopsy was performed at diagnosis and those patients without clinical lymphadenopathy at diagnosis were analyzed using nonadjusted (univariate) hazard ratios to determine the prognostic impact of age, lymph node stage, hematologic stage, and presence of visceral disease. Adjusted comparisons (multivariate analysis) were then performed with the identified significant univariate factors using the Cox model to establish their significance as independent prognostic factors. The likelihood ratio test was then used to determine the prognostic impact of each variable upon the other.
Results
We identified 57 patients with erythrodermic CTCL for the study. The mean age at diagnosis was 63 years (range, 38-84 years). The total follow-up period was 247 patient-years, with a mean follow-up of 4.3 years. During that time, 20 patients were alive, 28 patients died from CTCL-related disease, and 9 patients died from unrelated causes (3 patients from pneumonia; one patient from a myocardial infarction, congestive cardiac failure, and endometrial carcinoma; and 3 patients from lung carcinoma). The patients were staged according to the peripheral blood tumor burden at diagnosis using the staging system H0-H4 (Table 1). The number of patients in each stage and their clinical characteristics are summarized in Table3.
Three patients with erythrodermic CTCL did not have a peripheral blood T-cell clone detected by Southern blot analysis or PCR. Two patients were diagnosed prior to routine diagnostic use of PCR (1991), but analysis after this time demonstrated a T-cell clone in lesional skin but not in peripheral blood (H0). Eight patients had a peripheral T-cell clone detected by PCR and less than 5% circulating Sézary cells on a peripheral blood smear (H1). The mean age at diagnosis of erythrodermic CTCL was 47 years for patients with H0 stage and 65 years for patients with H1 stage, and the mean follow-up period from diagnosis was 11 years for patients with H0 stage and 6.6 years for patients with H1 stage. There were 3 deaths during follow-up: one death was related to CTCL with H1 stage disease, and 2 deaths were unrelated to CTCL (one from each stage, H0 and H1). This corresponded to a disease-specific death rate of 0.02 in stage H1 or 0.01 per year when both groups were analyzed together.
Fourteen patients were identified with H2 stage disease. The mean age at diagnosis was 60 years, and the mean follow-up from diagnosis was 3.9 years. There were 9 deaths in this group, of which 6 deaths were CTCL-related. This corresponds to a disease-specific death rate of 0.11 per year. The most frequent hematologic stage at diagnosis of erythrodermic CTCL was H3, and 24 patients were identified with this stage, with a mean age at diagnosis of 64 years. The mean follow-up was 3.4 years, and there were 19 deaths during this time, of which 15 deaths were CTCL-related. This equates to a disease-specific death rate of 0.18 per year. Eight patients had H4 stage disease with a Sézary count of more than 10 × 109 cells per liter at diagnosis; the mean age at diagnosis was 67 years, and the mean follow-up time was 3.0 years. There were 6 CTCL-related deaths in this stage, corresponding to a death rate of 0.25 per year. Serum LDH was only available in the 25 patients diagnosed after 1995. The mean serum LDH rose with increasing hematologic stage, from 656 U/L in H0-H1 stage disease (normal range, 286-580 U/L), 718 U/L in H2 stage, 772 U/L in H3 stage, and 918 U/L in H4 stage.
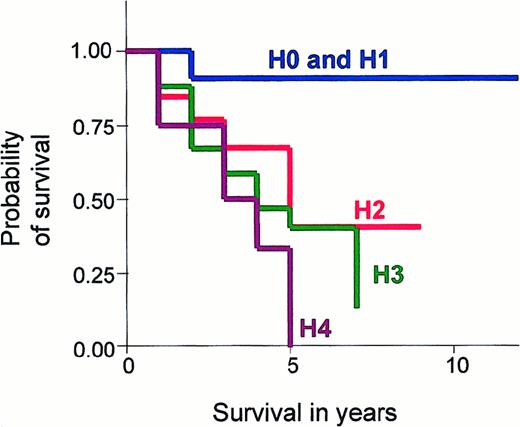
It is known that increasing age is associated with a worse survival in CTCL. Prior to statistical comparison between these hematological stages, we corrected for age variance by using the Cox proportional regression hazard ratio. The observed and expected CTCL-related deaths were compared using the log-rank test and were found to be significantly less than expected in H0 and H1 stage disease and significantly higher in H3 and H4 stage disease, after correcting for age (P = .010). The actuarial survival curves for each hematologic stage are shown in Figure 1, and outcome is defined as CTCL-related deaths.
Graph showing Kaplan-Meier survival estimates, given by hematologic stage in erythrodermic CTCL.
Comparison of Kaplan-Meier survival estimates in patients with different hematologic stages of disease (H0-H4): (1) H0 stage is no evidence of blood involvement; (2) H1 stage is a T-cell clone identified by PCR analysis of the T-cell receptor gene and less than 5% Sézary cells on a peripheral blood smear; (3) H2 stage is a T-cell clone identified by Southern blot analysis or a clone by PCR and more than 5% Sézary cells; (4) H3 stage is an absolute Sézary count of greater than 1 × 109 cells per liter; and (5) H4 stage is an absolute Sézary count of greater than 10 × 109 cells per liter. Graphs were truncated at 10 years due to little available data after this time.
Graph showing Kaplan-Meier survival estimates, given by hematologic stage in erythrodermic CTCL.
Comparison of Kaplan-Meier survival estimates in patients with different hematologic stages of disease (H0-H4): (1) H0 stage is no evidence of blood involvement; (2) H1 stage is a T-cell clone identified by PCR analysis of the T-cell receptor gene and less than 5% Sézary cells on a peripheral blood smear; (3) H2 stage is a T-cell clone identified by Southern blot analysis or a clone by PCR and more than 5% Sézary cells; (4) H3 stage is an absolute Sézary count of greater than 1 × 109 cells per liter; and (5) H4 stage is an absolute Sézary count of greater than 10 × 109 cells per liter. Graphs were truncated at 10 years due to little available data after this time.
The CTCL-related death rates between the hematologic stages were compared (Table 4). Death rates in patients with H2 stage disease were higher than in those with H0-H1 stage disease (hazard ratio, 6.6; P = .081). Similarly, the death rate in patients with H3 stage disease was higher than in patients with H0-H2 stage disease (hazard ratio, 2.6;P = .036), and the death rate in patients with H4 stage was higher than in patients with H0-H3 stage disease (hazard ratio, 2.2; P = .091). Similar values were also obtained comparing the overall death rates in these groups: H2 versus H0-H1 stage (hazard ratio, 5.5; P = .03), H3 versus H0-H2 stage (hazard ratio, 2.3; P = .032), and H4 versus H0-H3 stage (hazard ratio, 1.66; P = .266). Although the disease-specific death rates increased through H0 to H4 stages, there was no significant difference between CTCL-related death rates in these stages: H2 and H3 (P = .604), H3 and H4 (P = .637), and H2 and H4 disease stages (P = .422).
Lymph node stage at diagnosis was available in 22 patients and included 6 patients with N1 stage disease and 16 patients with N3 stage disease. In H0 and H1 stages, 6 of 11 patients had palpable lymphadenopathy. Two patients had a biopsy performed at diagnosis; one patient was assigned N1 stage, and the other patient was assigned N3 stage. Of note, the patient with N3 stage disease was the only patient with H1 stage who subsequently died from CTCL-related disease. In the H2 stage, 11 of 14 patients had palpable lymphadenopathy. Four patients had lymph node biopsies: one patient with N1 stage and 3 patients with N3 stage. In H3 and H4 stages, all patients had palpable lymphadenopathy. In H3 stage, 12 of 24 patients had lymph node biopsies: 4 patients with N1 stage, and 8 patients with N3 stage. In H4 stage lymph node, histology was available on 4 of 8 patients, all of whom had N3 stage disease. None of the patients had cutaneous tumors at initial presentation of erythrodermic CTCL or any clinical or histologic evidence of visceral spread.
There were 8 patients who had no palpable lymphadenopathy present at diagnosis of erythrodermic CTCL. Five patients had H0-H1 stage disease, and 3 patients had H2 stage disease; none of the patients had a lymph node biopsy performed at diagnosis. For the purposes of the study it is assumed that these patients were N0 stage. These 8 patients and the 22 patients in whom biopsies had been performed were then analyzed together to determine the prognostic impact of age at diagnosis, hematologic stage, and lymph node stage. The mean age in this cohort of 30 patients was 64 years, which was similar to the whole group. The mean follow-up time was slightly less, 3.8 years versus 4.3 years. There were 17 deaths among this subgroup; 14 deaths were CTCL-related. Of these, one patient had N0 stage disease, 2 patients had N1 stage, and 11 patients had N3 stage (Table 5).
In a univariate analysis, increasing lymph node stage was found to be significantly associated with a poor prognosis (hazard ratio, 2.86;P = .013). Increasing hematologic stage was also associated with a worse prognosis (hazard ratio, 1.89;P = .045), as was increasing age at diagnosis. However, the latter did not reach statistical significance (hazard ratio, 1.52;P = .136). In a multivariate analysis, lymph node stage at diagnosis was the only independent prognostic variable identified (hazard ratio, 2.69; CI = 1.11-6.50; P = .028). However, the confidence interval was wide enough to suggest that the hematologic stage and age may provide additional useful prognostic information. Likelihood ratio tests revealed that hematologic stage (hazard ratio, 2.00; P = .035), but not age (hazard ratio, 1.69;P = .097), provided further prognostic information in patients with an established lymph node stage.
Discussion
We have devised a detailed hematologic staging system to quantify blood involvement in erythrodermic CTCL. We found that with each increase in hematologic stage, there was a rise in the death rates, and this was found to be statistically significant comparing both H2 with H0-H1 stage disease and H3 with H0-H2 stage disease.
The group with the most favorable prognosis included patients with no evidence of hematological involvement at diagnosis (H0) and those with a T-cell clone detected by PCR and less than 5% circulating Sézary cells (H1). In this group, only one patient died from disease during a mean follow-up period of 7.8 years, which equates to a disease-specific death rate of 0.01. Patients with H2 stage disease, which is equivalent to the original B1 rating by the NCI and also the definition chosen by Russell-Jones and Whittaker,16 had a 5-times higher disease-specific death rate than those with H0 and H1 stage disease and were found to have a worse survival (P = .081). The H3 stage, defined as having more than 1 × 109 cells per liter circulating Sézary cells, was the largest group and included 24 patients. This stage correlates with the definition for blood involvement in Sézary syndrome used by the ISCL, and patients with H3 stage disease had a disease-specific death rate that was 3.5 times greater than the patients with H0-H2 stage disease (P = .036). Finally, patients with H4 stage disease, with leukemic involvement of more than 10 × 109 Sézary cells per liter, had the worst prognosis, which was 2.5 times higher than patients with H0-H3 stage disease (P = .091).
We distinguished between CTCL-related and CTCL-unrelated deaths as determined by cause of death entered on the death certificate. Unrelated deaths were recorded in 9 patients; 3 patients died of pneumonia, one patient died of myocardial infarction, congestive cardiac failure, and endometrial carcinoma; and 3 patients died of lung carcinoma. It is known that patients with Sézary syndrome may be immunosuppressed and have an increased risk of second malignancies,19 and some of these deaths, particularly those with pneumonia or carcinoma, could have been related to the disease. Furthermore, many treatments for erythrodermic CTCL are immunosuppressive and could have contributed to these other deaths. We therefore compared overall survival in patients with different hematologic stages of disease. The trend was similar, although the difference in survival between H3 and H4 stages was less pronounced (Table 3).
A CD4:CD8 ratio of greater than 10 has been proposed by the EORTC for defining patients with Sézary syndrome1 and is used for diagnosis in centers where Sézary counts are not available. We did not include CD4:CD8 ratios in our hematological staging system, as the value is dependent on the absolute CD8 count in addition to the CD4 count. Even so, we did find that the mean CD4:CD8 ratio increased with each stage, although the range within each group was extremely variable (Table 3). Our data indicate that a CD4:CD8 ratio of 6.6 is equivalent to H2 stage disease, whereas a CD4:CD8 ratio of more than 10 equates to an absolute Sézary count of 1 × 109cells per liter. This would explain why patients fulfilling the EORTC criteria of Sézary syndrome have a median survival of only 2 years.20
The serum LDH increased progressively with stage, from 656 U/L in H0-H1 stage disease to 918 U/L in H4 stage disease. This suggests that serum LDH may provide a simple low-cost means of determining the tumor burden in erythrodermic CTCL; however, data were only available on 25 patients. A rise in serum LDH to more than 10% of the normal value has previously been found to be a poor prognostic feature in mycosis fungoides and Sézary syndrome.21 However, in our cohort of 57 patients with erythrodermic disease, even those with a low peripheral blood tumor burden (H0 and H1 stages) had a mean serum LDH of more than 10% of the normal value (greater than 638 U/L).
Our data have found that the mean survival of patients with erythrodermic CTCL decreases with increasing hematological stage. In particular, maximum survival time was reduced from 7.8 years in H0 and H1 stages, 3.9 years in H2, 3.4 years in H3, and 3.0 years in H4 stage disease. However, the power of this study was not able to determine any statistical difference in the survival of patients with H2, H3, or H4 stage disease. A larger multicenter study would be required to determine if these hematological stages provide independent prognostic information. In addition, other important prognostic features need to be assessed in relation to hematological stage.
We only selected patients with erythrodermic CTCL (mycosis fungoides and Sézary syndrome) and thus eliminated any bias from cutaneous stage, which has consistently been found to be the most important determinant of outcome in CTCL.3,6,22,23 Increasing age has also been shown to be associated with a worse prognosis in CTCL,10,15,21 and therefore we corrected for this by using the Cox proportional regression hazard model. Other poor prognostic indicators identified in CTCL include lymph node stage and visceral spread.15,24 25
In this study, none of the patients had histologic evidence of visceral disease, although 49 of the 57 patients had peripheral lymphadenopathy, and the lymph node stage may have an impact on the analysis of survival data by hematologic stage. We therefore analyzed a subgroup of 30 patients. Eight patients without clinically palpable lymph nodes at diagnosis were assumed to be in N0 stage, as the presence of adenopathy correlates with advanced lymph node stage24; however, this is not absolute, and rarely, advanced stages may be present without adenopathy.24,26,27 Twenty-two patients had a histologically proven lymph node stage at diagnosis. We excluded the remaining 27 patients with peripheral adenopathy in whom a lymph node biopsy was not performed at diagnosis. Lymph node and hematologic stages, but not age at diagnosis, were found to be associated with a worse prognosis in univariate analysis. In a multivariate analysis, lymph node stage was the only independent variable associated with a worse outcome, although likelihood ratio tests did show that hematologic stage but not age provides additional prognostic information (P = .035). These data suggest that lymph node stage provides more useful prognostic information than hematologic stage. However, in erythrodermic CTCL, histologically proven lymph node involvement was only documented in 22 patients at diagnosis, and 27 patients with peripheral lymphadenopathy at presentation did not have a biopsy at diagnosis and were excluded from this analysis. This may have biased results because patients with more extensive cutaneous disease or a higher tumor burden in blood would be more likely to be referred for immediate lymph node biopsy, and those with less severe disease may have been biopsied at a later date. In addition, it was presumed that those patients without clinical lymphadenopathy at diagnosis, none of whom had a biopsy, were N0 stage, but they could have had N2 stage disease.24,26 27
Overt bone marrow involvement is considered a late feature occurring only in advanced stages of Sézary syndrome,28 and bone marrow biopsies have not been routinely used in our department as part of the staging procedure for erythrodermic CTCL. Bone marrow biopsies were only performed on 5 of 57 patients at initial presentation: 2 patients with H3 stage disease, both of whom had a normal trephine, and 3 patients with H4 stage disease, one of whom had a scanty infiltrate of Sézary cells, one patient had myelodysplasia with no evidence of Sézary cells, and the other patient had a normal trephine biopsy. Although bone marrow involvement has been associated with a reduced survival in CTCL, including Sézary syndrome,29 it has not been shown to be an independent poor prognostic indicator in multivariate analysis.21 25
The survival of patients with erythrodermic CTCL has previously been shown to be worse in patients with hematologic involvement than in patients without hematologic involvement6,10,15,21,25,30,31(Table 6). However, the differing criteria for both hematologic involvement in CTCL and a diagnosis of Sézary syndrome among different study groups has made accurate comparisons of survival data difficult in erythrodermic CTCL. Data published in the literature show a variation in survival from 1.25-3.5 years (Table 6). We recently found that in patients with mycosis fungoides, the presence of a peripheral blood T-cell clone was an independent prognostic factor for survival in patients with T1 to T3 stage disease.18 However, there were insufficient patients with T4 stage disease to determine if a peripheral blood T-cell clone was of any prognostic value in erythrodermic mycosis fungoides.
Here we have found a positive correlation between increasing peripheral blood tumor burden and a reduced survival in erythrodermic CTCL. It is likely that at diagnosis the hematologic stage could identify those patients with a poorer prognosis. Further studies are now required to confirm this finding. The lymph node stage provides more independent prognostic data but may require hospital admission, whereas hematologic staging is performed at the initial out-patient assessment, thereby enabling stratification in any future clinical trials. The development of a universal hematologic staging system for erythrodermic CTCL would also allow more accurate comparison of data between different study groups and may eventually be used alongside the TNM staging system to determine prognosis.
Acknowledgment
We would like to thank Dr Paul Seed, Department of Public Health, Guy's, King's and St Thomas' Medical School, London, England, for his statistical advice.
Supported by a fellowship grant (G/052/0617/***) (J.J.S.) from the Special Trustees of St Thomas' Hospital, London, England.
Submitted June 23, 2000; accepted October 3, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Julia J. Scarisbrick, Skin Tumour Unit, St John's Institute of Dermatology, St Thomas' Hospital, Lambeth Palace Rd, London, SE1 7EH, England.