The mechanism underlying the prothrombotic state that characterizes the primary antiphospholipid syndrome proves to be difficult to define mainly because of the variety of the phospholipid and protein targets of antiphospholipid antibodies that have been described. Much of the debate is related to the use of polyclonal antibodies during the different antiphospholipid assays. To better describe the antiphospholipid antibodies, a strategy was designed to analyze the reactivity of each one antibody making up the polyclonal anticardiolipin activity, breaking down this reactivity at the clonal level. This was performed in a single patient with primary antiphospholipid syndrome by combining (1) the antigen-specific selection of single cells sorted by flow cytometry using structurally bilayered labeled anionic phospholipids and (2) the cloning of immunoglobulin (Ig) variable (V) region genes originating from individual IgG anticardiolipin-specific B cells by a single-cell polymerase chain reaction technique. The corresponding V regions were cloned in order to express human recombinant antibodies in insect cells by a baculovirus expression system. The molecular analysis, the fine specificity, and the protein cofactor dependency of the first 5 monoclonal IgG anticardiolipins are reported here. This clonal analysis reveals the extreme heterogeneity of these antibodies, which could account for the difficulties in the previous attempts to define the pathogenic antiphospholipid response. This approach should help to unravel the complex antiphospholipid immune response and the mechanism of the prothrombotic state associated with these antibodies, but it could also shed some light on their possible origins.
Introduction
Antiphospholipid autoantibodies (aPLs)1 are associated with thrombosis, recurrent fetal loss, and thrombocytopenia in patients with antiphospholipid syndrome (APS).2-4 The mechanism of the characteristic prothrombotic state related to aPLs during APS is still under debate.5 Much of it is related to the definition of the precise nature of aPL specificity, which remains unclear because of the number of possible antigenic targets discovered over the last few years. It is now accepted that plasma proteins play an important role in aPL reactivity.6,7 The most common protein cofactors are β2GP18,9 and prothrombin.10-12Other phospholipid (PL)–binding proteins such as protein C9, protein S9, and annexin V13 have also been found to have cofactor properties. On one hand, studies have shown that the binding of aPLs to cardiolipin (CL) is enhanced by the addition of one cofactor (β2GP1, prothombin, protein C, protein S), which suggests that aPLs react to a complex compounded of anionic PLs and a cofactor8,10,11 or to an epitope that becomes exposed when a protein is bound to a PL.14-16 On the other hand, other studies have shown that aPLs are able to bind directly to immobilized β2GP117-19or PT20,21 in the absence of PL. In addition, some studies have shown that anti-β2GP1 antibodies (Abs) are primarily associated with thrombosis in APS22-24 although other reports have involved others Abs as anti-PT25 or lupus anticoagulant.26 It appears that most of these difficulties are related to the use of polyclonal Abs (serum or purified immunoglobulin [Ig])7,8,10,11 or a few monoclonal IgM15,27-29 or IgG30-33 aPLs originating from different patients. We therefore considered it necessary to concentrate our attention on aPL reactivity of each Ab making up the polyclonal aPL reactivity, breaking down this reactivity at the clonal level in a single patient.
The major methods currently in use for the production of human monoclonal antibodies (mAbs) are Epstein-Barr virus transformation,34 mouse-human hybridomas,35or human-human hybridomas36 and fusion of Epstein-Barr virus–transformed B lymphocytes with a malignant cell line.37 These methods have their drawbacks38: In particular, Epstein-Barr virus infection leads to the immortalization of selected B cells, which could engender problems of biased sampling. The culture of human hybridomas is not only laborious, but the hybridomas are difficult to obtain in a stable manner and the yield is very poor due to the limited numbers of sensitized B cells in peripheral blood. To overcome this problem, antigen preselection methods are used, the most common being the isolation of antigen-specific subpopulations by cell sorting. This approach is followed by the generation of variable (V) gene complementary DNA libraries39 or phage-display combinatorial libraries.40 However, these libraries in fact constitute a pool of Ig sequences, where the heavy (H) and light (L) chain are assembled randomly. The probability of selecting Abs formed by a few H and L chain genes occurring in vivo is probably low. To get around this problem, alternative approaches have been proposed: Original VH/VL combinations can be preserved by the technique of “in-cell PCR”41 or by a single-cell culture system of B cells involving clonal expansion.42
To circumvent all these problems, we propose a method based on a combination of both the antigen-specific selection of single cells sorted by flow cytometry using structurally bilayered anionic PL (CL) and, also, a cloning of VH and VL genes originating from individual anti-CL–specific B cells by a single-cell polymerase chain reaction (PCR) technique. For the generation of mAbs, the corresponding Ig genes are cloned in transfer vectors in order to express human recombinant Abs in insect cells using a baculovirus expression system. In this paper we describe our method for isolating monoclonal anticardiolipin (aCL) IgG Abs. We also report the specificity and the cofactor dependency of a series of monoclonal IgGs allowing breakdown of the polyclonal aCL response in a patient suffering from primary APS. This clonal analysis revealed the extreme heterogeneity of aCL Abs in terms of molecular structure, fine specificity, and cofactor dependency.
Patient, materials, and methods
The patient
Patient CIC is a 27-year-old woman suffering from a primary APS. She presented with 6 recurrent spontaneous miscarriages and 2 episodes of deep vein thrombosis of the legs within the last 2 years. The patient's serum consistently contained markedly elevated levels of aPL IgG (84 U GPL, according to manufacturer's internal standard, Asseromapa IgG, IgH, Stago, Annières, France), weak levels of aPL IgA (14 U APL, according to manufacturer's internal standard, Varelisa cardiolipin IgA, Pharmacia, Frieburg, Germany), and a weak and transient lupus anticoagulant. She had no systemic symptoms and no antinuclear Abs. After obtaining informed consent from the patient, 30 mL whole blood was drawn when she was hospitalized for a deep vein thrombosis of the leg. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Lymphoprep, Nycomed, Oslo, Norway). Cells were frozen in RPMI 1640 (Biowhittaker, Verviers, Belgium) containing 20% fetal calf serum (FCS) and 10% dimethyl sulfoxide for further cell staining and sorting.
Cell labeling for flow cytometry analysis
For cell-staining studies, we produced lipid vesicles using a mixture of CL (Sigma Chemical, St Louis, MO), phosphatidylcholine (PC) (Sigma), cholesterol, and PC coupled with a fluorochrome (Bodipy FL, Molecular Probes, Leiden, The Netherlands). The CL vesicles contained 29.1% CL, 58.3% PC, 11.6% cholesterol, and 0.9% labeled PC. The PC vesicles are composed of 87.4% PC, 11.7% cholesterol, and 0.9% labeled PC. These vesicles were prepared according to the method previously published.43 The final PL concentration of these vesicles was 1 mM, and they were used at a dilution of 1:10 000 in phosphate-buffered saline (PBS; 10 mM sodium phosphate, 150 mM NaCl, pH 7.4) containing 2% FCS for cell staining.
Cells were defrosted, washed in PBS, resuspended in PBS 2% FCS, and seeded into microtiter plates at a final concentration of 3.105 cells per well. Cells were incubated at 4°C for 1 hour in the presence of the lipid vesicle suspension, washed twice with 150 mL PBS 2% FCS, and stained with R-phycoerythrin–conjugated F(ab′)2 antihuman IgG Fcγ (Jackson Immunoresearch, West Grove, PA) for 15 minutes at 4°C. Finally, cells were washed twice with PBS 2% FCS and resuspended in 100 mL PBS 2% FCS containing propidium iodide at a final concentration of 5 mg/mL. Inhibition experiments were performed with unlabeled vesicles and an antihuman Fab fragment Ab (Bio-Yeda, Rehovat, Israel). For these inhibition experiments, cells were preincubated either with unlabeled CL or PC vesicles at different concentrations, 1:10 000, 1:1000, or 1:100, for 1 hour or with the antihuman Fab Ab (1, 10, or 20 mg/mL) for 15 minutes. The cells were analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Data acquisition and analysis were conducted with Cell-quest software.
Single-cell sorting
Cells were sorted using an Epics Altra flow cytometer (Beckman Coulter, Brea, CA) equipped with an automatic cell deposition unit. Single cells were directly sorted into thin-wall polycarbonate 96-well plates (Costar, Corning, NY) containing 6 mL water and 3 mL 5 × concentrated reverse transcriptase (RT)-PCR buffer (Gibco, Life Technologies, Gaithersburg, MD). Plates were immediately frozen by putting them on dry ice and were stored at −80°C until use for DNA amplification.
Preparation of complementary DNA
The RT reaction was performed using random hexamer pd(N)6 (Amersham Pharmacia Biotech, Germany) and Superscript RT (Gibco). To prepare complementary DNA, single cells were directly heated for 1 minute at 65°C, put on ice, and 6 mL RT mixture (dithiothreitol 1 mM, deoxyribonucleoside triphosphate 0.1 mM, ribonuclease inhibitor 40 U, pd(N)6, and Superscript RT [25 U]) immediately added. Samples were incubated at 37°C for 1.5 hours. The enzyme was inactivated for 3 minutes at 95°C. A β-actin PCR amplification was used as a control for the RT step.
PCR amplification
DNA amplification was carried out in 2 rounds of PCR (Figure1A,C) using a DNA Thermal Cycler 9600 (PerkinElmer, Foster City, CA). For PCR amplification of rearranged VH and Vκ genes, a seminested PCR approach was chosen. The sequences of the sets of oligonucleotides used as primers are from a previous publication.44
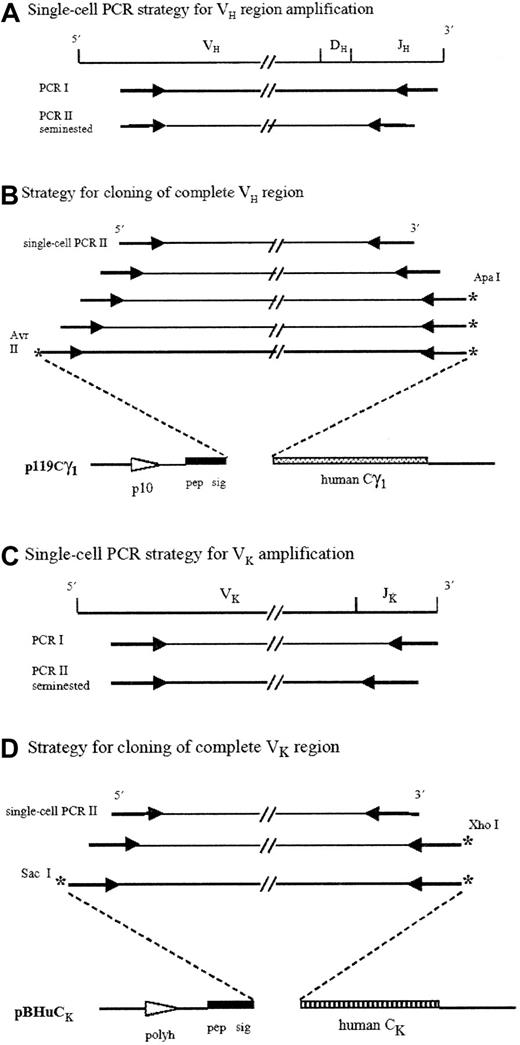
Strategies for V region amplifications and cloning.
VH and Vκ gene fragments were amplified by a single-cell PCR technique using a seminested PCR as second reaction (A,C). Four sets of PCR were necessary for the 5′ extension of VH regions (B), and 2 sets of PCR were required for the 5′ extension of Vκ regions (D). Gene fragments were cloned in a forced orientation (B,D). *Unique restriction cloning sites; Pep sig indicates signal peptide; polyh, polyhedrine promoter; p10, p10 promoter.
Strategies for V region amplifications and cloning.
VH and Vκ gene fragments were amplified by a single-cell PCR technique using a seminested PCR as second reaction (A,C). Four sets of PCR were necessary for the 5′ extension of VH regions (B), and 2 sets of PCR were required for the 5′ extension of Vκ regions (D). Gene fragments were cloned in a forced orientation (B,D). *Unique restriction cloning sites; Pep sig indicates signal peptide; polyh, polyhedrine promoter; p10, p10 promoter.
The first round of amplification was carried out in the same sample tube as the RT reaction and in a final volume of 90 mL containing 50 mM KCl; 10 mM Tris-HCl, pH 8.4; 2.5 mM MgCl2; 6 nM of each dATP, dCTP, dGTP, and dTTP; 7.5 pM of each VH, Vκ, 3′JH, and 3′Jκ primer; and 5 U Taq DNA polymerase High Fidelity (Roche Molecular Biochemicals, Mannheim, Germany). The conditions of the first round consisted of 1 cycle at 94°C for 1 minute; followed by 35 cycles at 94°C for 45 seconds, at 59°C for 60 seconds, and 72°C for 90 seconds; followed by 10 minutes at 72°C.
The second round of amplification was performed in separate reactions of 20 mL for each of the 6 VH and 6 Vκfamilies. A volume of 1 mL of the first PCR amplification was reamplified with one specific 5′ primer and the set of anchored 3′ primers. All the second PCR amplifications contained dATP, dCTP, dGTP, and dTTP at 200 mM each and 1U Taq DNA polymerase High Fidelity in PCR buffer (50 mM KCl; 10 mM Tris-HCl, pH 8.4; 1.5 mM MgCl2 for VH primers or 2.5 mM MgCl2 for Vκprimers; 0.125 mM of one of the 5′ primers; and 0.125 mM of the set of 3′ primers). The second round consisted of 1 cycle at 94°C for 1 minute; followed by 35 cycles at 94°C for 20 seconds, at 65°C for 30 seconds, and 72°C for 60 seconds; followed by 10 minutes at 72°C. A total of 10 mL of the second PCR reaction products was analyzed on 2% agarose gels stained with ethidium bromide.
PCR product sequencing
PCR products were purified by extraction of the DNA from 2% low-melt agarose gels. (Euromedex, France). They were then sequenced using the Big Dye Terminator cycle DNA sequencing kit (Applied Biosystems, Foster City, CA) and an automated DNA sequencer (310 Genetic Analyzer, PerkinElmer).VH and Vκ products were sequenced with the primers used in the second round of amplification. The V genes were analyzed using the international ImmunoGeneTics (IMGT) database45,46 (http:// imgt.cines.fr).61
Cloning of PCR products
The resulting amplified VH and Vκgenes originating from single-cell PCR were cloned in the transfer vectors p119Cg1 and pBHuCk47, respectively, for expression in recombinant baculovirus–infected insect cells.
Preparation of fragments to be inserted
Because the 5′ end of the amplified products did not contain the original extremity of the rearranged V regions, we used overlapping oligonucleotides and PCR to reconstitute the 15 lacking 5′ codons (Figure 1B). The primers were designed according to the identified germline gene (VH or Vκ) and are accessible through our website (http://aloes.u-strasbg.fr/). The PCR was performed with a Taq High Fidelity DNA polymerase (Roche). The VH and Vκ extended fragments were cleaved with AvrII/ApaI andSacI/XhoI, respectively. The fragments were then cloned in a forced orientation into the cassette transfer vectors (Figure 1B,D). Integrity of the cloned fragment sequences was verified by sequencing of the plasmids (automated DNA sequencer).
Construction of recombinant baculovirus–producing human mAbs
To obtain double-recombinant virus expressing the H and L chains, plasmidic DNA, p119Cg1, and pBHuCk containing the VH and VL gene fragments from the original cell were cotransfected with the baculovirus DNA. The cotransfections of plasmidic DNA, virus propagations, and plaque assays were performed using published methods.47 48 The efficiency of the cotransfection ranged from 20% to 50%. Assembled and secreted Abs were detected in the insect cell supernatant by a conventional antihuman IgG enzyme-linked immunosorbent assay (ELISA). Finally, mAbs were produced by Sf9 insect cell infection in culture medium without FCS.
Purification of IgG
Supernatants containing IgG were dialyzed, first against Tris ethylenediaminetetraacetic acid buffer (10 mM Tris HCl, 1 mM ethylenediaminetetraacetic acid) to increase the pH to 7.8 and, finally, against PBS, pH 7.8. IgG was purified with a protein A column (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. The eluate was dialyzed in PBS or in Tris buffer saline (TBS; 50 mM Tris buffer, 120 mM NaCl, 2.7 mM KCl, pH 7.5) and 0.1 mm filtered. The purity of human IgG was checked by gel migration under undenatured conditions showing no isolated H or L chains (data not shown), and the quantity of purified IgG was measured by a direct ELISA.
Immunologic assays for IgG antiphospholipid antibodies
Anti-PLs were measured by means of a modified ELISA. Polystyrene microtitration plates (Polysorp, Nunc, Roskilde, Denmark) were coated with CL, phosphatidylserine (PS; Sigma), or PC in 95% ethanol at 50 mg/mL. Uncovered plates were left to evaporate overnight at room temperature and saturated at 4°C for 2 hours with PBS containing 10% FCS. After washing with PBS, samples diluted in PBS 10% FCS were added to each well (in duplicate) and incubated at room temperature for 3 hours. After 3 washing steps in PBS 0.01% Tween 20, Abs were revealed by peroxidase-conjugated goat antihuman IgG (Jackson ImmunoResearch) diluted in PBS 0.01% Tween 20 for 50 minutes at 37°C, followed by O-phenylenediamine dihydrochloride peroxidase substrate (Sigma) after 3 washing steps. Plates were read at 492 nm using a Titertek Multiskan (Labsystems, Helsinki, Finland). The background resulting from binding to wells treated with ethanol alone was subtracted from that of the PL-coated wells.
Purified aCLs were tested for their serum independency. Briefly, plates were coated with anionic PLs in ethanol and blocked with 1% bovine serum albumin (BSA) (Euromedex) in PBS. Test samples were diluted in PBS containing 1% BSA.
Monoclonal aCLs were tested for their cofactor dependency. Their reactivity with CL and PS was tested in the presence of purified human cofactors: β2GP1, prothrombin (PT), protein S (Prot S), protein C (Prot C), and annexin V (An V). For this, plates were coated with CL or PS as described above and blocked with PBS containing 0.25% gelatin. Samples were diluted in PBS 0.25% gelatin, 2 mM CaCl2, containing either 10 mg/mL human PT, 5 mg/mL human Prot S, 5 mg/mL human Prot C (Kordia, Leiden, The Netherlands), 5 mg/mL human An V, or 10 mg/mL human β2GP1 (in PBS 0.25% gelatin) added to the wells in duplicate and incubated for 3 hours at room temperature.
The patient serum and purified monoclonal IgGs were also tested directly with each of the previous human cofactors, β2GP1, PT, Prot S, Prot C, and An V, without addition of PL. Microtiter plates (Costar) were coated with 10 mg/mL human β2GP1 in PBS alone, 10 mg/mL PT, 5 mg/mL Prot S, 5 mg/mL Prot C, 5 mg/mL An V in PBS, 3 mM CaCl2, for 2 hours at 37°C. After blocking with 0.25% gelatin in PBS, samples were distributed in duplicate and incubated for 2 hours at 37°C.
Inhibition experiments
For the inhibition studies, CL vesicles (1 mM) were incubated 30 minutes at 4°C with the purified cofactors before they were mixed at various dilutions (1:10 000, 1:50 000, 1:100 000, 1:200 000) with the mAbs at a fixed concentration (10 mg/mL) for 30 minutes at 37°C. A total of 50 mL of the mixture was transferred to the wells of plates coated as above with CL and purified cofactors. The assays were performed as described above. Data were expressed as optical density (OD) values of mAb binding to solid-phase CL/cofactor complexes.
Lupus anticoagulant test
The lupus anticoagulant (LA) activity of the mAbs was determined by a modified dRVVT (dilute Russell's viper venom test (LAC Screen Instrumentation Laboratory, Milano, Italy) according to the manufacturer's instructions. LA activity is considered as positive when the clotting time ratio of LAC Screen/LAC Confirm is higher than 1.2.
Prothrombinase functional assay
The prothrombinase assay used here was adapted from a previous publication.49
The inhibition of the prothrombinase assay was measured using irrelevant human polyclonal IgG representative of 100% activity. Microtiter plates (Maxisorp, Nunc) were coated overnight at room temperature with 5 mg/mL human serum albumin in TBS 1 mM CaCl2. PL liposomes (3.8 mM) composed of 33% PS and 67% PC were diluted at 1:200 000 in TBS CaCl2, 3 mg/mL human serum albumin. A total of 50 mL IgG Abs was mixed with 250 mL PL liposome suspension and incubated for 15 minutes at room temperature. After 3 washing steps of the precoated plates with TBS CaCl2, 100 mL of the mixture was added to the well (in duplicate). Then, each well was supplied with factor V, factor Xa, factor II, and CaCl2 at a final concentration of 30 pM, 5 pM, 1 mM, and 2 mM, respectively. Plates were incubated for 15 minutes at 37°C. The conversion of PT to thrombin was revealed by adding 50 mL per well of chromozyme TH (Roche), a chromogenic substrate for thrombin, at a final concentration of 0.75 mM. Linear absorbance changes were recorded at 405 nm using a microtitration plate reader equipped with kinetics software.
Results
Flow cytometry analysis and sorting
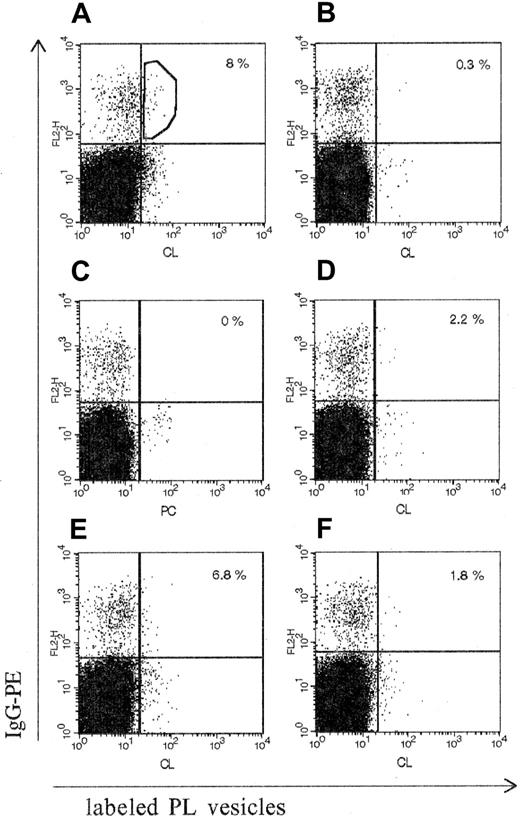
PBMCs originating from patient CIC were analyzed in their ability to react with CL vesicles. Figure 2A shows that fluorochrome-labeled lipid vesicles reacted with a low proportion of IgG-bearing cells (8%). This reactivity was specific for CL-expressing vesicles because (1) PC vesicles were unreactive with IgG-bearing cells (Figure 2C), (2) unlabeled CL vesicles were able to inhibit up to 70% the CL staining of IgG+ cells (Figure2D) and, finally, (3) F(ab′)2 antihuman Fab Abs were able to block up to 80% the binding of CL vesicles to IgG-bearing B cells (Figure 2F). The cell sorting was performed on the double-positive cells (labeled CL vesicles or IgG) as described in “Patient, materials, and methods” (Figure 2A). Notably, cells from a healthy control donor contained only 0.3% of such double-positive cells (Figure 2B).
Flow cytometry analysis of IgG-bearing B cells with labeled PL vesicles.
The percentage of double-positive cells is expressed as the percentage of IgG-binding B cells and is indicated in the upper right quadrant of each panel. (A) PBMCs from patient CIC: binding of labeled CL vesicles. (B) PBMCs from a healthy donor: binding of labeled CL vesicles. (C) PBMCs from patient CIC: binding of PC vesicles. (D-F) PBMCs from patient CIC: inhibition of labeled CL vesicle binding with, respectively, nonlabeled CL vesicles, nonlabeled PC vesicles, or antihuman Fab Ab. Single-cell sorting was performed in the gate of the double-stained population shown in the upper right quadrant in panel A. IgG-PE indicates IgG conjugated with R-phycoerythrin.
Flow cytometry analysis of IgG-bearing B cells with labeled PL vesicles.
The percentage of double-positive cells is expressed as the percentage of IgG-binding B cells and is indicated in the upper right quadrant of each panel. (A) PBMCs from patient CIC: binding of labeled CL vesicles. (B) PBMCs from a healthy donor: binding of labeled CL vesicles. (C) PBMCs from patient CIC: binding of PC vesicles. (D-F) PBMCs from patient CIC: inhibition of labeled CL vesicle binding with, respectively, nonlabeled CL vesicles, nonlabeled PC vesicles, or antihuman Fab Ab. Single-cell sorting was performed in the gate of the double-stained population shown in the upper right quadrant in panel A. IgG-PE indicates IgG conjugated with R-phycoerythrin.
Single-cell variable region gene amplifications
Sorted single cells were subjected to RT-PCR for IgG V region genes. Ninety-eight CL+/IgG+ single cells were analyzed for their VH and Vκ gene rearrangements. Our method was able to amplify the VHregion in 58% of the cells and the Vκ region in 61% of the cells. Both V regions were amplified in 48 single cells representing 49% of the analyzed cells.
The PCR products were directly sequenced, and the first 5 couples of VH and Vκ regions were further analyzed. All the sequences were found functional and were aligned with their closest respective germline gene sequence using the IMGT database.
Monoclonal anticardiolipin antibody production and specificities
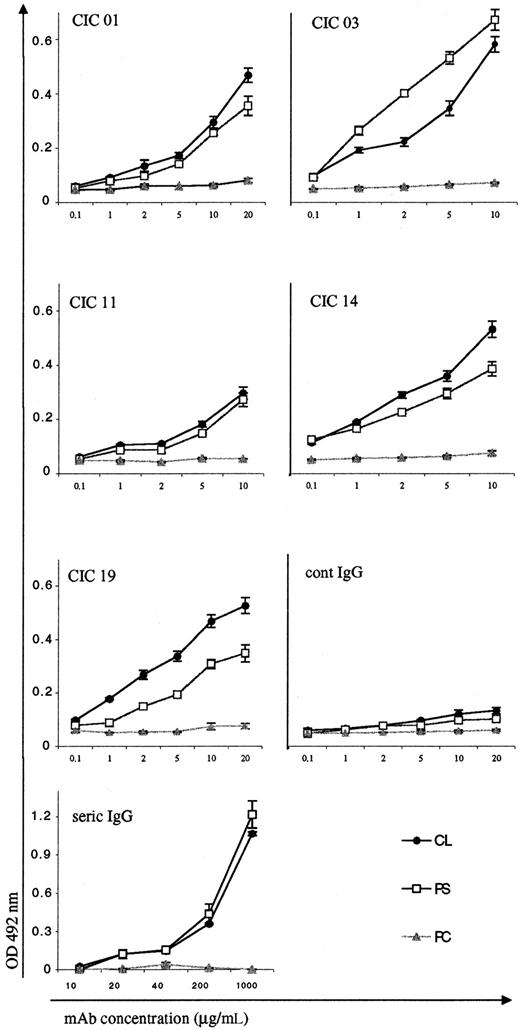
As described in Figure 1, we first extended the 5′ and 3′ regions of the amplified V products to clone the entire VH/D/JH and Vκ/Jκregions into transfer vectors for the baculovirus insect cell expression system. The plasmids were sequenced to ensure the integrity of the cloned fragments. The first 5 pairs of VH and VL regions gave rise to 5 different monoclonal IgG Abs (CIC 01, CIC 03, CIC 11, CIC 14, CIC 19) whose specificity was determined after protein A purification. Figure 3shows the reactivity of the 5 mAbs with anionic PL (CL and PS) and with a neutral PL (PC). All of them reacted to various degrees in a dose-dependent manner with the anionic PL in the presence of FCS. None of them reacted with the neutral PL. In addition, none of them recognized PL alone (without FCS). For comparison, we also purified by the same procedure (protein A column) the serum patient IgG fraction and showed that the aCL activity was detectable at an IgG concentration of 20 mg/mL (Figure 3). These results clearly demonstrate the efficiency of the sorting and amplification strategies that were employed to break up the aCL polyclonal Ab response in this single patient.
PL binding activities of the purified CIC monoclonal IgG Abs.
Monoclonal Abs were tested for their reactivities with 2 anionic PLs, CLs, and PSs and with a neutral PL, PC. Protein A–purified human IgG (cont IgG) was used as the negative control. Protein A–purified plasma IgG from patient CIC (seric IgG) was used as positive control. The results are expressed as OD at 492 nm minus background (mean ± SE of 2 experiments).
PL binding activities of the purified CIC monoclonal IgG Abs.
Monoclonal Abs were tested for their reactivities with 2 anionic PLs, CLs, and PSs and with a neutral PL, PC. Protein A–purified human IgG (cont IgG) was used as the negative control. Protein A–purified plasma IgG from patient CIC (seric IgG) was used as positive control. The results are expressed as OD at 492 nm minus background (mean ± SE of 2 experiments).
Cofactor dependency
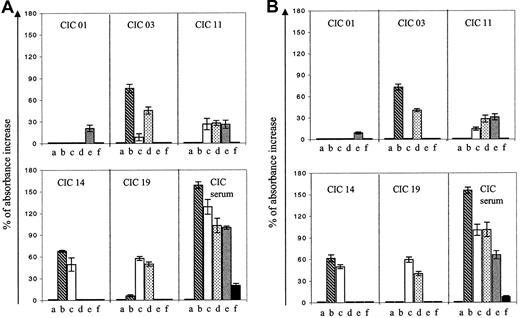
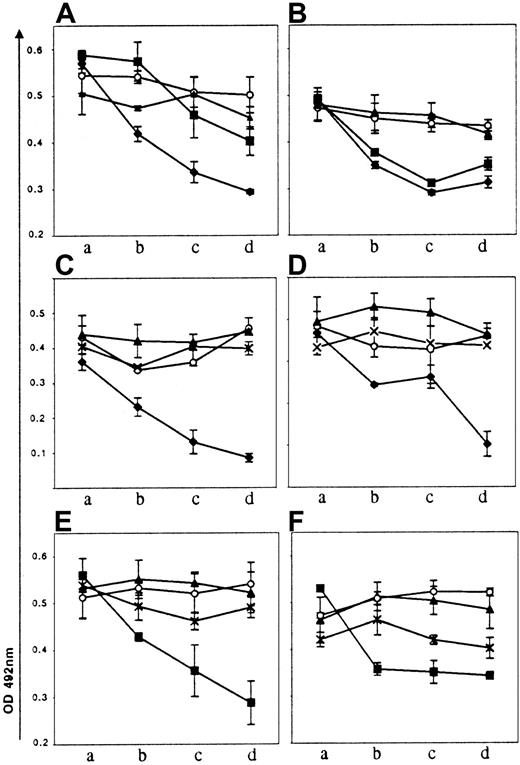
The 5 aCL monoclonal IgGs were tested for their cofactor requirement in anti-PL binding and compared with the patient's serum reactivity. Purified human β2GP1, PT, Prot S, Prot C, and An V were added during the ELISA procedure of anti-PL (CL or PS) binding (Figure4A), and mAb cofactor dependencies were considered significant when they increased the IgG binding to PL by at least 30%. The cofactor dependency of the mAbs was extremely heterogeneous: β2GP1 enhances the binding of CIC 03 to anionic CL-coated plates, PT enhanced the binding of CIC 19 to CL, and both cofactors enhance CIC 14 anti-CL activity. CIC 01 was not influenced by any one of the purified proteins. Interestingly, the cofactor dependency was identical with that of PS (Figure 4B).
Cofactor dependency of the purified CIC monoclonal IgG Abs and of the CIC serum.
Monoclonal Abs were tested for their reactivities with anionic PL, CL (A) and PS (B) in the presence of purified human proteins. The results are expressed as the percentage of increase of OD in the presence of the purified cofactors. The patient's serum was tested diluted at 1:100, and the purified IgG was tested at a concentration of 5 mg/mL. Lane a: BSA (control); lane b: β2GP1; lane c: prothrombin; lane d: Prot S; lane e: Prot C; lane f: An V.
Cofactor dependency of the purified CIC monoclonal IgG Abs and of the CIC serum.
Monoclonal Abs were tested for their reactivities with anionic PL, CL (A) and PS (B) in the presence of purified human proteins. The results are expressed as the percentage of increase of OD in the presence of the purified cofactors. The patient's serum was tested diluted at 1:100, and the purified IgG was tested at a concentration of 5 mg/mL. Lane a: BSA (control); lane b: β2GP1; lane c: prothrombin; lane d: Prot S; lane e: Prot C; lane f: An V.
The apparent multireactivity of 3 mAbs (CIC 03, CIC 14, CIC 19) was further tested by performing binding inhibition experiments where the binding of the Abs to solid-phase CL-purified cofactor complexes was measured after incubating the Abs with liquid-phase CL vesicles previously coated either with the same cofactor (homologous inhibition) or a different cofactor (heterologous inhibition). The results are described in Figure 5. The inhibition curves of CIC 14 binding on solid-phase CL-β2GP1 or CL-PT with liquid-phase CL-β2GP1– or CL-PT–coated vesicles (Figure 5A,B) clearly show that CIC 14 is bireactive but also that it has a higher affinity for CL-β2GP1 than for CL-PT, because the homologous and heterologous inhibition curves are identical on solid-phase CL-PT, but the inhibition on solid-phase CL-β2GP1 by CL-PT in solution is significantly lower than that induced by CL-β2GP1 (30% vs 50%). The same analysis of the inhibition curves with CIC 19 and CIC 03 (Figure 5C-F) also shows that these 2 mAbs are also bireactive but CL–Prot S vesicles were unable to induce both homologous and heterologous inhibitions, suggesting that the conformation of the vesicle complexes cannot be recognized by the Abs and/or that the affinities of the Abs for solid-phase CL–Prot S are higher than for liquid-phase complexes. Finally, considering the mode of B-cell selection and the use of CL-containing vesicles in the presence of FCS, we should bear in mind that this screening approach would potentially miss certain B cells producing Abs that are human cofactor–specific, also explaining that none of the 5 mAbs reacted with the purified proteins without the presence of anionic PL (data not shown).
Inhibition studies of the binding of mAbs to solid-phase CL/cofactor.
Binding inhibition experiments were performed as described in “Patient, materials, and methods” with β2GP1 (♦), PT (▪), Prot S (*), Prot C (○) coated vesicles or vesicles alone (▴). The vesicles were used at a dilution of 1:200 000 (a), 1:100 000 (b), 1:50 000 (c), and 1:10 000 (d). Inhibition curves of the CIC 14 binding on CL/β2GP1 (A), of the CIC 14 binding on CL/PT (B), of the CIC 03 binding on CL/β2GP1 (C), of the CIC 03 binding on CL/Prot S (D), of the CIC 19 binding on CL/PT (E), and of the CIC 19 binding on CL/Prot S (F).
Inhibition studies of the binding of mAbs to solid-phase CL/cofactor.
Binding inhibition experiments were performed as described in “Patient, materials, and methods” with β2GP1 (♦), PT (▪), Prot S (*), Prot C (○) coated vesicles or vesicles alone (▴). The vesicles were used at a dilution of 1:200 000 (a), 1:100 000 (b), 1:50 000 (c), and 1:10 000 (d). Inhibition curves of the CIC 14 binding on CL/β2GP1 (A), of the CIC 14 binding on CL/PT (B), of the CIC 03 binding on CL/β2GP1 (C), of the CIC 03 binding on CL/Prot S (D), of the CIC 19 binding on CL/PT (E), and of the CIC 19 binding on CL/Prot S (F).
In vitro functional tests of aCL antibodies
LA activity was performed using a dRVVT. One (CIC 01) of the 5 mAbs was considered positive, prolonging the clotting time. This prolongation was reversed by adding platelet PL (Table1). We also tested the ability of the mAbs to inhibit the generation of thrombin in a prothrombinase activity test. The mechanism of this assay involves the Ab binding to PL and is mainly dependent on PS. The results are shown in Table 1. CIC 01 and CIC 03 markedly inhibit in a dose-dependent manner the generation of thrombin; CIC 11 is unable to do it, and CIC 14 as well as CIC 19 are intermediate. Notably, CIC 01 has both the capacity to prolong the clotting time during the dRVVT and to inhibit the thrombin generation during the prothrombinase activity test, which was not the case for CIC 03 effective only in the second test.
Molecular analysis of the aCL IgG antibodies
The analysis of the V region sequences is summarized in Table2, leading to a few remarks. First, there is no apparent bias in the gene family usage: 3 of the autoantibodies use a VH gene originating from the main human VH family (VH3, CIC 14, CIC 11, CIC 19), one uses a VH4 gene (CIC 03), and the last one uses a VH5 gene. Second, the sizes of the third heavy chain complementary determining regions (CDRs) are heterogeneous, ranging from 11 to 18 amino acids. Third, the ratios of replacement-to-silent changes (R/S) in the CDRs compared with the frameworks show that the CIC 01 and CIC 11 VH regions have significant R/S ratios in the CDRs, as well as the CIC 14 Vκ region, possibly indicating an antigen-driven process in the selection of the Ab production B cells. All the sequences are available through GenBank (AF301481, AF301482, AF301483, AF301484, AF301485, AF301486,AF301487, AF301488, AF301489, AF301490). Their detailed analysis with the others originating from the same patient is beyond the scope of this paper and will be described elsewhere (manuscript in preparation). Notably, however, the activity of the most mutated Ab (CIC 01) was not influenced in vitro by one of the tested cofactors but still had LA activity and inhibited the generation of thrombin.
Discussion
The precise characterization of aPL associated with an increased thrombotic state is an important goal in order to understand the mechanism that links such autoantibodies to thrombosis and to better stratify the risk of thrombotic event in subjects with APS. The analysis of polyclonal purified aPL or serum aPL already suggests the complexity of the immune response against PL or PL complexed to proteins.6-8,10 Such a complexity at the polyclonal level certainly explains the difficulties in the mechanistic approach of aPL associated with thrombosis. The description of a few mAbs directed against PL, originating from different patients, also suggests that these Abs are indeed heterogeneous.32,33 Our approach combining a step of specific B-cell selection, a step of amplification of the IgG V region genes, and a step of in vitro Ab production confirms and extends previous observations, showing, in a single patient suffering from APS, that aCL Abs are extremely heterogeneous at the clonal level. It is clear that this situation is not peculiar to patient CIC because the molecular analysis of the VH gene usage by aCL Abs obtained following the same approach in a second patient suffering from APS gives the same picture of extreme molecular heterogeneity of aCL (to date, from the first 20 analyzed single cells, 4 different VH families were amplified and the first 7 sequences obtained were distinct; data not shown).
The methodology described here could be applied to the general analysis of any specific B-cell response, providing that the antigen is purified. The specificity of the sorting of aCL B cells is already suggested by the binding inhibition experiments (Figure 2) and definitely demonstrated by the binding of the first 5 produced Abs to CL. As described in “Patient, materials, and methods,” the process of the V region cloning implicated a preliminary extension, mainly on the 5′ side, of the products of amplification. This extension was performed according to the previously determined germline genes, making it possible that a few mutations located in the first framework were missing in the final entire V region. However, aCL specificity was preserved even though we cannot totally rule out that rare missing mutations could affect Ab affinity. This method appears extremely powerful in breaking down the polyclonal Ab response revealing, in this case, at an unexpected rate the functional and molecular diversities of aCL Abs. The diversities could be even more important, given that we did not try to analyze λ-bearing B cells.
The CIC serum reactivity is in fact very difficult to interpret (Figure4): How many different Abs are involved; how frequent is the multireactivity of the different aCLs; which of these Abs are potentially pathogenic? The analysis of the first 5 aCLs shows that each one has a different reactivity profile with CL, PS in the presence or absence of cofactors. No Abs react with cofactors alone. Finally, only one Ab (CIC 01) was found positive in the in vitro functional tests: even though these tests could not be sufficient to identify the prothrombotic activity of an Ab, CIC 01 could be a candidate for further analysis of thrombogenic potential. This last Ab could also be responsible for the weak and transient LA activity detected in patient's plasma.
The molecular analysis also gives a picture of high heterogeneity: different VH and Vκ families and gene segments, different JH and Jκ genes, different lengths of CDR3, different degrees of mutations. No clear structure-function relationship can be deduced from the analysis of these first Abs. For instance, at the opposite of the description of a single almost germline-encoded mAb with a LA activity,30our less mutated Ab (CIC 19) has no functional activity, and the most mutated (CIC 01) has LA activity as well as the ability to inhibit the thrombin generation in the prothrombinase activity test. Clearly, the diversity of aCL Abs will render the study of their structure-function relationships more difficult and will necessitate comparison of numerous Abs with homogeneous reactivity profiles. Finally, both the diversity of the molecular structures and the fine specificity of these autoantibodies question the origin of this immune response. At this point in our work, we can only suggest some hypotheses: (1) A number of serum proteins can bind anionic PL and could induce the formation of PL-protein complexes, which offer many different targets for autoantibodies. For instance, the cofactors that we have tested are probably not the only ones51-54 in patient CIC, the choice of the cofactors being dictated by previous published data and their implication in hemostasis. (2) The heterogeneity of the aCL Abs could be related to the mechanism of their appearance: some aCLs could constitute primary events in the thrombosis, the others being just a byproduct of the pathology. (3) Some of the aCL Abs (CIC 19) are almost germline-encoded, but others (CIC 01, CIC 11, CIC 14) are mutated in a way that suggests an antigen-driven process. Anti-CL Abs can be detected in normal individuals and are therefore components of natural Abs, which play a crucial role in the initial fighting against exogeneous pathogens.55 Whether the mutations of some aCL Abs represent recent events directly linked to the autoimmune pathology (APS) or constitute the molecular imprint of the patient's past history of infectious diseases—the producing B cells being just reactivated by the autoimmune state—remains unknown and will be further evaluated.
The observed diversity of aCL Abs could explain some of the known difficulties in defining in vivo pathogenic Abs. Thus, it will be important to test our monoclonal aCL IgGs in their ability to bind to endothelial cells,56-58 to induce tissue factor expression on monocytes,59 to interfere with the function of activated Prot C24 60 and, finally, to help in elaborating a murine in vivo model of thrombosis.
We thank Professor P. De Groot for help and assistance and for welcoming P.L. in his laboratory.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM CRI 9702). P.L. was supported by fellowships from the Fondation pour la Recherche Médicale, France, and the Association Alsacienne pour la Recherche et la Formation en Médecine Interne, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thierry Martin, Laboratoire d'Immunopathologie, Institut d'Hématologie et d'Immunologie, Hôpital Civil, 1, place de l'Hôpital, 67091 Strasbourg Cedex, France; e-mail: thierry.martin@hemato-ulp.u-strasbg.fr.