Sickle cell anemia (SCA) is an inherited disorder of β-globin, resulting in red blood cell rigidity, anemia, painful crises, organ infarctions, and reduced life expectancy. Allogeneic blood or marrow transplantation (BMT) can cure SCA but is associated with an 8% to 10% mortality rate, primarily from complications of marrow-ablative conditioning. Transplantation of allogeneic marrow after less intensive conditioning reduces toxicity but may result in stable mixed hematopoietic chimerism. The few SCA patients who inadvertently developed mixed chimerism after BMT remain symptom free, suggesting that mixed chimerism can reduce disease-related morbidity. However, because the effects of various levels of mixed chimerism on organ pathology have not been characterized, this study examined the histologic effects of an increasing percentage of normal donor hematopoiesis in a mouse model of BMT for SCA. In lethally irradiated normal mice that were reconstituted with varying ratios of T-cell–depleted marrow from normal and transgenic “sickle cell” mice, normal myeloid chimerism in excess of 25% was associated with more than 90% normal hemoglobin (Hb). However, 70% normal myeloid chimerism was required to reverse the anemia. Organ pathology, including liver infarction, was present in mice with sickle Hb (HbS) levels as low as 16.8% (19.6% normal myeloid chimerism). Histologic abnormalities increased in severity up to 80% HbS, but were less severe in mice with more than 80% HbS than in those with 40% to 80% HbS. Therefore, stable mixed chimerism resulting from nonmyeloablative BMT may reduce the morbidity from SCA, but prevention of all disease complications may require minimizing the fraction of circulating sickle red cells.
Introduction
Sickle cell anemia (SCA) is a disorder of the β-globin subunit of hemoglobin (Hb) resulting from an amino acid substitution of valine in place of glutamic acid at position 6. Polymerization of the mutated Hb molecule under hypoxic conditions causes red blood cells (RBCs) to have a sickle-shaped deformity, become dehydrated and rigid, and adhere to vascular endothelium. Anemia, growth delay, frequent infection with encapsulated bacteria, bone infarctions, and chronic organ damage involving the brain, heart, lungs, joints, gallbladder, kidneys, and retina are common complications of SCA and account for most of the morbidity and mortality. Median life expectancy is estimated to be 42 years for men and 48 years for women with homozygous sickle Hb (HbSS).1Organ damage may occur at an early age, contributing to a mortality of 15% by 18 years.1 2
Allogeneic blood or marrow transplantation (BMT) is the only known cure for SCA. The first successful BMT for SCA, in a patient with coexisting leukemia, was reported in 1984.3 A recent review of the worldwide literature reported only 127 patients with SCA who have been treated with BMT.4 Rejection occurred in 16 of 127 (12.6%) and death in 11 of 127 (8.7%). In the United States, where the prevalence of HbSS is estimated to be 60 000, only 50 allografts for SCA have been reported.5 Despite promising results, the significant toxicity of myeloablative BMT has restricted its application to patients who have already experienced severe and irreversible complications. Toxicity must be substantially reduced if BMT is to be offered to younger patients as a way of preventing life-limiting complications of SCA.
Recent evidence that stable mixed hematopoietic chimerism can result from nonmyeloablative BMT (NM-BMT) in patients with hematologic malignancies6 has led many to speculate that this could be an effective treatment for SCA, with substantially reduced transplant-related morbidity and mortality. Small amounts of donor bone marrow chimerism may result in high levels of normal Hb because sickled RBCs have a survival disadvantage.7 In the report of Walters and coworkers,5 5 patients with SCA developed stable but partial donor myeloid chimerism after myeloablative BMT. Fractions of HbS in blood were 0%, 0%, and 7% in the 3 patients whose donors had a normal Hb genotype (corresponding to donor myeloid chimerism level of 67%, 75%, and 20%, respectively). None of the patients experienced painful events or other clinical complications of sickle cell disease after BMT.8 Hence, a significant reduction in morbidity and mortality from SCA may occur with even low levels of donor myeloid chimerism.
To reduce the toxicity and optimize the therapeutic effect of NM-BMT in patients with SCA, it would be important to characterize the level of donor chimerism that is therapeutic, resulting in sufficient levels of normal Hb to ameliorate anemia and acute complications such as vaso-occlusive crises and chest syndrome, as well as chronic, progressive organ damage. The level of hematopoietic chimerism that alleviates painful crises, however, may differ from that which prevents silent organ damage. Moreover, the level of chimerism that prevents damage in one organ, such as the brain, may be insufficient in the retina, kidney, lung, or heart. Therefore, simple clinical parameters, such as frequency of painful crises, may underestimate the level of donor chimerism that will prevent ongoing organ damage. To gain insight into the level of HbS below which pathology would not be observed, we used a mouse model of BMT for SCA to relate the level of sickle-derived myeloid chimerism and HbS fraction in the blood to hematologic indices and histopathologic changes.
Materials and methods
Mice
Sickle cell transgenic mice were developed by Paszty and colleagues9 and kindly provided by Dr E. M. Rubin (Lawrence Livermore Laboratories, Berkeley, CA). These mice were created by inactivating the genes for mouse globin by homologous recombination and by transgenic insertion of human genes for α, β, and γ globin. The sickle cell transgenic mice carry a mixed genetic background with contributions from DBA/2, FVB/N, Black Swiss, 129, and C57BL/6 strains; major histocompatibility complex (MHC) typing of the animals in our colony revealed all mice to be H-2Kb+, H-2Dd−, and H-2Kq−. These mice express only human globin molecules and have an excess α-globin chain synthesis (α/βS, 1.26 ± 0.02), indicating a thalassemic component to this sickle cell mouse model.9 All transgenic sickle mice used for these experiments were demonstrated to express the CD45.1 allele of the CD45 common leukocyte antigen. C57BL/6 (H-2b, CD45.2+) and C3H.SW (H-2b, CD45.2+) mice, normal inbred strains, were obtained from the National Cancer Institute (Frederick Cancer Research Facility, Frederick, MD) and Jackson Laboratories (Bar Harbor, ME), respectively. All mice were maintained in microisolator cages and were fed with autoclaved chow and acidified water ad libitum. The sickle cell transgenic mice were fed with a special diet containing folate.
Bone marrow chimeras
C57BL/6 male mice were exposed to 950 cGy total body irradiation and, 6 hours later, received a total of 4 million T-cell–depleted bone marrow cells mixed from sickle cell transgenic female and C3H.SW male donors. T-cell depletion was performed as previously described.10 Nucleated cells in the bone marrow were counted manually using a hemacytometer. Ratios of sickle cell to normal bone marrow, 5 to 10 per group, were as follows: 100:0, 64:1, 32:1, 16:1, 8:1, 4:1, 1:1, and 0:100.
High-pressure liquid chromatography
The Hb content, including quantitation of HbS, was measured using high-pressure liquid chromatography (HPLC) on blood from all experimental mice at 4, 5, and 6 months after transplantation. Controls included at least 5 per group of sickle transgenic, C57BL/6, and C3H.SW mice. All HPLC measurements were made using the Bio-Rad Variant Hemoglobin Testing System (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. Using a mean corpuscular hemoglobin (MCH) of 9.3 pg/cell in sickle cell transgenic mice and 14.5 pg/cell in C57BL/6 mice, a standard curve of the percentage of circulating sickle erythrocytes was calculated based on the measured HbS as follows: % Hemoglobulin S = {[(% sickle RBCs)(9.3 pg/cell)] × 100}/{[(% sickle RBCs)(9.3 pg/cell)] + [(% normal RBCs)(14.5 pg/cell)]}.
Flow cytometry
Flow cytometry was performed on peripheral blood to assess donor myeloid and lymphoid chimerism, on all experimental and control mice at 4 and 6 months after transplantation. Sickle cell transgenic donor cells were distinguished from both normal donor C3H.SW and C57BL/6 recipient cells by differential expression of alleles of the CD45 pan-leukocyte surface protein. Sickle cell donor leukocytes were CD45.1+ and CD45.2−, whereas C3H.SW and C57BL/6 leukocytes are CD45.2+ and CD45.1−. Sickle donor lymphoid chimerism was assessed by staining peripheral blood lymphocytes with fluorescein isothiocyanate (FITC)-conjugated antibody against CD45.1 and phycoerythrin (PE)-conjugated antibodies to CD4 and CD8 (all antibodies from BD Pharmingen, San Diego, CA) followed by flow cytometry. Myeloid chimerism was measured using FITC-conjugated antibody against CD45.1 and antibodies to CD11b, which is expressed on mature granulocytes, macrophages, dendritic cells, and natural killer (NK) cells. Anti-CD11b antibody was biotin conjugated and required an additional incubation with PE-conjugated streptavidin. Blood (approximately 50-100 μL) was obtained from the tail vein or from the heart (at the time of sacrifice) and was collected into plastic tubes coated with sodium EDTA as an anticoagulant. RBCs were lysed using a hyperosmolar solution prior to cell sorting. Cells were counted on a hemacytometer and one million cells per sample were stained for analysis by flow cytometry using a FACScan (Becton Dickinson, Franklin Lakes, NJ).
Complete blood count measurement
Hb concentrations (g/dL), white blood cell (WBC) counts (number × 10−3/cu mm), platelet counts (number × 10−3/cu mm), and reticulocyte counts (%) were determined on peripheral blood 6 months after transplantation (the time of sacrifice) using the Sysmex 9500 (Sysmex of America, Long Grove, IL). At least 5 per group of sickle transgenic, C57BL/6, and C3H.SW mice were included as controls.
Evaluation of peripheral blood smears
Independent, blinded assessments of peripheral blood smears were made by a single hematologist (J.F.C.) for each animal at the time of sacrifice. Smears were scored from 0 (most like normal mice) to 16 (most like sickle cell mice) based on the degree of hypochromia (0-4), polychromasia (0-4), abnormal RBC shapes (0-4), and nucleated RBCs (0-4). Controls included at least 5 per group of sickle transgenic, C57BL/6, and C3H.SW mice. The same hematologist also made a visual estimation of RBC chimerism based on the distinct appearance of sickle versus normal RBCs in mice with mixed hematopoietic chimerism (eg, Figure 1B).
Progressively severe hematologic and pathologic changes in mixed hematopoietic chimeras containing an increasing percentage of HbS.
Histologic images are shown for a representative sample of chimeric mice as follows: lethally irradiated C57BL/6 mouse reconstituted with normal C3H.SW bone marrow (left column), mixed chimeric mouse with 16.8% HbS (19.6% normal myeloid chimerism) generated by lethal irradiation followed by reconstitution with a mixture of transgenic sickle and C3H.SW bone marrow (middle column), and mixed chimeric mouse with 91% HbS (0.2% normal myeloid chimerism) generated by lethal irradiation and reconstitution with only transgenic sickle bone marrow (right column). Mice were humanely killed for histologic analysis 6 months after transplantation. (A-C). Peripheral blood smears demonstrate increasing hypochromia and polychromasia with increasing levels of HbS. Two morphologically distinct populations of RBCs are apparent in the 16.8% sickle chimera, as indicated by arrows in panel B. The 91% sickle chimera, panel C, has nucleated RBCs (dark arrow) and sickle forms (open arrow). (D-I) Sections of spleen are shown at low power (D-F) and high power (G-I). Low power illustrates loss of follicular architecture in mice with a majority of HbS. High power illustrates increasing extramedullary hematopoiesis as well as increasing congestion and hemosiderin deposition in mice with larger proportions of HbS. (J-L) Liver infarcts (arrows) are present in mice with as little as 16.8% HbS in the blood, as well as in mice with a majority of HbS. Magnifications: panels A-C, 160 ×; D-F, 40 ×; G-L, 100 ×.
Progressively severe hematologic and pathologic changes in mixed hematopoietic chimeras containing an increasing percentage of HbS.
Histologic images are shown for a representative sample of chimeric mice as follows: lethally irradiated C57BL/6 mouse reconstituted with normal C3H.SW bone marrow (left column), mixed chimeric mouse with 16.8% HbS (19.6% normal myeloid chimerism) generated by lethal irradiation followed by reconstitution with a mixture of transgenic sickle and C3H.SW bone marrow (middle column), and mixed chimeric mouse with 91% HbS (0.2% normal myeloid chimerism) generated by lethal irradiation and reconstitution with only transgenic sickle bone marrow (right column). Mice were humanely killed for histologic analysis 6 months after transplantation. (A-C). Peripheral blood smears demonstrate increasing hypochromia and polychromasia with increasing levels of HbS. Two morphologically distinct populations of RBCs are apparent in the 16.8% sickle chimera, as indicated by arrows in panel B. The 91% sickle chimera, panel C, has nucleated RBCs (dark arrow) and sickle forms (open arrow). (D-I) Sections of spleen are shown at low power (D-F) and high power (G-I). Low power illustrates loss of follicular architecture in mice with a majority of HbS. High power illustrates increasing extramedullary hematopoiesis as well as increasing congestion and hemosiderin deposition in mice with larger proportions of HbS. (J-L) Liver infarcts (arrows) are present in mice with as little as 16.8% HbS in the blood, as well as in mice with a majority of HbS. Magnifications: panels A-C, 160 ×; D-F, 40 ×; G-L, 100 ×.
Pathologic assessment
All experimental animals were humanely killed 6 months after transplantation (n = 59). A single experienced pathologist (F.B.A.) made blinded assessments of the liver, spleen, lungs, and kidneys from each experimental mouse. In addition to the 59 experimental mice, at least 5 per group of sickle transgenic, C57BL/6, and C3H.SW mice were included as controls. Scores of 0 (unaffected) to 16 (most like sickle cell mice) were assigned based on the extent of sinusoidal congestion and hemosiderin deposition in both spleen and liver, (each feature rated 0-2); loss of follicular architecture and extramedullary hematopoiesis within the spleen (0-2 each); and severity and frequency of ischemia and infarcts in the liver (0-4; 3-4 assigned only when overt infarcts were present).
Statistical considerations
Correlation coefficients were calculated using StatView (SAS Institute, Cary, NC) statistical software. Graphs were prepared andP values (unpaired Student t test) and SEMs were calculated using Sigmaplot statistical software (SPSS Science, Chicago, IL). The change in HbS percentage over time within individual mice was assessed with linear regression, with a categorical term for mouse and with month treated as a continuous variable.
Results
Sickle cell donor mice (H-2b) were generated previously by Paszty and colleagues9 by breeding mouseglobin gene knockout mice with mice transgenic for human α, sickle β, and γ globin. Consistent with prior descriptions, transgenic sickle cell mice in our colony were anemic: Hb concentration averaged 4.65 g/dL (SEM = 0.32) in transgenic sickle mice, compared to 14.1 g/dL in C3H.SW (SEM = 0.2) and 15.0 g/dL in C57BL/6 (SEM = 0.1) mice. Spleens were grossly enlarged (mean 1.75 g, SEM = 0.16) compared to C3H.SW (mean 0.12 g, SEM = 0.02) and C57BL/6 (mean 0.07, SEM = 0.01) mice. Peripheral blood smears showed marked hypochromia (reflecting a thalassemic component in these mice9), polychromasia, nucleated RBCs (nRBCs), and sickle and other abnormal forms (data not shown, but Figure 1C shows an animal with 91% circulating HbS). The mean blood smear score for transgenic sickle mice was 14.2 (SEM = 0.66), compared to 0 in C3H.SW and C57BL/6 mice. Brain, heart, lung, liver, kidney, spleen, and bone marrow were examined microscopically. Consistent with previous observations,9 hematopoietic organs were most severely affected. Spleens showed increased extramedullary hematopoiesis, loss of follicular architecture, sinusoidal congestion, and hemosiderin deposition. Livers also showed sinusoidal congestion and hemosiderin deposition. Liver ischemia and overt infarcts were the most dramatic and consistent pathologic findings (data not shown, but Figure 1L shows an animal with 91% circulating HbS). The mean pathology score for transgenic sickle mice was 12.1 (SEM = 0.77), compared to 4.4 in C3H.SW (SEM = 0.4) and 3 in C57BL/6 mice (SEM = 0.37).
To characterize the histologic and hematologic effects of mixed hematopoietic chimerism in a mouse model of SCA, C57BL/6 (H-2b, CD45.1−) mice were exposed to 950 cGy total body irradiation and were reconstituted the following day with a mixture of T-cell–depleted marrow from MHC-identical C3H.SW (H-2b, CD45.1−) and sickle cell transgenic mice (H-2b, CD45.1+). Reconstituting ratios of sickle/normal marrow were 100:0, 64:1, 32:1, 16:1, 8:1, 4:1, 1:1, and 0:100 (5-10 per group), and were chosen, based on previous experiments, to provide a broad range of Hb chimerism. After transplantation, serial determinations of peripheral blood WBC chimerism, by flow cytometry (4 and 6 months) and Hb chimerism, by HPLC (4, 5, and 6 months) were made. The level of HbS showed minimal change in each mouse over this time frame, with an estimated average monthly increase of 1.7% (95% CI, 0.5%-2.9%). Table 1 lists Hb fractions, myeloid chimerism, spleen weights, WBC counts, Hb concentrations, reticulocyte counts, peripheral blood smear scores, and pathology scores (n = 59), as assessed at the time of sacrifice (6 months after BMT) for all experimental mice. The presence or absence of liver infarcts is noted for each mouse. The ranges of values for each of these assessments were as follows: HbS (HPLC), 0% to 94.1%; fraction of myeloid cells of transgenic sickle origin, less than 1% (background) to 99.9%; spleen weights, less than 0.1 to 0.9 g; WBC counts, 6.0 to 25.7 × 103/cu mm (uncorrected for nRBCs); Hb levels, 2.9 to 13.7 g/dL; reticulocyte counts, 2.1% to 26.1%; peripheral blood smear scores, 0 to 15; and pathology scores, 1 to 14.
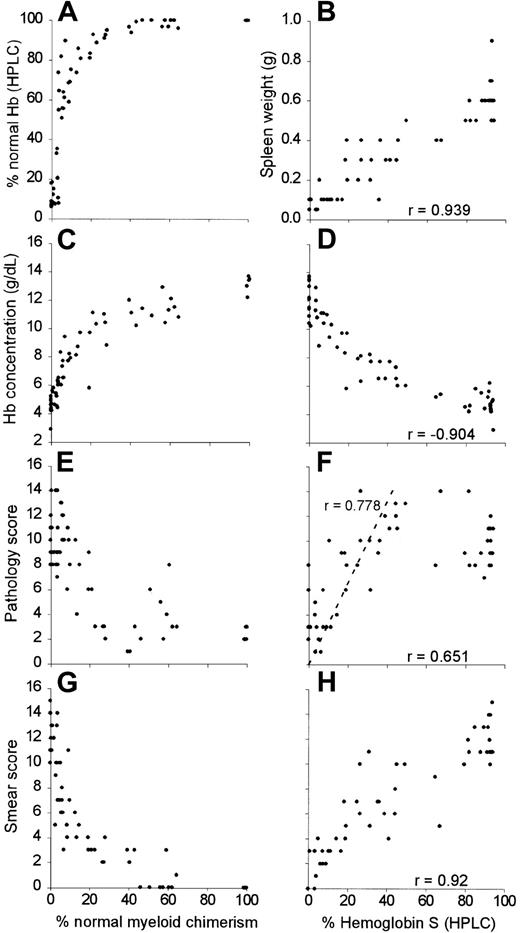
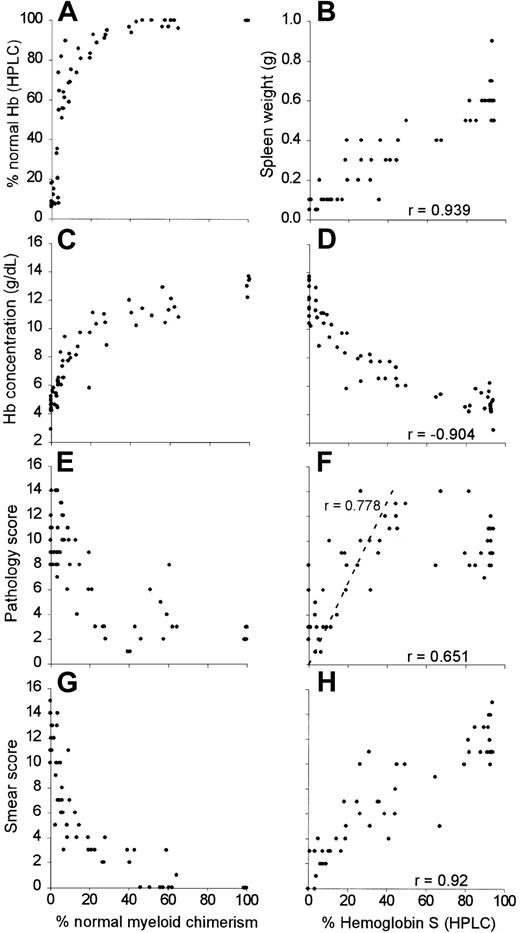
Figure 1 illustrates progressively severe blood, spleen, and liver changes that occurred in experimental mice with increasing HbS levels (0%, 16.8%, and 91%, corresponding to 100%, 19.6%, and 0.2% normal myeloid chimerism). Microscopic liver infarcts were noted in several mice whose HbS was about one third of total, and in one mouse with 16.8% HbS (Table 1 and Figure 1K).
As shown in Figure 2, all mice with more than 25% normal myeloid chimerism had more than 90% normal Hb, as measured by HPLC (Figure 2A). This finding may be due to the known survival advantage of normal mouse over sickle cell transgenic mouse RBCs.11 There were strong positive correlations between the HbS fraction in the blood and spleen weight (Figure 2B; r = 0.939, 95% CI, 0.964-0.899), pathology score (Figure 2F; r = 0.651, 95% CI, 0.777-0.474) and blood smear score (Figure 2H; r = 0.920, 95% CI, 0.952-0.869). Hb concentration was negatively correlated to HbS fraction (Figure 2D; r = −0.904, 95% CI, −0.844 to −0.942). Mixed chimeras with 40%-70% normal myeloid chimerism had 100% normal circulating Hb (Figure 2A) but were more anemic (mean Hb concentration 11.26 g/dL, SEM = 0.25; Figure 2C) than animals with > 70% normal myeloid chimerism (mean Hb concentration 13.2 g/dL, SEM = 0.27; P = .004). Pathology scores (Figure 2F) increased in a linear fashion in the range of 0% to 80% HbS (r = 0.778, 95% CI, 0.882-0.600). However, pathology scores were significantly higher in mice with 40% to 80% HbS compared to those with more than 80% HbS (11.38 ± 2.1 versus 9.63 ± 1.82, respectively; P = .045). As in homozygous sickle donors, organ pathology was most apparent in organs such as liver and spleen.
Progressive normalization of hematologic and histologic parameters in chimeric mice with increased normal myeloid chimerism and decreased fractions of sickle Hb.
For percent normal myeloid chimerism, the percentage of normal myeloid chimerism, as measured by dual color flow cytometry of peripheral blood, is plotted along the x-axis with the following outcomes along the y-axis: (A) % normal Hb (HPLC), (C) Hb concentration, (E), pathology score, and (G) blood smear score. For percent HbS (HPLC), the percentage of HbS is plotted along the x-axes with the following outcomes measures along the y-axes: (B) spleen weight, (D) hemoglobin concentration, (F) pathology score, and (H) blood smear score. Correlation coefficients (r) are shown for each of the data sets.
Progressive normalization of hematologic and histologic parameters in chimeric mice with increased normal myeloid chimerism and decreased fractions of sickle Hb.
For percent normal myeloid chimerism, the percentage of normal myeloid chimerism, as measured by dual color flow cytometry of peripheral blood, is plotted along the x-axis with the following outcomes along the y-axis: (A) % normal Hb (HPLC), (C) Hb concentration, (E), pathology score, and (G) blood smear score. For percent HbS (HPLC), the percentage of HbS is plotted along the x-axes with the following outcomes measures along the y-axes: (B) spleen weight, (D) hemoglobin concentration, (F) pathology score, and (H) blood smear score. Correlation coefficients (r) are shown for each of the data sets.
Visual chimerism estimate of sickle RBCs on blood smear was positively correlated to HbS levels (Figure 3; r = 0.968, 95% CI, 0.947-0.981), as measured by HPLC. However, the curve is nonlinear. In general, the slope of the curve is steepest between 0% and 20% HbS (HPLC), and then decreases as the fraction of the HbS increases, such that the measured HbS (x-axis) underestimates the fraction of sickle RBCs. In Figure 3, a solid line is drawn to represent a predicted curve that is based on the difference in MCH between sickle cell mice (MHC = 9.3 pg/cell) and normal donor mice (MHC = 14.5 pg/cell). At higher levels of measured HbS, the visual estimate of sickle RBCs fraction does not follow the predicted curve as well for lower levels of HbS.
Nonlinear relationship of fraction of sickle Hb and percentage of sickle RBCs.
A graph of the percent HbS as measured by HPLC versus a visual estimate of the percent sickle cell mouse–derived RBCs is compared to a predicted curve based on the difference in MCH between transgenic sickle cell mice (MHC = 9.3 pg/cell) and normal donor mice (MHC = 14.5 pg/cell). The predicted curve is based on the following formula: % Hemoglobin S = {[(% sickle RBCs)(9.3 pg/cell)] × 100}/{[(% sickle RBCs)(9.3 pg/cell)] + [(% normal RBCs)(14.5 pg/cell)]}.
Nonlinear relationship of fraction of sickle Hb and percentage of sickle RBCs.
A graph of the percent HbS as measured by HPLC versus a visual estimate of the percent sickle cell mouse–derived RBCs is compared to a predicted curve based on the difference in MCH between transgenic sickle cell mice (MHC = 9.3 pg/cell) and normal donor mice (MHC = 14.5 pg/cell). The predicted curve is based on the following formula: % Hemoglobin S = {[(% sickle RBCs)(9.3 pg/cell)] × 100}/{[(% sickle RBCs)(9.3 pg/cell)] + [(% normal RBCs)(14.5 pg/cell)]}.
Discussion
The potential utility of inducing mixed hematopoietic chimerism to treat SCA was first validated by clinical experience with chronic blood transfusions, which reduce the incidence of painful crises and the risk of recurrent stroke.12,13 Subsequently, 3 patients with SCA inadvertently achieved stable mixed chimerism after conventional BMT from donors with normal Hb.5 Although none of these patients with mixed hematopoietic chimerism has experienced symptoms or complications of their disease to date, these data reflect a limited experience and follow-up in patients who have 7% or fewer circulating sickle erythrocytes. The long-term effects on various organs of low to moderate levels (eg, 5%-40%) of HbS after NM-BMT remain incompletely understood. Chimerism sufficient to prevent acute complications, such as painful crises or acute chest syndrome, may not prevent chronic and progressive damage to organs such as brain, kidney, or retina. Apparent differences in the susceptibility of distinct organs to dysfunction in SCA may be attributed to local conditions, such as oxygen tension or vascularity, an organ's regenerative capacity, or the amount of damage that is required to produce clinical manifestations. For example, chronic blood transfusions, to reduce HbS to less than 30% of total Hb, reduce the incidence of painful crises12 but do not eliminate the risk of stroke. In a retrospective analysis, recurrent stroke occurred in 53 of 157 patients receiving chronic blood transfusions for a history of stroke (Michael DeBaun, written communication, March 10, 2000), which extends prior observations to this effect by Pegelow et al.13
In our mixed chimeric sickle mouse model, 25% normal myeloid chimerism results in more than 90% normal Hb in the blood. Reducing the fraction of HbS below 80% resulted in progressive normalization of hematologic and histologic effects linearly until 0% HbS was achieved. Overt liver infarction occurred with as little as 16.8% HbS. This suggests that that standard of reducing HbS to less than 30% with blood transfusions of NM-BMT may not completely eliminate pathologic effects of SCA in this model. Furthermore, a higher threshold of normal myeloid chimerism (70%) was required to eliminate anemia, compared to the approximate 40% normal myeloid chimerism needed to eliminate sickle RBCs from the blood. This suggests that ineffective erythropoiesis by sickle RBC precursors is substantial and prevents the achievement of normal Hb concentrations in mixed chimeric mice with less than 70% normal myeloid chimerism. We think that these hematologic and histologic changes occurred during a relatively steady state of HbS. Linear regression analysis demonstrated that the level of HbS showed minimal change in each mouse over this time frame, with an estimated average monthly increase of 1.7% (95% CI 0.5%-2.9%).
Also, it is possible that mild to modest decreases in HbS and increased Hb concentration from mixed hematopoietic chimerism may exacerbate the pathologic effects of SCA, as was observed in our experimental mice (HbS range 40%-80%, corresponding to 2.7%-8.8% normal myeloid chimerism). This was an unexpected finding that we cannot fully explain in the context of this study. It is possible that increasing the Hb concentration without substantially reducing the proportion of HbS resulted in increased viscosity and decreased oxygen-carrying capacity, as has been described in viscosity studies of sickle cells.14 To verify this observation and investigate the etiology, we are pursuing additional studies that will measure blood viscosity in mice with 40% to 100% HbS and make correlations to observed histopathology.
A number of features of the BMT model used here, especially the use of normal and sickle cell transgenic mice together, differ from the situation in humans and make it difficult to extrapolate directly. Fundamental differences such as RBC size, oxygen affinity, life span, and the presence of α and β globin chain imbalance (thalassemia trait), in the sickle cell transgenic mice used in this study, probably contribute to differences in the phenotype of this mouse model and the clinical manifestations of human disease. For instance, anemia and liver infarcts occur in this mouse model, but no effects on the brain have been noted. Also, the spleen is a site of extramedullary hematopoiesis in the mouse, whereas patients with sickle cell disease become functionally asplenic due to infarctions.
On the one hand, this model may be predisposed to underestimating the curative level of normal hematopoietic chimerism. For example, mouse Hb has a lower affinity for oxygen (P50 = 40 mm Hg) than human Hb (P50 = 25 mm Hg).15 The P50 of Hb has not been characterized in the sickle mouse model that we studied, though another transgenic sickle mouse model has an oxygen affinity that is slightly higher than that of normal mice but lower than human Hb (P50 = 33.5-37.4 mm Hg).16 Thus, in our chimeric mice, unpolymerized human HbS may “steal” oxygen from normal mouse Hb under hypoxic conditions. This “steal” phenomenon would minimize the pathophysiologic effects of HbS and thereby underestimate the level of normal erythrocyte chimerism needed to ameliorate the disease in humans.
On the other hand, some features of this model might tend to overestimate the level of normal hematopoiesis needed to prevent the pathologic effects of sickle erythropoiesis. Because the MCH is lower in sickle transgenic mice compared to the normal donor mice, the measured HbS may correspond to a slightly higher fraction of sickle RBCs in the blood (Figure 3). For example, the mouse with 16.8% HbS (HPLC) and a liver infarct was assessed by visual estimate to have 20% sickle mouse–derived RBCs, about what would be predicted based on differences in MHC between transgenic sickle and normal mice (Figure3). If this were to lower the threshold of measured HbS that is associated with organ pathology, it would overestimate the level of normal hematopoiesis needed to cure the disease. Additionally, the survival advantage of normal Hb compared to HbS is more pronounced in the human (approximately 2 weeks versus 120 days) than in this sickle mouse model (16 versus 40 days).7 11 On this basis, our model may overestimate the myeloid chimerism that is associated with a reduction in HbS sufficient to ameliorate symptoms.
Mindful of these differences in this mouse model compared to the situation in humans, we would emphasize 2 points. First, a minority fraction of normal donor hematopoietic chimerism is probably sufficient to eliminate HbS from the blood, perhaps due to the survival advantage of normal Hb versus HbS, even though a higher fraction of normal hematopoiesis may be needed to eliminate anemia. Second, there does not appear to be a threshold of HbS below which pathophysiologic effects are prevented. Rather, the physiologic benefit of NM-BMT, in this model, is proportional to the reduction in HbS. This finding is consistent with the observation that RBC transfusions, which reduce the fraction of HbS, ameliorate but do not eliminate complications of SCA.13
Based on the clinical evidence of progressive disease in SCA patients receiving chronic RBC transfusions, and the results described herein, we conclude that mixed hematopoietic chimerism resulting from NM-BMT may significantly ameliorate SCA, but that near elimination of HbS from the blood may be necessary to derive this potential benefit, and the degree of benefit may correlate with the degree of normal donor hematopoiesis. Furthermore, small fractions of donor bone marrow that result in a minimal or modest reduction in HbS may actually exacerbate complications of SCA. Thus, if stable but low-level normal donor hematopoiesis occurs after NM-BMT in a patient with SCA, minimally toxic means of boosting donor hematopoiesis must be available.
The authors would like to thank Dr George Dover for his review of this manuscript.
Supported in part by an institutional pilot project grant from Fujisawa Healthcare, Deerfield, IL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ephraim J. Fuchs, Bunting-Blaustein Cancer Research Bldg, Rm 488, 1650 Orleans St, Baltimore, MD 21231; e-mail:ejf@jhmi.edu.


![Fig. 3. Nonlinear relationship of fraction of sickle Hb and percentage of sickle RBCs. / A graph of the percent HbS as measured by HPLC versus a visual estimate of the percent sickle cell mouse–derived RBCs is compared to a predicted curve based on the difference in MCH between transgenic sickle cell mice (MHC = 9.3 pg/cell) and normal donor mice (MHC = 14.5 pg/cell). The predicted curve is based on the following formula: % Hemoglobin S = {[(% sickle RBCs)(9.3 pg/cell)] × 100}/{[(% sickle RBCs)(9.3 pg/cell)] + [(% normal RBCs)(14.5 pg/cell)]}.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/12/10.1182_blood.v97.12.3960/6/m_h81211164003.jpeg?Expires=1766597630&Signature=GH3eeNd0sIcorDeMDxI8zL-19omhGOxuLCPOAaH7bnYFTqkgjPDP8ILj2wOsmAJlHX46bdaUfdtf9yaDv6UJ-xJh0NQ9fKLRBEDyqT9eS1G0bFT-Pk9wlQ9YJjHnRp3mywysH1yC6nTJIlMGQFTmSuiqbhmKX7Iv0yS~XFvY9C-oGUTQI8ZA4y3wpJX5ztXKjcTcNF1YSyCtBAdRYBDhZ-7gmGD1R0QGCUwBBF8GdhUiTBLuZof5fnqMJ9FvBFRuHVuwjbbhTbWreEcx0jq05nvDUji3Wm~OBwJqa6oeZVt0vbCQs~F-DVECmxOhl2LKxMVSCKUuefxMveq5wIu20A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 3. Nonlinear relationship of fraction of sickle Hb and percentage of sickle RBCs. / A graph of the percent HbS as measured by HPLC versus a visual estimate of the percent sickle cell mouse–derived RBCs is compared to a predicted curve based on the difference in MCH between transgenic sickle cell mice (MHC = 9.3 pg/cell) and normal donor mice (MHC = 14.5 pg/cell). The predicted curve is based on the following formula: % Hemoglobin S = {[(% sickle RBCs)(9.3 pg/cell)] × 100}/{[(% sickle RBCs)(9.3 pg/cell)] + [(% normal RBCs)(14.5 pg/cell)]}.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/12/10.1182_blood.v97.12.3960/6/m_h81211164003.jpeg?Expires=1766597631&Signature=GW0Fn3KHl3MLoHa3pj-n54q9B03Zo8ygcSnWFd~JhYJ92hXgBye1fUgQMm1AI4DIpoeqL2kMKZKXxlAw3H67mdxBZRM9KkLrNo5MEfB4nP7pJa4x9ng797ggljmwCSXseU50MVMjb2cr-2JBEEuEqJ2jNOuQ~5Q6ER8ZOJnH2Lbyi2tkgt8nDwbJm30Qn46Ec4y0NzEEPWcFnXI1nnB2wquSFfMaP9nvDRWC0alvpofhRuI-AgG-c5-S6wDB32eGERq3WJ1YsmLtilma2sMz3Y5xYhM1XmGR5Gguce63OMC~vrjatQXMrU1AjTmax9cZt4DPcCR-MUQaY58VsVPv9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)