CD100/Sema4D is a 150-kd transmembrane protein that belongs to the semaphorin family. The interaction of CD100 with CD72 is critical for the immune system. In CD100-deficient mice, the production of specific antibodies against T-cell–dependent antigens is severely impaired, but not against T-cell–independent antigens. Here, a functional soluble CD100 protein (sCD100) released from activated lymphocytes is reported. sCD100 was detected in culture supernatants of activated lymphocytes. Either affinity-purified from supernatants of activated T-cells, or produced as a recombinant sCD100 protein consisting of the extracellular region of the mouse CD100 fused to the human IgG1 Fc (CD100-Fc), sCD100 significantly enhanced CD40-induced B-cell responses. Furthermore, sCD100 was detected either in sera of mice immunized with T-cell–dependent antigens, or in sera of MRL/lpr mice, but not in sera of mice immunized with T-cell–independent antigens. A significant correlation was observed between the level of sCD100 and the titer of autoantibodies in the serum of MRL/lpr mice. This study's findings suggest a potential role for sCD100 in immune responses, including production of antibody, and autoimmune diseases.
Introduction
The semaphorin family consists of a large number of phylogenetically conserved proteins, carrying a large “Sema domain” (approximately 500 amino acid residues) in their extracellular regions.1,2 Many members of the semaphorin family are known to be critically involved in axon pathfinding, acting as chemorepulsion factors during neuronal development.2-6Although the function of semaphorins has been initially addressed with respect to neuronal guidance as mentioned, it is becoming clear that semaphorins play important roles in organogenesis, vascularization, angiogenesis, and progression of cancers.7-10
The semaphorin family is divided into 8 groups which are defined based on structural properties.1 CD100, which belongs to group IV, is the first semaphorin member expressed physiologically in lymphocytes to be identified.11-13 CD100 is expressed abundantly by T cells but weakly by B cells.11,12,14 Its expression is significantly enhanced in both T and B cells, following their respective activation.15 Human CD100–expressing transfectants have been reported to promote aggregation and survival of B cells in vitro.12 A recombinant soluble mouse CD100 protein, consisting of the extracellular region of CD100 and the Fc portion of human IgG1 (CD100-Fc), exerts an effect on the production of antibodies in vivo, as well as B-cell responses in vitro.15 Collectively, these findings indicate that CD100, like other semaphorin proteins, can function through a receptor on the surface of lymphocytes.
Two types of receptors with distinct binding affinities have been identified for CD100. Plexin-B1, which is widely expressed at prominent levels in the fetal brain and kidney, is a receptor which has been shown to have a high affinity for CD100.16,17 In addition, we have recently identified CD72, a receptor found on the surface of lymphocytes.15 Our recent study has shown that CD100-deficient mice have multiple defects in lymphoid tissues, but not in other tissues where plexin-B1 is abundantly expressed, suggesting that the interaction of CD100 with CD72, but not with plexin-B1, is rather important in the immune system.18 CD72 has been shown to recruit the protein tyrosine phosphatase SHP-1, via its immunoreceptor tyrosine–based inhibitory motifs (ITIM) of its cytoplasmic region.19 B cells from CD72-deficient mice have been reported to be hyperproliferative in response to various stimuli.20 CD72 thus appears to be a negative regulator of B-cell response. Recently, we have shown that CD100-binding induces the dephosphorylation of tyrosines on CD72 and the dissociation of SHP1 from the latter.15 Therefore, CD100 appears to turn off the negative signaling effects of CD72, resulting in an enhancement of B-cell activation. Indeed, CD100-deficient mice displayed hyporesponsiveness of B cells. This is almost symmetric in CD72-deficient mice, whose B cells were hyperreactive to various stimuli.18 20
Soluble forms of various transmembrane-type factors and receptors such as CD21, CD23, CD27, CD44, CD106, and Fas have been reported.21-26 For instance, CD27, which is a member of the tumor necrosis factor (TNF) receptor superfamily and expressed on the surface of lymphocytes as a 55-kd protein, is cleaved proteolytically into a 32-kd soluble form after activation of the lymphocyte.23 The quantity of soluble CD27 has been shown to be elevated in the sera from patients of infectious and autoimmune diseases.23 Similarly, soluble CD23 is generated by proteolytic cleavage, and has been shown to be biologically active.22 On the other hand, a soluble form of Fas (sFas) has been shown to be generated by alternative splicing after the activation of peripheral blood mononuclear cells (PBMCs).26 sFas has been reported to be elevated in sera from patients of system lupus erythematosus (SLE),27suggesting its involvement in the progression of autoimmunity. Also, in the case of human CD100, Herold et al28 29 reported the presence of a soluble form of CD100 (sCD100) in culture supernatants of PBMCs treated with anti-CD100 or anti-CD45 monoclonal antibodies (mAbs). However, the physiologic and pathologic significance of the activity of sCD100 in immune response has not been elucidated.
Here, we demonstrate the presence, the activities, and the elevation of the levels of sCD100 protein, either in culture supernatants of activated lymphocytes, or in the sera of immunized mice and of mice with autoimmune disease. The physiologic and pathologic roles of sCD100 in the regulation of immune responses are also discussed.
Materials and methods
Mice and antibodies
MRL/lpr, MRL/n, C57BL/6, and BALB/c mice were purchased from SLC (Shizuoka, Japan). Rat antimouse CD100 monoclonal antibodies (anti-CD100 mAbs) (BMA-12; rat IgG1, BMA-8; rat IgG2a) were established by immunizing rats with CD100-Fc as previously described.15 Anti-CD100 mAbs were biotinylated using a biotinylation kit (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's protocol. Rat antimouse CD16/32 antibody (2.4G2), allophycocynanin (APC)–conjugated streptavidin, fluorescein isothiocyanate (FITC)-conjugated anti-B220 (RA3-6B2), phycoerythin (PE)–conjugated anti-Thy1.2 (30-H12), FITC-conjugated anti-CD8 (Ly-2), PE-conjugated anti-CD4 (GK1.5), and anti-CD40 mAb (HM40-3) were purchased from Pharmingen (San Diego, CA). Alkaline phosphatase (AP)–conjugated streptavidin was from Sigma (St Louis, MO), AP-labeled antimouse IgG antibodies were from Southern Biotechnology (Birmingham, AL), and streptavidin-peroxidase (POD) conjugate was from Boehringer Mannheim.
Flow cytometric analysis
Single-cell suspensions were prepared from the inguinal and popliteal lymph nodes. One million cells were stained with the following antibodies: PE-conjugated anti-CD4 and FITC-conjugated anti-CD8, or PE-conjugated anti-Thy1.2 and FITC-conjugated anti-B220, followed by biotinylated antimouse CD100 mAb (BMA-12) plus APC-conjugated steptavidin in the presence of Fc block (anti-CD16/CD32 mAb). Cells were then washed and analyzed by a flow cytometer. Data analysis was performed using the Flow Jo software (Treestar, San Carlos, CA).
Enzyme-linked immunosorbent assay
sCD100.
Sandwich enzyme–linked immunosorbent assay (ELISA) for detecting sCD100 was performed as follows: 96-well microplates (Maxisorp Nunc-Immuno plate, Rochester, NY) were coated with BMA-12 (100 μL of 5 μg/mL in 0.1 mM NaHCO3, pH 9) at 4°C for 15 hours. The wells were incubated in blocking solution (200 μL of 50 mM Tris-HCl, pH 8.1; 1 mM MgCl2; 0.15 M NaCl; 1% BSA; 0.05% Tween-20) for 1 hour at room temperature. Samples or the standards (a recombinant sCD100 protein consisting of the extracellular region of mouse CD100 fused with FLAG peptide15) diluted in blocking solution were added to the wells (100 μL/well) and incubated for 2 hours at room temperature. The wells were washed 3 times with phosphate buffered saline (PBS) containing 0.05% Tween-20 and incubated with biotinylated BMA-8 for 1 hour at room temperature. The bound Abs were incubated with AP-conjugated streptavidin and subsequently developed by phosphatase substrate (Sigma-104; Sigma). Absorbance values were obtained at 405 nm (correction wavelength set at 620 nm).
Anti–single-stranded DNA Ab.
Calf thymus DNA (Sigma) was purified with a Sepa Gene kit (Nippon Gene, Tokyo, Japan). Single-stranded DNA (ssDNA) was prepared by boiling the DNA solution for 15 minutes followed by immediate immersion on ice. Serially diluted serum samples were incubated in the ssDNA-coated (5 μg/mL) plates, followed by the addition of AP-labeled antimouse IgG antibodies. We defined serum titers of MRL/lpr mice at 7 months of age as 1000 U/mL.
Immunoprecipitation
sCD100 in culture supernatants.
Primary T (Thy1.2+) or B (B220+) cells were purified from the spleen of C57BL/6 mice (6-8 weeks of age) by Magnetic Cell Sorting (MACS) (Miltenyi Biotech, Bergisch Gladbach, Germany). The resulting primary T cells, B cells, or T-cell hybridoma 2B4 cells were biotinylated by a Cellular Labeling and Immunoprecipitation kit (Boehringer Mannheim) and stimulated with concanavalin A (ConA) (4 μg/mL). The cells were then harvested, washed, and solubilized in 1% NP40. The culture supernatants and the cell lysates were further centrifuged at 100 000g for 1 hour to remove membrane debris. The contents of the supernatants and cell lysates were immunoprecipitated with anti-CD100 mAb (BMA-12), subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and electrophoretically transferred to nitrocellulose membranes. The membranes were blotted with streptavidin-POD conjugates, and developed by enhanced chemiluminescence (ECL) reagent (Amersham Pharmacia Biotech, Uppsala, Sweden) following the manufacturer's protocol.
Affinity purification of sCD100 protein
2B4 cells were stimulated with ConA (4 μg/mL) for 20 hours in serum-free culture medium, GIT (Wako, Tokyo, Japan). sCD100 in the culture supernatants was affinity-purified by using a column made of CNBr-activated Sepharose 4 Fast Flow resin (Amersham Pharmacia Biotech) conjugated with anti-CD100 mAb (BMA-12). The CNBr-activated Sepharose 4 Fast Flow gel was conjugated with BMA-12 according to the manufacturer's protocol. The supernatants were applied to the column. After extensive washing with PBS, the bound protein was eluted using glycine-Cl buffer (pH 2.5).
B-cell proliferation assays
Nonadherent splenic B cells were isolated using a combination of anti-Thy1.2 (F7D5; Serotec, Oxford, United Kingdom) and rabbit complement (Wako) to remove T cells. The remaining B cells were further fractionated through a 50%, 60%, 66%, and 70% step-gradient of percoll, and cells at the interface between 66% and 70% were collected. The resulting small splenic B cells (1 × 105cells/mL) were stimulated with or without anti-CD40 mAb (1 μg/mL), interleukin 4 (IL-4) (10 U/mL), CD100-Fc (20 μg/mL) or sCD100 (5 μg/mL) in flat-bottomed 96-well microtiter plates for 3 days. Cells were pulsed with 2 μCi of [3H]-thymidine for the last 16 hours.
Immunization and serum antibody assays
To induce antibody responses to T-cell–dependent antigen (TD Ag), 8-week-old mice were immunized intraperitoneally with 100 μg of 4-hydroxy-3-nitrophenylacetyl-chicken-γ-globulin (NP-CGG) as an alum-precipitated complex at day 0 and boosted at day 21. Animals were bled before and 7, 14, 21, and 28 days after the first immunization. NP-specific antibodies were detected with NP12-BSA–coated plates as described.18 To induce antibody responses to T-cell–independent antigen (TI Ag), 8-week-old mice were immunized intraperitoneally with 50 μg of 2, 4, 6-trinitrophenyl (TNP) conjugated-LPS (Sigma) in PBS and were bled before and 7 and 14 days after immunization. The anti-TNP–specific antibodies were detected using 2,4-dinitrophenylated bovine serum albumin (DNP-BSA)–coated ELISA plates and quantified by isotype-specific ELISA.18
Results
Activated T and B lymphocytes release sCD100
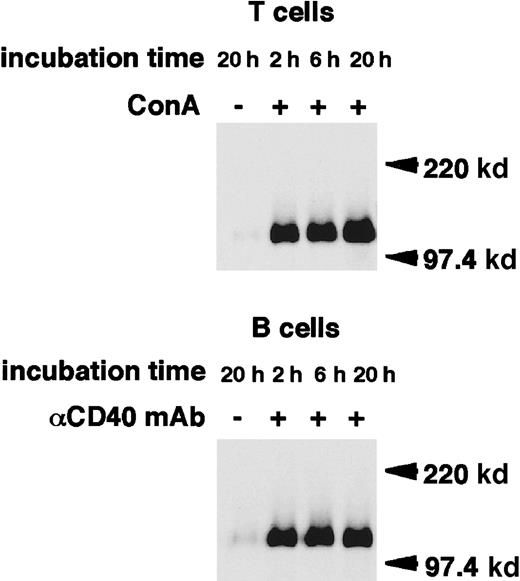
Cross-linking of CD100 on the surface of human PBMCs using antihuman CD100 mAb has been shown to induce the release of a 120-kd isoform of CD100.29 We thus tested whether sCD100 is also generated after lymphocyte activation. Mouse T-cell hybridoma 2B4 cells were biotinylated. The fate of the biotinylated CD100 on the cell surface was chased over a time course after ConA stimulation. Immunoprecipitates with an anti-CD100 mAb, BMA-12 from supernatants, or cell lysates after various periods of stimulation were analyzed on SDS-PAGE under reducing or nonreducing conditions (Figure1). From the culture supernatants of ConA-stimulated 2B4 cells, a single band of 120 kd was detected with BMA-12 under reducing conditions. This was 30 kd smaller than the molecular weight of full-length membrane-bound CD100. The intensity of this band increased with longer culture times with ConA, while unstimulated cells released only trace amounts of sCD100, even after 20 hours culture. Under nonreducing conditions, sCD100 was detected as 2 bands of 240 kd and 120 kd in the culture supernatant of ConA-stimulated cells. The larger is probably a homodimer of sCD100 since CD100 has been shown to form a homodimer via an intermolecular disulfide bond in the extracellular domain.13-15,28,29 In cell lysates of ConA-stimulated 2B4 cells, 2 species of CD100 with molecular weights of about 150 kd and 120 kd were observed in reducing conditions. The intensity of the 150-kd band corresponding to the full-length membrane-bound CD100 decreased gradually, whereas the 120-kd band emerged after stimulation with ConA, suggesting that the 120-kd protein in lysates of stimulated cells may be an intermediate form: sCD100 may be cleaved but remains membrane-associated and not released in the extracellular milieu. The expression of CD100 has been previously shown to be upregulated in activated primary T and B cells.15 We next examined whether sCD100 is also expressed in primary T and B cells after activation. As shown in Figure2, the single band of 120 kd appeared in the culture supernatants of either ConA-stimulated T cells or CD40-stimulated B cells in reducing conditions, whereas the release of sCD100 was hardly detected for unstimulated T or B cells even after 20 hours culture. These results indicate that sCD100 is also generated from activated primary T and B cells.
Release of sCD100 from the surface of 2B4 cells stimulated with ConA.
2B4 cells (1 × 107 cells/lane) were surface-labeled with biotin and then cultured in the presence or absence of ConA (4 μg/mL) for the time indicated, at 37°C. Cell lysates (1% NP-40) or culture supernatants (sup) were immunoprecipitated with BMA-12. Immunoprecipitates were subjected to SDS-PAGE in reducing (2ME [+]) or nonreducing (2ME [−]) conditions, electrophoretically transferred to nitrocellulose membranes, and blotted with streptavidin-POD. Molecular weight markers (Mr) are indicated on the right.
Release of sCD100 from the surface of 2B4 cells stimulated with ConA.
2B4 cells (1 × 107 cells/lane) were surface-labeled with biotin and then cultured in the presence or absence of ConA (4 μg/mL) for the time indicated, at 37°C. Cell lysates (1% NP-40) or culture supernatants (sup) were immunoprecipitated with BMA-12. Immunoprecipitates were subjected to SDS-PAGE in reducing (2ME [+]) or nonreducing (2ME [−]) conditions, electrophoretically transferred to nitrocellulose membranes, and blotted with streptavidin-POD. Molecular weight markers (Mr) are indicated on the right.
Release of sCD100 from the surface of activated primary T and B cells.
Primary T and B cells were stimulated with ConA (4 μg/mL) or anti-CD40 mAb (HM40-3, 1 μg/mL), respectively, for 20 hours at 37°C. Cell lysates (1% NP-40) or culture supernatants were immunoprecipitated with BMA-12. Immunoprecipitates were subjected to SDS-PAGE in reducing conditions, and then electrophoretically transferred to nitrocellulose membranes and blotted with streptavidin-POD. Molecular weight markers (Mr) are indicated on the right.
Release of sCD100 from the surface of activated primary T and B cells.
Primary T and B cells were stimulated with ConA (4 μg/mL) or anti-CD40 mAb (HM40-3, 1 μg/mL), respectively, for 20 hours at 37°C. Cell lysates (1% NP-40) or culture supernatants were immunoprecipitated with BMA-12. Immunoprecipitates were subjected to SDS-PAGE in reducing conditions, and then electrophoretically transferred to nitrocellulose membranes and blotted with streptavidin-POD. Molecular weight markers (Mr) are indicated on the right.
Both CD100-expressing transfectants and recombinant soluble CD100 have been shown to have a synergistic effect on CD40-stimulated B cells.12 15 To determine whether sCD100 released from activated lymphocytes retains such a biologic activity, we purified sCD100 from culture supernatants of ConA-stimulated 2B4 cells, using an anti-CD100 mAb-conjugated affinity column. Analysis of SDS-PAGE revealed the single band of 120 kd (Figure3A), indicating that sCD100 was purified near homogeneity. Then we examined in vitro the effect of sCD100 on B-cell responses mediated by CD40. As shown in Figure 3B, sCD100 as well as CD100-Fc synergistically augmented B-cell proliferation induced by optimal doses of anti-CD40 mAb and IL-4. This activity was not due to contamination by 2B4-derived cytokines, by particularly IL-2 in the sCD100 preparation, because sCD100 did not induce any proliferation in an IL-2–dependent CTLL2 cell line (data not shown). These results thus demonstrate that purified natural sCD100 released from activated lymphocytes also has a costimulatory activity on lymphocytes.
Natural sCD100 released from activated T cells retains activities to enhance B-cell response.
(A) SDS-PAGE analysis of natural sCD100 purified from culture supernatants of ConA-stimulated 2B4 cells. 2B4 cells were stimulated with ConA (4 μg/mL) for 24 hours, and the resulting culture supernatants were harvested and passed through the affinity column to which anti-CD100 mAbs (BMA-12) were covalently bound. The bound protein eluted from the column was subjected to SDS-PAGE in reducing conditions and visualized by silver staining. Mr markers are indicated on the right. (B) The enhancing effect of sCD100 on B-cell proliferation. Small resting B-cells purified from C57BL/6 mice were cultured with or without anti-CD40 mAb (1 μg/mL), IL-4 (10 U/mL), CD100-Fc (20 μg/mL), or sCD100 (5 μg/mL) as indicated, for 3 days. Cells were pulsed with 2 μCi of [3H]-thymidine for the last 16 hours.
Natural sCD100 released from activated T cells retains activities to enhance B-cell response.
(A) SDS-PAGE analysis of natural sCD100 purified from culture supernatants of ConA-stimulated 2B4 cells. 2B4 cells were stimulated with ConA (4 μg/mL) for 24 hours, and the resulting culture supernatants were harvested and passed through the affinity column to which anti-CD100 mAbs (BMA-12) were covalently bound. The bound protein eluted from the column was subjected to SDS-PAGE in reducing conditions and visualized by silver staining. Mr markers are indicated on the right. (B) The enhancing effect of sCD100 on B-cell proliferation. Small resting B-cells purified from C57BL/6 mice were cultured with or without anti-CD40 mAb (1 μg/mL), IL-4 (10 U/mL), CD100-Fc (20 μg/mL), or sCD100 (5 μg/mL) as indicated, for 3 days. Cells were pulsed with 2 μCi of [3H]-thymidine for the last 16 hours.
Elevation of the amount of sCD100 in sera of immunized mice
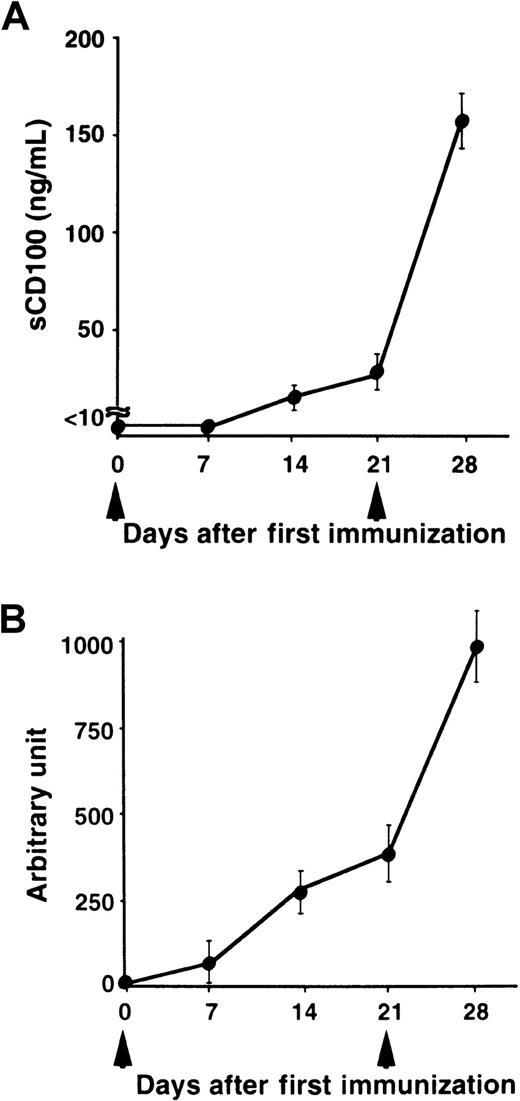
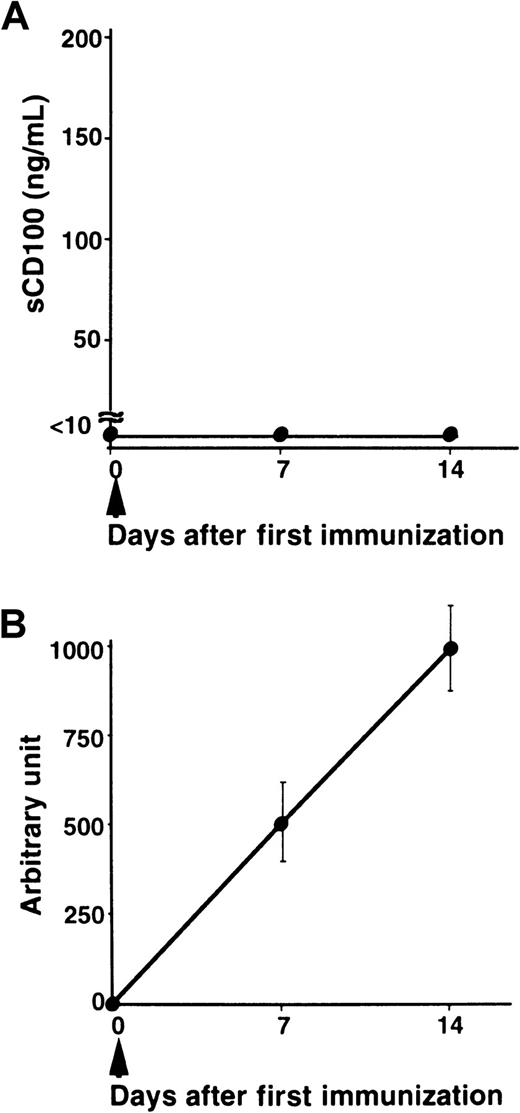
We have previously shown that administration of recombinant sCD100-Fc enhanced the production of specific antibodies in vivo,15 and the deficiency of CD100 resulted in impaired production of specific antibodies against TD Ag but not against TI Ag.18 To explore the physiologic relevance of natural sCD100 in the antibody response in vivo, we measured the levels of sCD100 in the serum after immunization with a TD Ag, NP-CGG. As shown in Figure 4, although sCD100 was not detected before immunization, significant amounts of sCD100 were detected in the sera of immunized mice. Furthermore, the kinetics of the appearance of sCD100 in the serum is very similar to the kinetics of the production of specific antibodies. The results suggest the involvement of sCD100 released from activated lymphocytes in antibody responses in vivo. However, we could not detect sCD100 in sera of mice immunized with a TI Ag, TNP-LPS (Figure 5). These results suggest a possible involvement of sCD100 in antibody responses against TD Ag but not against TI Ag.
Serum levels of sCD100 and titers of antibodies in mice immunized with a TD Ag.
C57BL/6 mice (6-8 weeks old) were immunized intraperitoneally with 100 μg of NP-CGG as an alum precipitated complex (n = 5) at day 0, boosted at day 21 (arrowheads), and bled at the indicated days. Concentrations of sCD100 (A) and serum titers of anti-NP antibodies (B) were determined by ELISA. The concentrations of anti-NP IgG1 antibodies were established by comparison with standard curves created from pooled sera of C57BL/6 mice 28 days after immunization. The concentration of anti-NP IgG1 present in a 1:1000 dilution of pooled immune sera of C57BL/6 mice was defined as one arbitrary unit.18
Serum levels of sCD100 and titers of antibodies in mice immunized with a TD Ag.
C57BL/6 mice (6-8 weeks old) were immunized intraperitoneally with 100 μg of NP-CGG as an alum precipitated complex (n = 5) at day 0, boosted at day 21 (arrowheads), and bled at the indicated days. Concentrations of sCD100 (A) and serum titers of anti-NP antibodies (B) were determined by ELISA. The concentrations of anti-NP IgG1 antibodies were established by comparison with standard curves created from pooled sera of C57BL/6 mice 28 days after immunization. The concentration of anti-NP IgG1 present in a 1:1000 dilution of pooled immune sera of C57BL/6 mice was defined as one arbitrary unit.18
Serum levels of sCD100 and titers of antibodies in mice immunized with a TI Ag.
C57BL/6 mice (8 weeks old) each received an intraperitoneal injection of 50 μg TNP-LPS in PBS (n = 5) at day 0. Serum from individual mice was collected at indicated times. Concentrations of sCD100 (A) and serum titers of anti-TNP IgG1 antibodies (B) were determined by ELISA. The concentrations of anti-TNP IgG1 antibodies were established by comparison to standard curves created from pooled sera of C57BL/6 mice 14 days after immunization. The concentration of anti-TNP antibodies present in a 1:1000 dilution of pooled immune sera of C57BL/6 mice was defined as one arbitrary unit.18
Serum levels of sCD100 and titers of antibodies in mice immunized with a TI Ag.
C57BL/6 mice (8 weeks old) each received an intraperitoneal injection of 50 μg TNP-LPS in PBS (n = 5) at day 0. Serum from individual mice was collected at indicated times. Concentrations of sCD100 (A) and serum titers of anti-TNP IgG1 antibodies (B) were determined by ELISA. The concentrations of anti-TNP IgG1 antibodies were established by comparison to standard curves created from pooled sera of C57BL/6 mice 14 days after immunization. The concentration of anti-TNP antibodies present in a 1:1000 dilution of pooled immune sera of C57BL/6 mice was defined as one arbitrary unit.18
Enhanced expression of membrane-bound CD100 in MRL/lpr mice
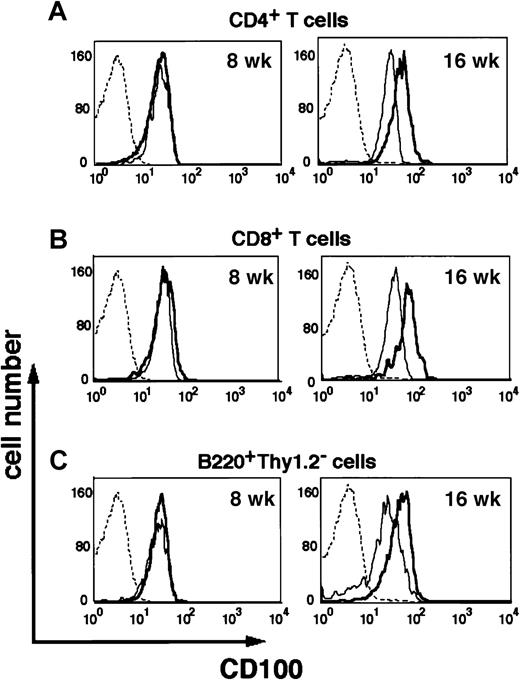
The MRL/lpr mouse is an autoimmune model that produces various autoantibodies and develops autoimmune diseases including nephritis, arthritis, sialodenitis, and skin lesions.30-33 To examine the pathologic relevance of CD100 in the development of autoimmune diseases, we analyzed the cell surface expression of CD100 and the serum sCD100 levels in these mice. Figure 6shows that T-cell surface expression of CD100 is significantly increased for either B or T cells of MRL/lpr mice compared with 16-week-old MRL/n mice, although there was no difference before 8 weeks of age. Among the subpopulations of lymphocytes in MRL/lpr mice, there was no difference in CD100 expression between the B220−Thy1.2+ and B220+Thy1.2+ cell-subpopulations of MRL/lpr mice (data not shown).
Increased expression of CD100 by B and T cells of MRL/lpr mice.
Lymphocytes from the inguinal and popliteal lymph nodes of 8-week-old (8 wk) (left) or 16-week-old (16 wk) (right) MRL/lpr mice (thick lines) or MRL/n mice (thin lines) were stained with PE-conjugated anti-CD4 and FITC-conjugated anti-CD8 (A,B) or PE-conjugated Thy1.2 and FITC-conjugated anti-B220 (C), and biotinylated anti-CD100 mAb plus APC-conjugated steptavidin (A-C). CD4+ (A), CD8+ (B) or B220+Thy1.2− (C) cells were gated and CD100 expression was analyzed by flow cytometer. The profiles of cells stained with APC-conjugated steptavidin alone are also shown (A-C, dashed line).
Increased expression of CD100 by B and T cells of MRL/lpr mice.
Lymphocytes from the inguinal and popliteal lymph nodes of 8-week-old (8 wk) (left) or 16-week-old (16 wk) (right) MRL/lpr mice (thick lines) or MRL/n mice (thin lines) were stained with PE-conjugated anti-CD4 and FITC-conjugated anti-CD8 (A,B) or PE-conjugated Thy1.2 and FITC-conjugated anti-B220 (C), and biotinylated anti-CD100 mAb plus APC-conjugated steptavidin (A-C). CD4+ (A), CD8+ (B) or B220+Thy1.2− (C) cells were gated and CD100 expression was analyzed by flow cytometer. The profiles of cells stained with APC-conjugated steptavidin alone are also shown (A-C, dashed line).
Correlation between the levels of sCD100 and autoantibodies in the serum of MRL/lpr mice
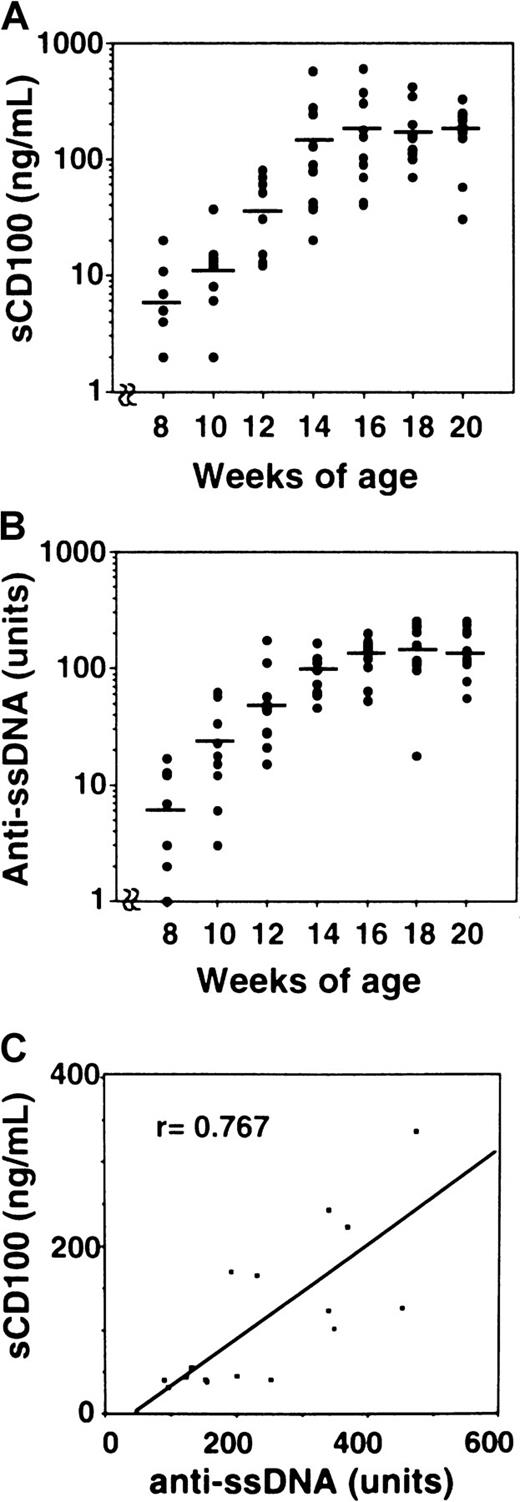
We then measured the levels of sCD100 in the serum of MRL/lpr mice (Table 1). The concentrations of sCD100 in the serum were significantly elevated for MRL/lpr mice, whereas C57BL/6, BALB/c, and MRL/n mice did not have detectable levels of sCD100 in the serum. Collectively, these findings demonstrate the existence of sCD100 in sera of MRL/lpr mice with an autoimmune disease. From 8 weeks of age, an increase in the amount of autoantibodies such as anti-ssDNA is observed in MRL/lpr mice.31 We analyzed the levels of sCD100 and anti-ssDNA antibody in each MRL/lpr mice at various weeks of age. Although neither sCD100 nor anti-ssDNA autoantibodies were detected in MRL/lpr mice younger than 8 weeks (data not shown), the levels of sCD100 as well as anti-ssDNA autoantibodies became detectable after 8 weeks (Figure7A,B). The level of sCD100 gradually increased with age and peaked to 196.6 ± 157.2 ng/mL at 16 weeks. The levels of sCD100 and anti-ssDNA in the serum for each MRL/lpr mouse at 16 weeks of age seemed to be well correlated with ssDNA levels (r = 0.767, P < .01), showing a potential utility of sCD100 as an indicator for the development of autoimmunity.
Age-dependent increase of the amount of sCD100 and autoantibodies in MRL/lpr mice.
Sera from MRL/lpr mice were collected at 8, 10, 12, 14, 16, 18, and 20 weeks of age. Concentrations of serum sCD100 (A) and titers of anti-ssDNA (B) were measured by ELISA as described in “Materials and methods.” The horizontal lines represent the mean value for each group. (C) Correlation was observed between the concentration of sCD100 and the titers of anti-ssDNA antibodies in 16-week-old MRL/lpr mice (n = 16) with a probability of P < .01.
Age-dependent increase of the amount of sCD100 and autoantibodies in MRL/lpr mice.
Sera from MRL/lpr mice were collected at 8, 10, 12, 14, 16, 18, and 20 weeks of age. Concentrations of serum sCD100 (A) and titers of anti-ssDNA (B) were measured by ELISA as described in “Materials and methods.” The horizontal lines represent the mean value for each group. (C) Correlation was observed between the concentration of sCD100 and the titers of anti-ssDNA antibodies in 16-week-old MRL/lpr mice (n = 16) with a probability of P < .01.
Discussion
In this study, we have demonstrated the existence of naturally produced sCD100, not only in the culture supernatants of activated lymphocytes, but also in the sera of immunized mice or of mice with an autoimmune disease. Although the semaphorin family consists of secreted-type and transmembrane-type proteins, most of their functions have been reported on secreted-type members acting as soluble factors.2,4 On the other hand, accumulating evidence suggests that transmembrane-type semaphorins may also function as factors,12 although it is not clear whether transmembrane-type semaphorins can retain their activities when converted into a soluble form. We have recently demonstrated that administration in vivo of recombinant soluble CD100-Fc protein had an effect on humoral and cellular immune responses, suggesting a function of CD100 as a soluble ligand. However, the existence and functions of naturally produced sCD100 in vivo have not been well clarified. In the present study, we provide direct evidence that, like recombinant CD100-Fc, naturally produced sCD100 after lymphocyte activation has a potent costimulatory function to induce proliferation of CD40-stimulated B cells.
Our previous study with CD100-deficient mice exhibited reduced production of specific antibodies against TD Ag but not against TI Ag, implying a role of CD100 especially in T-cell–mediated humoral immune responses.18 Here, we observed significant levels of sCD100 in the serum of immunized mice and a good correlation between the levels of sCD100 and titers of specific antibodies produced against a TD Ag. Thus, sCD100 generated in vivo after immunization seems to play a role in humoral immune responses against TD Ag. In contrast, sCD100 was not detectable in the sera of mice immunized with a TI Ag, although the expression of membrane-bound CD100 and the release of sCD100 were upregulated on B cells after LPS-stimulation (data not shown).18 The abundant expression of CD100 by T cells may explain why sCD100 could be detected in the sera of mice immunized with TD Ag but not with TI Ag. Alternatively, CD100 on the surface of T cells may be more easily cleaved into sCD100 after activation, compared with B cells.
Human sCD100 is thought to be generated by proteolytic cleavage, not by alternative splicing.14 Our surface biotinylation-chase experiment demonstrates that mouse sCD100 is a product of proteolytic cleavage. Biotinylated sCD100 was immediately released into the culture supernatants after activation of 2B4 cells (Figure 1). The release of sCD100 from activated lymphocytes seems to parallel a decrease in the expression of the full-length membrane-bound CD100. This kinetic strongly suggests that sCD100 is generated by a cleavage and a release from the cell surface. The molecular weight of sCD100 identified here in vitro and in vivo was almost the same as the human sCD100.29 Recently, Adams et al34 have demonstrated that Sema3A is converted from proSema into active Sema via proteolytic cleavage by a furin-like protease. Their findings imply that activation of proteases is involved in the regulation of semaphorin functions. Proteases including metalloproteases are known to be involved in the shedding from the surface of lymphocytes of many receptors such as CD62L, IL-6 receptor, and TNFα.35-37Although the proteases involved in the shedding of CD100 are unknown, they might be activated under certain conditions such as cell activation, resulting in a modulation of immune responses.
This is the first report showing the elevations of the level of serum sCD100 in antibody responses in vivo and in an autoimmune disease. Although it is not clear whether the elevations of the level of serum sCD100 might be a cause or a consequence of the progression of autoimmunity, sCD100 should not only be a good indicator for monitoring the production of antibodies in vivo, but also for following the development of autoimmune diseases. Moreover, sCD100 might be relevant to the progression of autoimmunity in MRL/lpr mice, for several reasons. First, a large amount of sCD100 was detected in the sera of MRL/lpr mice producing autoantibodies, whose levels exhibited a good correlation with the titers of the autoantibodies. Second, sCD100 displays synergistic effects on CD40-induced B-cell responses, which might promote abnormal B-cell proliferation, resulting in the production of autoantibodies in MRL/lpr mice. B cells also play a significant role in the development of autoimmunity, through the production of pathogenic autoantibodies and costimulation of autoreactive T cells.38 Also, in MRL/lpr mice, autoantibodies have been shown to be involved in the progression of autoimmunity, since anti-DNA antibodies derived from the mice are shown to bind to glomerular basal membrane, resulting in exacerbation of kidney damage.39-41 Because neutralizing antibodies against mouse CD100 have not yet been established, further analysis using CD100-deficient mice in MRL background will be required for dissecting the pathogenic role of CD100.
In conclusion, we have shown that a soluble isoform of a transmembrane-type semaphorin, sCD100, can be generated from activated lymphocytes. The levels of sCD100 are increased either in the sera of immunized mice or in the sera of mice with an autoimmune disease, suggesting the implication of sCD100 in physiologic and pathologic immune responses.
We gratefully acknowledge Ms Kubota for excellent secretarial assistance.
Supported by research grants from the Ministry of Education, Science and Culture, Japan, to H.K. and A.K.
X.W. and A.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hitoshi Kikutani, Department of Molecular Immunology, Research Institute for Microbial Diseases, Osaka University, 3-1 Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail:kikutani@ragtime.biken.osaka-u.ac.jp

![Fig. 1. Release of sCD100 from the surface of 2B4 cells stimulated with ConA. / 2B4 cells (1 × 107 cells/lane) were surface-labeled with biotin and then cultured in the presence or absence of ConA (4 μg/mL) for the time indicated, at 37°C. Cell lysates (1% NP-40) or culture supernatants (sup) were immunoprecipitated with BMA-12. Immunoprecipitates were subjected to SDS-PAGE in reducing (2ME [+]) or nonreducing (2ME [−]) conditions, electrophoretically transferred to nitrocellulose membranes, and blotted with streptavidin-POD. Molecular weight markers (Mr) are indicated on the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/11/10.1182_blood.v97.11.3498/6/m_h81111099001.jpeg?Expires=1769176535&Signature=U363-UQr9iBFvWoKc6~y1LO-plUUSq4TpmDCXSzeDMLAQYlZomuSrrrdolv1FLvR-qtUhCOYFQLSoID0ELqSCM0MpsujpmEE~NqxXC74O2uOgXUj5-W5KgB2r-C3Y9h78pqWvsE760N25RVoZnX7VpPOxCu3OlXtnGzLQKbfgCAAcj1-CDvg2gqvM4zd1rmKjNGdVn87blggVcm0E8A3FVHJxsvoZqmtcof8NOB0MkhtafaiT~e-DxekXM-4EbclhZv3ORb7UeOTMxmwzHKSNYLF8cPyN8pyBLwe6sRDF1Pa2dElHUmYR3jHK4xhp4G5MwcZJnRzwg~sbgMNo6NBJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Natural sCD100 released from activated T cells retains activities to enhance B-cell response. / (A) SDS-PAGE analysis of natural sCD100 purified from culture supernatants of ConA-stimulated 2B4 cells. 2B4 cells were stimulated with ConA (4 μg/mL) for 24 hours, and the resulting culture supernatants were harvested and passed through the affinity column to which anti-CD100 mAbs (BMA-12) were covalently bound. The bound protein eluted from the column was subjected to SDS-PAGE in reducing conditions and visualized by silver staining. Mr markers are indicated on the right. (B) The enhancing effect of sCD100 on B-cell proliferation. Small resting B-cells purified from C57BL/6 mice were cultured with or without anti-CD40 mAb (1 μg/mL), IL-4 (10 U/mL), CD100-Fc (20 μg/mL), or sCD100 (5 μg/mL) as indicated, for 3 days. Cells were pulsed with 2 μCi of [3H]-thymidine for the last 16 hours.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/11/10.1182_blood.v97.11.3498/6/m_h81111099003.jpeg?Expires=1769176535&Signature=w-AJzxC7tfLAk~7PW~tiRqw2JIdAkMOlezLyI8ZFhfVxEcGvhIgcI4YNmXxZFP4H4ahNpledIVRtij57NxZJ1zc2gGeFh-BFeypPEYVJN7NRVA8SJvrDfLwWVR~JQyZdCcOt1NlLxaPE8P5tggDlD5MnNL0fU4CBMMD3vXP6~5-xoFeoiKfc0J9rU6DJEMZaUVdRKAgKOGh9V4w3jQ73uJD86egXSJXzM2r9qcSaeQJ3IePA7HA~bUkNuomBffM8G4QB06ul63xf9R2lQ~GK0RmAr5lI4-kk3hvG85IMHa9GoG4fhbYGJKAd2dW9eq5GvQ~IvHacjToE233SqYcKJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)