The fibroblast growth factor (FGF) family has an important role in processes such as angiogenesis, wound healing, and development in which precise control of proteinase activity is important. The human plasma proteinase inhibitor α2-macroglobulin (α2M) regulates cellular growth by binding and modulating the activity of many cytokines and growth factors. These studies investigate the ability of native and activated α2M (α2M*) to bind to members of the FGF family. Both α2M and α2M* bind specifically and saturably to FGF-1, -2, -4, and -6, although the binding to α2M* is of significantly higher affinity. Neither α2M nor α2M* bind to FGF-5, -7, -9, or -10. FGF-2 was chosen for more extensive study in view of its important role in angiogenesis. It was demonstrated that FGF-2 binds to the previously identified TGF-β binding site. The α2M* inhibits FGF-2–dependent fetal bovine heart endothelial cell proliferation in a dose-dependent manner. Unexpectedly, α2M* does not affect FGF-2–induced vascular tubule formation on Matrigel basement membrane matrix or collagen gels. Further studies demonstrate that FGF-2 partitions between fluid-phase α2M* and solid-phase Matrigel or collagen. These studies suggest that the ability of α2M* to modulate the activity of FGF-2 is dependent on an interplay with extracellular matrix components.
Introduction
Fibroblast growth factors (FGF) constitute a family of heparin-binding proteins that exert pleiotropic effects on cells from all embryonic lineages.1,2 Sequencing of FGF-1 and FGF-2 has led to the identification of at least 19 proteins that are members of this mammalian family.3,4 The FGFs are expressed during both embryogenesis and in mature organisms.5 They play important roles in angiogenesis, mitogenesis, embryonic pattern formation and development, cellular differentiation, and wound healing.1,6-10 Only FGF-1 and FGF-2 are expressed at high levels in adults. FGF-1 expression is predominantly confined to the central nervous system, but FGF-2 is ubiquitously expressed throughout all adult tissues.5FGF-2 is a potent mitogen for mesoderm-derived cells, such as endothelial cells.1,11 FGF-2 induces cell proliferation in endothelial cells derived from large vessels or capillaries with a median effective concentration of 1.5 to 2.6 pM.12 In model systems of angiogenesis, such as the rabbit cornea, the chick chorioallantoic membrane, and the hamster cheek pouch, FGF-2 exerts a potent angiogenic effect.6,13-15 FGF-1 and -2 are both involved in vasculogenesis, because epiblast cells can be induced to differentiate into endothelial cells by incubation with either of these FGFs.8
FGF-2 up-regulates the urinary plasminogen activator receptor and stimulates the release of urinary plasminogen activator and collagenase in endothelial cells.16-18 Additionally, FGFs act as chemoattractants for endothelial cells.19 The ability of the FGFs to exert their effects depends on their interaction with both cell-surface receptor tyrosine kinases and extracellular and cell-surface–bound heparan sulfate proteoglycans.20 21However, the nature of the interactions of the FGFs with proteins and molecules that are predominantly fluid-phase has not been extensively investigated.
The plasma protein α2-macroglobulin (α2M) is a 718-kd homotetrameric glycoprotein that inactivates proteinases from all 4 mechanistic classes.22 Proteinase inhibition involves a major conformational change and compaction of the native α2M structure.23 This conformational change results in exposure of receptor binding domains that mediate interaction of α2M with its receptors, the low-density lipoprotein-related protein,24 and the α2M signaling receptor.24-26 Ammonia or small amines such as methylamine can directly attack the thiolester bonds present in each α2M subunit. This converts native α2M into a conformational variant indistinguishable from activated α2M-proteinase complexes with respect to receptor recognition27 and cytokine binding.28 Forms of α2M activated by either small nucleophiles or proteinases are considered functionally equivalent and are designated α2M*.
In vitro and in vivo, α2M binds to numerous cytokines and growth factors.29,30 Binding to α2M and α2M* typically inhibits the ability of a given growth factor to regulate cellular functions in vitro, although such binding may enhance the activity of platelet-derived growth factor (PDGF).31,32 An important function of α2M is to serve as a reservoir for cytokines and growth factors. Examples of this include tumor growth factor (TGF)-β and PDGF, where 80% to 90% of these growth factors are associated with α2M in the circulation.33-36 α2M binds FGF-2 in serum,16 and an equilibrium dissociation constant (Kd) is available for its binding to α2M* but not α2M.28
In the present study, we sought to determine whether α2M and α2M* interact with other members of the FGF family and to derive equilibrium Kds for these interactions. Further, given the high expression of FGF-2 in adult tissues and its important role in angiogenesis, we investigated whether α2M or α2M* regulate the ability of FGF-2 to induce proliferation and differentiation of endothelial cells, both important steps in angiogenesis.37 We report that binding to α2M and α2M* is restricted to certain members of the FGF family. We also demonstrate that although α2M* is able to inhibit the activity of FGF-2 in proliferation assays, it does not block the ability of FGF-2 to induce endothelial tubule formation on Matrigel basement membranes or on collagen gels. These studies indicate that α2M* and, to some extent, α2M can regulate the activity of FGF-2 in the fluid phase. The basement membrane, however, appears slightly more important than α2M* in modulating the activity of FGF-2. This may serve to confine FGF activity to the site of ongoing proteinase activity associated with angiogenesis, inflammation, and tissue repair because only basement membrane or extracellular matrix (ECM)-bound FGF-2 can evade α2M* inhibition.
Materials and methods
Materials
125I-labeled Bolton-Hunter reagent and [methyl-3H]thymidine were purchased from NEN Life Science Products (Boston, MA). RPMI 1640, Dulbecco phosphate-buffered saline (PBS), L-glutamine, penicillin/streptomycin, and fetal bovine serum (FBS) were purchased from Life Technologies (Gaithersburg, MD). Endothelial basal medium (EBM), human endothelial growth factor, bovine pituitary extract, and hydrocortisone were purchased as the endothelial growth medium kit from Clonetics (San Diego, CA); 5,5′-dithio-bis(2-nitrobenzoic acid), methylamine (CH5NHCl), gelatin, and bovine serum albumin (BSA) were purchased from Sigma (St Louis, MO). Electrophoresis reagents were purchased from Bio-Rad Laboratories (Richmond, CA). All other reagents were of the highest quality commercially available.
Proteins
Human native α2M was purified from plasma according to a previously described protocol.38 The α2M was at least 90% active against proteinases as determined by thioester titration using the 5,5′-dithio-bis(2-nitrobenzoic acid) assay.39 The protein concentration was based on A280nm (1%/1 cm) = 8.93 and molecular mass = 718 kd. The α2M-methylamine (α2M*) was prepared as previously described40 and employed throughout these experiments. Recombinant, carrier-free TGF-β1 and FGF-1, -2, -4, -5, -6, -7, -9, and -10 were purchased from R&D Systems (Minneapolis, MN). FGFs and α2M* were labeled with125I-labeled Bolton-Hunter reagent according to the manufacturer's recommended protocol. Both labeled and unlabeled FGFs were reconstituted with 0.5% BSA in PBS, pH 7.4.125I-labeled FGFs were used within 2 weeks of labeling.
125I-labeled FGF binding to α2M and α2M*
125I-FGFs (15 ng) were incubated with α2M or α2M* for 2 hours at 37°C in the presence of 0.1% BSA in PBS, pH 7.4. Following incubation, the mixture was subjected to electrophoresis on nondenaturing pore-limit gels to separate 125I-FGF bound to α2M or α2M* from unbound 125I-FGF. Following electrophoresis, gels were stained with Coomassie brilliant blue to verify the location of α2M or α2M*. Gels were subsequently dried and exposed to a Phosphorimager 410A (Molecular Dynamics, Sunnyvale, CA) plate. The plate was developed, and the radioactivity associated with the bands corresponding to α2M or α2M* was quantified employing ImageQuant analysis software (version 3.3, Molecular Dynamics). Simultaneously, 125I-FGFs were electrophoresed under denaturing conditions to determine total radioactivity added. Equilibrium Kds were determined by direct fit of the Phosphorimager counts to a one-site binding model employing SigmaPlot (version 3.02, Jandel Scientific, San Rafael, CA). TheKd determined for the binding of FGF-2 to α2M and α2M* was corrected for nonspecific binding.
Nonspecific binding of 125I–FGF-2 to α2M and α2M* was determined as described previously.41 Briefly, 96-well plates were coated with 1 μg of either α2M or α2M* by incubation in 0.015 M Na2CO3, 0.035 M NaHCO3, and 0.04% NaN3 for 4 hours at 25°C. The coated wells were blocked overnight with 0.02 M HEPES and 0.15 M NaCl, pH 7.5, containing 0.1% (vol/vol) Tween 20 and stored at 4°C prior to performing binding studies with FGF-2. The total amount of α2M and α2M* in the wells was determined in concurrent experiments that determined the amount of125I-α2M and125I-α2M* that could be coated onto the wells. 125I–FGF-2, 10 ng, was added to the wells in the presence or absence of a 1000-fold molar excess of unlabeled FGF-2, and the wells were then incubated at 37°C for 2 hours. The wells were then washed with 0.02 M HEPES and 0.15 M NaCl, pH 7.5, to remove any unbound 125I–FGF-2. The radioactivity associated with each well was determined by γ-counting. Nonspecific binding of125I–FGF-2 to α2M and α2M* was 15%, which is similar to previous determinations of nonspecific binding of cytokines and growth factors to α2M.42 43
Competition binding assays
Ninety-six–well plates were coated with 1 μg of either α2M or α2M* by incubation in 0.015 M Na2CO3, 0.035 M NaHCO3, and 0.04% NaN3 for 4 hours at 25°C. The coated wells were blocked overnight with 0.02 M HEPES and 0.15 M NaCl, pH 7.5, containing 0.1% (vol/vol) Tween 20 and stored at 4°C prior to performing competition binding studies with FGF-2 and TGF-β1. The total amount of α2M and α2M* in the wells was determined in concurrent experiments that determined the amount of125I-α2M and125I-α2M* that could be coated onto the wells. 125I–FGF-2, 10 ng, was added to the wells in the presence or absence of a 100-fold molar excess of unlabeled TGF-β1, and the wells were then incubated at 37°C for 2 hours. The wells were then washed with 0.02 M HEPES and 0.15 M NaCl, pH 7.5, to remove any unbound 125I–FGF-2. The radioactivity associated with each well was determined by γ-counting.
Cell proliferation assays
Fetal bovine heart endothelial (FBHE) cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in gelatinized 75-cm2 flasks in RPMI 1640 medium supplemented with 50 ng/mL FGF-2, 10% FBS, 2 mM L-glutamine, and 3% penicillin/streptomycin. Forty-eight hours prior to experimentation, FBHE cells were trypsinized from flasks and incubated in gelatinized 96-well plates at 2000 cells per well in FGF-2–deficient medium to reach quiescence. On the day of experimentation, the cell medium was replaced with one containing 0.5 ng/mL FGF-2 that had been incubated with varying concentrations of α2M or α2M* for 2 hours at 37°C, and the plates were then allowed to incubate for 48 hours at 37°C. Following incubation, each well was pulsed with [methyl-3H] thymidine (3.7 × 104 Bq) and harvested with a Combi Cell Harvestor (Skatron, Norway) onto glass fiber filters for scintillation counting.
This assay was also performed with human umbilical vein endothelial cells (HUVECs) obtained from Clonetics (San Diego, CA) and employed at passage 4. HUVECs were cultured in gelatinized 75-cm2flasks in EBM supplemented with 500 ng/mL endothelial growth factor, 10% FBS, 1 ng/mL hydrocortisone, 24 ng/mL bovine pituitary extract, and gentamicin/amphotericin. Forty-eight hours prior to experimentation, HUVECs were trypsinized from flasks and incubated in gelatinized 96-well plates at 2000 cells per well in EBM supplemented with 2% FBS, 1 ng/mL hydrocortisone, and gentamicin/amphotericin to reach quiescence. On the day of experiment, this quiescent cell medium was replaced with one containing 0.5 ng/mL FGF-2 that had been incubated with varying concentrations of α2M or α2M* for 2 hours at 37°C. The plates were then allowed to incubate for 48 hours at 37°C, after which the wells were pulsed with [methyl-3H] thymidine (1 μCi) and harvested with a Combi Cell Harvestor onto glass fiber filters for scintillation counting.
Endothelial tubule formation
Growth factor–reduced Matrigel was purchased from Collaborative Biomedical Products (Bedford, MA) and employed according to the manufacturer's recommended protocol to coat 24-well plates. FBHE cells that had been cultured in FGF-2–deficient medium for 48 hours were trypsinized from flasks and plated onto the Matrigel layers at 40 000 cells per well in the presence of varying concentrations of FGF-2. Where the effect of α2M* on the ability of FGF-2 to induce endothelial tubule formation was tested, varying concentrations of α2M* were incubated with FGF-2 for 2 hours at 37°C prior to addition to the cells. The FBHE cells were then incubated for 96 hours at 37°C and photographed.
Endothelial tubule formation was also studied employing collagen gels. Rat tail collagen type I was purchased from Collaborative Biomedical Products and employed according to the manufacturer's protocol to coat 48-well plates. HUVECs at passage 4 that had been cultured in EBM supplemented with 2% FBS, 1 ng/mL hydrocortisone, and gentamicin/amphotericin for 48 hours were trypsinized from flasks and plated onto the collagen gels in EBM supplemented with 1 ng/mL hydrocortisone and gentamicin/amphotericin at 30 000 cells per well in the presence of varying concentrations of FGF-2. Where the effect of α2M* on the ability of FGF-2 to induce endothelial tubule formation was tested, varying concentrations of α2M* were incubated with FGF-2 for 2 hours at 37°C prior to addition to the cells. The HUVECs were then incubated for 24 hours at 37°C and photographed.
Binding of 125I–FGF-2 and125I-α2M* to Matrigel
Matrigel layers were prepared as above in 24-well plates.125I–FGF-2 (15 ng) was added to the layers in cell medium and incubated for 48 hours at 37°C. The Matrigel layers were then washed 3 times with cell medium and solubilized with Matrisperse (Collaborative Biomedical Products) according to the manufacturer's instructions. The solubilized Matrigel was subjected to nonreducing 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), stained, and dried. The gels were exposed to a Phosphorimager plate, and the radioactivity associated with the125I–FGF-2 was quantified employing ImageQuant. The α2M* was tested for its ability to inhibit binding of I125–FGF-2 to the Matrigel layer by incubating 0.3 mg/mL α2M* with 125I–FGF-2 for 2 hours at 37°C prior to addition to the Matrigel. Studies were performed to determine the amount of 125I–FGF-2 that was associated with α2M* on the Matrigel layer. The solubilized Matrigel, following incubation as above with 125I–FGF-2 and α2M*, was electrophoresed on nondenaturing pore-limit gels to allow separation of 125I–FGF-2 bound to α2M* from free 125I–FGF-2, and the radioactivity associated with the α2M* band was quantified as above. In all cases, 15 ng 125I–FGF-2 alone was subjected to nonreducing SDS-PAGE and exposed simultaneously with the nondenaturing gels for which it served as 100% of total125I–FGF-2 added. Binding of125I-α2M* to Matrigel was performed by incubating 4 μg 125I-α2M* with Matrigel layers in cell medium for 48 hours at 37°C. The supernatant was aspirated, and the Matrigel layers were washed 3 times with cell medium and solubilized with Matrisperse. The solubilized Matrigel and supernatant were electrophoresed on nondenaturing pore-limit gels, and the radioactivity associated with the α2M* band was quantified as above and compared with 4 μg125I-α2M* alone.
The ability of FGF-2 to bind to collagen gels was also studied. Collagen gels were prepared as above in 48-well plates.125I–FGF-2 (100 ng) was added to the gels in cell medium and incubated for 24 hours at 37°C. The collagen gels were then washed 3 times with cell medium and solubilized according to the manufacturer's instructions. The solubilized collagen was subjected to nonreducing 5% SDS-PAGE, stained, and dried. The gels were exposed to a Phosphorimager plate, and the radioactivity associated with the125I–FGF-2 was quantified employing ImageQuant. The α2M* was tested for its ability to inhibit binding of I125–FGF-2 to the collagen gel by incubating 0.5 mg/mL α2M* with 125I–FGF-2 for 2 hours at 37°C prior to addition to the collagen gel. In all cases, 100 ng125I–FGF-2 alone was subjected to nonreducing SDS-PAGE and exposed simultaneously with the nondenaturing gels for which it served as 100% of total 125I–FGF-2 added.
Results
Binding of FGF-1, -2, -4, -5, -6, -7, -9, and -10 to α2M and α2M*
The FGFs are a family of highly homologous growth factors.5,44 Previous studies have determined that FGF-2 binds to α2M and α2M*16,28 and that FGF-1 can compete for this binding.16 To determine whether binding to α2M or α2M* is a conserved feature of the FGF family, we performed in vitro binding experiments with the following members of the FGF growth factor family: FGF-1, -2, -4, -5, -6, -7, -9, and -10. We screened these growth factors for binding to α2M and α2M* by radiolabeling each FGF with 125I and incubating them with a fixed concentration of α2M or α2M*, 0.5 mg/mL (about 700 nM), as described in “Materials and methods.” The amount of each 125I-FGF bound to either α2M (Figure 1A) or α2M* (Figure1B) was determined by reference to the total radioactivity of each125I-FGF alone.
Binding of FGF-1, -2, -4, -5, -6, -7, -9, and -10 to α2M and α2M*.
FGFs were labeled with 125I, as described in “Materials and methods,” and incubated with 0.5 mg/mL α2M (A) or α2M* (B) for 2 hours at 37°C followed by nondenaturing pore-limit electrophoresis. Radioactivity associated with the α2M band was quantified on the Phosphorimager and compared with the radioactivity of the appropriate 125I-FGF alone, which was defined as 100%, to determine the percentage of each FGF that bound to α2M. Each bar represents the mean of at least 2 independent determinations ± SD, except for FGF-10, where n = 1.
Binding of FGF-1, -2, -4, -5, -6, -7, -9, and -10 to α2M and α2M*.
FGFs were labeled with 125I, as described in “Materials and methods,” and incubated with 0.5 mg/mL α2M (A) or α2M* (B) for 2 hours at 37°C followed by nondenaturing pore-limit electrophoresis. Radioactivity associated with the α2M band was quantified on the Phosphorimager and compared with the radioactivity of the appropriate 125I-FGF alone, which was defined as 100%, to determine the percentage of each FGF that bound to α2M. Each bar represents the mean of at least 2 independent determinations ± SD, except for FGF-10, where n = 1.
Interestingly, there were dramatic differences in the amount of each FGF that bound to α2M and α2M*. A substantial fraction of FGF-1, -2, -4, and -6 bound to both α2M and α2M*. However, only a very small fraction of FGF-5, -7, -9, and -10 bound to α2M or α2M*. To ensure that FGF-5, -7, -9, and -10 had adequate time to achieve equilibrium, these labeled FGFs were incubated with α2M and α2M* for 24 hours at 37°C. However, the binding was not improved following this prolonged period of incubation (data not shown).
Concentration dependence of FGF-1, -2, -4, and -6 binding to α2M and α2M*
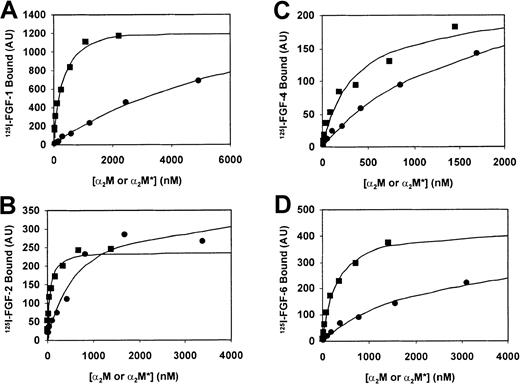
Because FGF-5, -7, -9, and -10 displayed only a very slight interaction with α2M or α2M*, we chose to focus our binding studies on FGF-1, -2, -4, and -6 (Figure2). These radiolabeled ligands were incubated with α2M or α2M*, and the binding of each 125I-FGF to α2M and α2M* was detected and quantitated as described in “Materials and methods.” A summary of the equilibriumKds derived from these experiments is presented in Table 1.
Concentration dependence of FGF-1, -2, -4, -6 binding to α2M and α2M*.
A total of 15 ng 125I–FGF-1 (A), 125I–FGF-2 (B), 125I–FGF-4 (C), or 125I–FGF-6 (D) was incubated with varying concentrations of α2M (●) or α2M* (▪) for 2 hours at 37°C followed by nondenaturing pore-limit electrophoresis. Radioactivity was quantified on a Phosphorimager and represented by arbitrary units (AU). Binding and lines that represent the best least square fit (R2 > 0.95) are plotted in the figure. The data are representative of 3 (FGF-1 and FGF-6) or 4 (FGF-2 and FGF-4) experiments. Table 1 contains a summary of theKd values derived from these binding experiments.
Concentration dependence of FGF-1, -2, -4, -6 binding to α2M and α2M*.
A total of 15 ng 125I–FGF-1 (A), 125I–FGF-2 (B), 125I–FGF-4 (C), or 125I–FGF-6 (D) was incubated with varying concentrations of α2M (●) or α2M* (▪) for 2 hours at 37°C followed by nondenaturing pore-limit electrophoresis. Radioactivity was quantified on a Phosphorimager and represented by arbitrary units (AU). Binding and lines that represent the best least square fit (R2 > 0.95) are plotted in the figure. The data are representative of 3 (FGF-1 and FGF-6) or 4 (FGF-2 and FGF-4) experiments. Table 1 contains a summary of theKd values derived from these binding experiments.
The Kd value we determined for the binding of FGF-2 to α2M* is consistent with previous studies.28 In all cases where the FGF studied bound to α2M or α2M*, binding was of higher affinity to α2M*. This result is in line with previous work, where differential binding affinities of cytokines and growth factors to α2M and α2M* is typically observed.28 45 In addition, we observed that members of the FGF family displayed varying ability to bind to α2M or α2M*. Of those FGFs that bound to α2M and α2M*, the binding affinities differed. The ranking (from greatest to least affinity) was FGF-2 > FGF-4 = FGF-6 > FGF-1.
TGF-β1 is able to compete for the binding of FGF-2 to α2M and α2M*
Given that FGF-2 had the greatest affinity for α2M and α2M*, we chose to focus on it to further explore the ability of α2M and α2M* to regulate the activity of the FGF family. An important issue is the α2M and α2M* binding site for the FGFs. Typically, cytokines and growth factors bind to similar or identical sites on both α2M and α2M*.42,43,46 However, recent work in our laboratory has indicated that α2M and α2M* can employ distinct sites to bind certain growth factors, such as vascular endothelial growth factor.41Previous work has localized the α2M binding site of TGF-β1 to a stretch of the amino acids that includes the bait region.43 46 To investigate whether FGF-2 binds to α2M and α2M* at this TGF-β1binding site, we incubated α2M and α2M* with 125I–FGF-2 in the presence or absence of a 100-fold molar excess (relative to FGF-2) of TGF-β1, as described in “Materials and methods.” Competition by TGF-β1reduced the binding of FGF-2 to α2M by 49% and to α2M* by 43%.
Effect of binding to α2M or α2M* for FGF-2–induced endothelial cell proliferation
Previous studies demonstrated the ability of α2M to inhibit in vitro induction by FGF-2 of plasminogen activator release in endothelial cells.16 We sought to determine whether α2M or α2M* could inhibit the ability of FGF-2 to induce proliferation of FBHE cells. Initial studies were conducted to determine concentrations of FGF-2 that were effective in inducing proliferation in FBHE cells (data not shown). The optimal concentration for our studies was 0.5 ng/mL FGF-2. Therefore, we incubated 0.5 ng/mL FGF-2 with varying concentrations of α2M or α2M* for 2 hours at 37°C and added this mixture to quiescent FBHE cells. The α2M* reduced the proliferation of FBHE cells in a dose-dependent manner, inhibiting about 70% of the proliferation observed with FGF-2 alone (Figure3). The α2M was less inhibitory, reducing proliferation by about 20% at the highest concentration tested. Similar studies employing HUVECs demonstrated that α2M* was also able to inhibit the ability of FGF-2 to stimulate HUVEC proliferation in a dose-dependent manner, inhibiting about 64% of the proliferation observed with FGF-2 (data not shown). Again, α2M was less inhibitory, reducing proliferation by about 20% at the highest concentrations tested.
Effect of binding to α2M or α2M* on FGF-2–induced endothelial cell proliferation.
FGF-2, 0.5 mg/mL, was incubated with the indicated concentrations of α2M or α2M* for 2 hours at 37°C. This mixture was then added to quiescent FBHE cells, and cell proliferation was determined by [methyl-3H]thymidine incorporation as described in “Materials and methods.” FGF-2 activity in the absence of α2M or α2M* was defined as 100%. Data presented are the mean ± SD from 4 experiments.
Effect of binding to α2M or α2M* on FGF-2–induced endothelial cell proliferation.
FGF-2, 0.5 mg/mL, was incubated with the indicated concentrations of α2M or α2M* for 2 hours at 37°C. This mixture was then added to quiescent FBHE cells, and cell proliferation was determined by [methyl-3H]thymidine incorporation as described in “Materials and methods.” FGF-2 activity in the absence of α2M or α2M* was defined as 100%. Data presented are the mean ± SD from 4 experiments.
Effect of α2M* on FGF-2–induced endothelial tubule formation
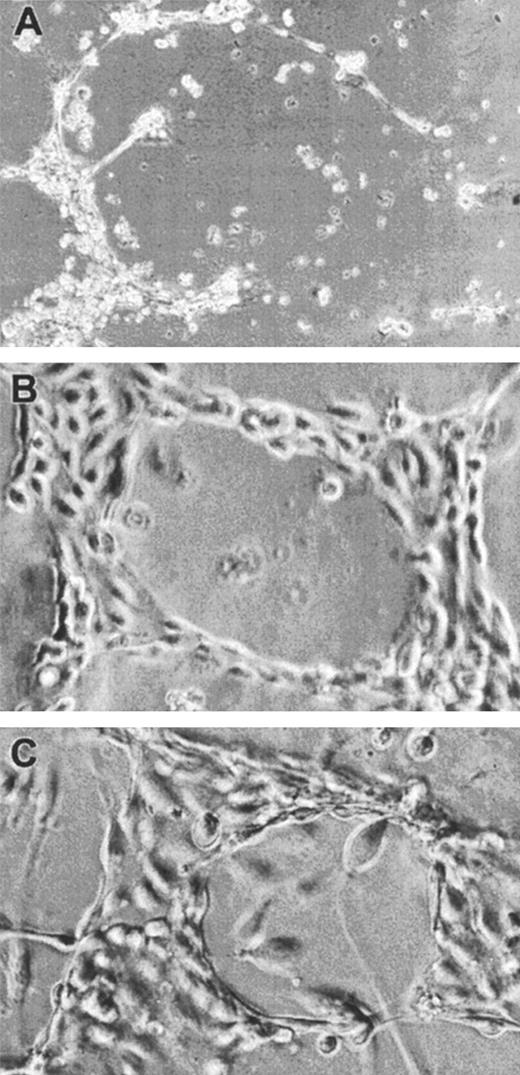
We then sought to determine whether α2M*, which was able to significantly inhibit endothelial cell proliferation in vitro (Figure 3), could also regulate the ability of FGF-2 to induce the differentiation of endothelial cells, such as formation of endothelial tubules. Matrigel is a solubilized basement membrane matrix extracted from Engelbreth-Holm-Swarm mouse tumor47 that has been demonstrated to induce endothelial tube formation by a variety of endothelial cells, including FBHE cells.48Twenty-four–well plates were coated with Matrigel as described in “Materials and methods,” and 40 000 FBHE cells per well were plated in either media alone, media with varying concentrations of FGF-2, or media containing varying concentrations of FGF-2 incubated with 0.5 mg/mL α2M* for 2 hours at 37°C prior to addition to the cells. Initially, under all conditions, FBHE cells formed endothelial tubules on Matrigel. However, by 24 hours, cells not treated with FGF-2 began to regress. After 96 hours of incubation, only those FBHE cells incubated on Matrigel in the presence of at least 0.5 ng/mL FGF-2 remained organized into endothelial tubules (Figure4A,B). We then examined the effect of 0.5 mg/mL α2M* on the activity of 0.625 ng/mL FGF-2 (Figure4C). Interestingly, the presence of α2M* had no effect on either the timing of endothelial tubule formation or the survival of the endothelial tubules in the presence of FGF-2. Increasing the concentration of α2M* had no effect on the activity of FGF-2, and FBHE cells treated with α2M* alone behaved as those cells treated with media alone (data not shown).
Effect of α2M* on FGF-2–induced endothelial tubule formation on Matrigel.
FBHE cells were plated on Matrigel and incubated at 37°C for 96 hours in the absence of FGF-2 (A), 0.625 ng/mL FGF-2 (B), and 0.625 ng/mL FGF-2 incubated with 0.5 mg/mL α2M* (C). Photographs are representative fields from 4 experiments at 25 × (A) or 50 × (B,C) magnification.
Effect of α2M* on FGF-2–induced endothelial tubule formation on Matrigel.
FBHE cells were plated on Matrigel and incubated at 37°C for 96 hours in the absence of FGF-2 (A), 0.625 ng/mL FGF-2 (B), and 0.625 ng/mL FGF-2 incubated with 0.5 mg/mL α2M* (C). Photographs are representative fields from 4 experiments at 25 × (A) or 50 × (B,C) magnification.
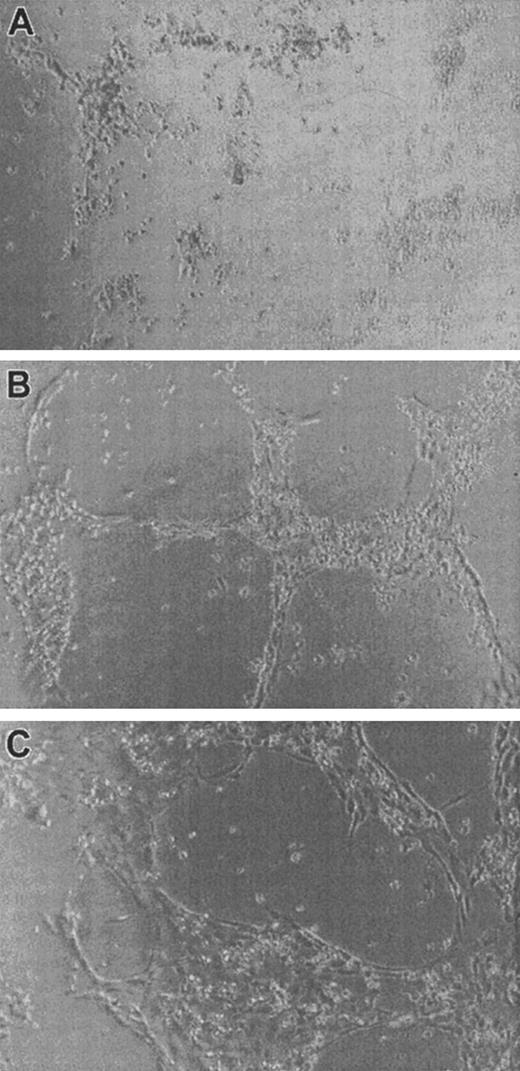
Matrigel is a complex mixture of basement membrane components and growth factors.47 To ensure that the inability of α2M* to inhibit the induction of endothelial tubules by FGF-2 was not due to the presence of other growth factors present in Matrigel, we employed collagen type I gels, which have been demonstrated to support the formation of endothelial tubules by HUVECs.49 Forty-eight–well plates were coated with collagen gel as described in “Materials and methods,” and 40 000 FBHE cells or 30 000 HUVECs per well were plated in either media alone, media with varying concentrations of FGF-2, or media containing varying concentrations of FGF-2 incubated with 0.5 mg/mL α2M* for 2 hours at 37°C prior to addition to the cells. FBHE cells did not organize into endothelial tubules on collagen gels under any of the conditions tested—nor did HUVECs plated onto collagen in the absence of FGF-2 (Figure5A). However, in the presence of at least 50 ng/mL FGF-2, HUVECs were stimulated to organize into endothelial tubules (Figure 5B). The α2M* was unable to inhibit the ability of FGF-2 to induce the formation of endothelial tubules by HUVECs (Figure 5C). Again, increasing the concentration of α2M* had no effect on the activity of FGF-2, and HUVECs treated with α2M* alone behaved as those HUVECs treated with media alone (data not shown).
Effect of α2M* on FGF-2–induced endothelial tubule formation on collagen gel.
HUVECs were plated on collagen type I gels and incubated at 37°C for 24 hours in the absence of FGF-2 (A), 100 ng/mL FGF-2 (B), and 100 ng/mL FGF-2 incubated with 0.5 mg/mL α2M* (C). Photographs are representative fields at 10 × magnification.
Effect of α2M* on FGF-2–induced endothelial tubule formation on collagen gel.
HUVECs were plated on collagen type I gels and incubated at 37°C for 24 hours in the absence of FGF-2 (A), 100 ng/mL FGF-2 (B), and 100 ng/mL FGF-2 incubated with 0.5 mg/mL α2M* (C). Photographs are representative fields at 10 × magnification.
Partitioning of FGF-2 between Matrigel or collagen gels and α2M*
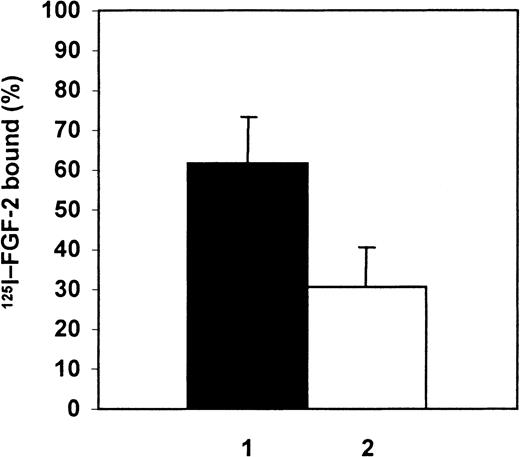
We then sought to understand why α2M* appeared to have no effect on the ability of FGF-2 to promote endothelial tubule formation (Figures 4 and 5), given that α2M* profoundly reduces the ability of FGF-2 to induce endothelial cell proliferation (Figure 3). One possibility is that FGF-2 can partition away from its complex with α2M* and bind to components of the Matrigel basement membrane or to collagen gels, thereby evading inhibition by α2M*. We tested whether FGF-2 was able to bind to Matrigel by coating 24-wells plates with Matrigel, as described in “Materials and methods,” and then incubating radiolabeled FGF-2 alone with the Matrigel for 48 hours at 37°C or radiolabeled FGF-2 incubated with α2M* for 2 hours at 37°C prior to addition to Matrigel. The Matrigel layers were washed to remove any unbound 125I–FGF-2 and solubilized, followed by electrophoresis on nonreducing 15% SDS-PAGE, and then compared with the total radioactivity of the 125I–FGF-2 alone. The percentage of 125I–FGF-2 bound to Matrigel in the presence or absence of α2M* was quantitated as described in “Materials and methods” (Figure 6). A substantial fraction, 62% ± 11%, of the FGF-2 added in the solution phase over Matrigel layers partitioned out of solution and bound to Matrigel. The percentage of FGF-2 that bound to Matrigel was reduced by approximately 50% (to 30% ± 10% of the125I–FGF-2 added) in the presence of α2M*.
Partitioning of FGF-2 between Matrigel and α2M*.
Partitioning of 125I–FGF-2 to Matrigel in the absence (1) or presence of α2M* (2). 125I–FGF-2, 15 ng, alone or 15 ng 125I–FGF-2 incubated with 0.3 mg/mL α2M* was added to Matrigel for 48 hours followed by solubilization of the Matrigel and SDS-PAGE. Radioactivity associated with the FGF-2 band was quantified on the Phosphorimager and expressed as a percentage of total 125I–FGF-2 added. Data are mean of 4 experiments ± SD.
Partitioning of FGF-2 between Matrigel and α2M*.
Partitioning of 125I–FGF-2 to Matrigel in the absence (1) or presence of α2M* (2). 125I–FGF-2, 15 ng, alone or 15 ng 125I–FGF-2 incubated with 0.3 mg/mL α2M* was added to Matrigel for 48 hours followed by solubilization of the Matrigel and SDS-PAGE. Radioactivity associated with the FGF-2 band was quantified on the Phosphorimager and expressed as a percentage of total 125I–FGF-2 added. Data are mean of 4 experiments ± SD.
Because it is possible that α2M* may bind to Matrigel carrying bound 125I–FGF-2, we determined whether the FGF-2 bound to the Matrigel layer was free or bound to α2M*. We incubated 125I-α2M* with Matrigel for 48 hours at 37°C and determined the amount of α2M* that had bound to the Matrigel. The amount of α2M* was 15% ± 4% of the total 125I-α2M* added. We then incubated 125I–FGF-2 with α2M* for 2 hours at 37°C and added this mixture to Matrigel for 48 hours at 37°C. The radioactivity associated with the α2M* band in the Matrigel fraction was quantified with a Phosphorimager. The percentage of 125I–FGF-2 bound to α2M* on the Matrigel layer was 6% ± 1% (n = 3) of the total125I–FGF-2.
Similarly, we tested whether FGF-2 was able to partition away from its complex with α2M* and bind to collagen gels by coating 48-well plates with collagen type I, as described in “Materials and methods,” and then incubating radiolabeled FGF-2 alone with the collagen gel for 24 hours at 37°C or radiolabeled FGF-2 incubated with α2M* for 2 hours at 37°C prior to addition to collagen gel. The collagen gels were washed to remove any unbound125I–FGF-2 and solubilized, followed by electrophoresis on nonreducing 5% SDS-PAGE, and then compared with the total radioactivity of the 125I–FGF-2 alone. The percentage of125I–FGF-2 bound to collagen gel in the presence or absence of α2M* was quantified as described in “Materials and methods.” We found that 76% of the FGF-2 bound to α2M* added in the solution phase over collagen gels partitioned out of solution and bound to collagen gels.
Discussion
In the present study, we have shown that FGF-1, -2, -4, and -6 bind to α2M and α2M* in solution. The ability of α2M and α2M* to bind to certain members of the FGF family and not to others was unexpected. The FGFs are highly homologous in certain regions, which provides the basis for assigning membership to this family.5 However, of the FGFs we tested, only for FGF-1, -2, -4, and -6 was a significant fraction of the FGF complexed to α2M and α2M* at equilibrium. FGF-5, -7, -9, and -10 bound insignificantly to α2M or α2M*.
A binding site for α2M and α2M* may therefore be present on certain FGFs but missing on other FGFs, accounting for their differences in binding activity. Binding to α2M and α2M* may provide an additional level of regulation of FGF family growth factor activities. Analyses of the evolutionary relationships between the FGFs show that FGF-1 and FGF-2 are more closely related to each other than to other FGFs. Similarly, FGF-4 and FGF-6 are most closely related to each other.3,50 Thus, it is not surprising that ability to bind to α2M or α2M* is shared by these pairs of FGFs. However, FGF-7 and -10 are more closely related to FGF-1/FGF-2 than FGF-4/FGF-6, yet they do not interact significantly with α2M.3 This may support the concept of a latent binding site for α2M that is active only in certain members of the FGF family.
The FGFs that we investigated bind to α2M* with higher affinity than to α2M. Such differential binding to α2M or to α2M* has been observed for many growth factors and cytokines.28 Interestingly, we found that TGF-β1 competed with FGF-2 for binding to α2M and α2M*. Previous studies have demonstrated that TGF-β and PDGF bind to a 20-kd fragment of α2M that includes the bait region.43 TGF-β and PDGF also bind to a synthetic peptide comprising a 16–amino acid portion of this same region.46 The higher affinity of binding of the FGFs to α2M*, as compared with α2M, must presumably be due to the major conformational change that occurs upon the conversion of α2M to α2M* by proteinases or small-amine nucleophiles, such as methylamine. This conformational change may permit more ready access of the FGF to the binding site in α2M*. The hydrophobic nature of the binding site46 suggests that the FGFs are interacting with α2M and α2M* via a mechanism that is primarily hydrophobic in nature and, therefore, exquisitely sensitive to changes in temperature and pH. Further studies exploring these questions and whether physiologic pH and temperature variations may dramatically alter the ability of α2M and α2M* to modulate the activity of the FGFs are ongoing in our laboratory.
α2M may bind a significant fraction of FGF-1, -2, -4, -6 in vivo despite the high-nanomolar equlibriumKds for their interaction because α2M is present in micromolar concentrations in vascular fluids and in the extravascular space, especially in states of increased vascular permeability.51-56 α2M may also serve as a carrier in serum for FGF-1, -2, -4, and -6, a role played by heparan sulfate proteoglycans on the cell surface or in the ECM.57 At sites of ongoing proteolysis, such as tissue remodeling during angiogenesis,58 α2M encounters large amounts of activated proteinases that interact with it and convert it to α2M*, which is rapidly cleared from such sites by binding to lipoprotein-related protein.24,59Cytokines and growth factors bound to α2M* can thereby also be cleared,29 possibly allowing α2M* to directly down-regulate FGF activity.
Among many other activities, FGF-2 plays an important role in angiogenesis.60,61 Angiogenesis is a complicated process that can be divided into a series of events, including digestion of the basement membrane surrounding a blood vessel, migration and proliferation of endothelial cells, and endothelial tube formation.37,62,63 We chose to study whether α2M might regulate angiogenesis by investigating the ability of α2M to affect certain angiogenic properties of FGF-2. Previous studies demonstrated that α2M inhibited FGF-2–stimulated plasminogen activator release.16 The present study demonstrates that α2M* is able to inhibit FGF-2–stimulated endothelial cell proliferation in a dose-dependent fashion, whereas α2M is less able to inhibit this activity of FGF-2.
Because α2M* may be present in high concentrations in inflammatory lesions due to proteinase inhibition by α2M, FGF-2 must be able to escape inhibition by α2M* when and where appropriate. We demonstrate here that α2M* is unable to block the ability of FGF-2 to induce the formation and survival of endothelial tubules by FBHE cells on Matrigel basement membranes or by HUVECs on collagen gels. This appears to be because FGF-2 partitions away from α2M*–FGF-2 complexes to bind to Matrigel basement membranes or to the collagen gel. Matrigel contains heparan sulfate proteoglycans47 that would provide binding sites for FGF-2 and allow presentation of FGF-2 to FGF receptor tyrosine kinase on the surface of the FBHE cells, enabling FGF-2 to evade α2M*-mediated inhibition. The presence of heparan sulfate proteoglycans in the ECM may therefore serve to protect FGF-2 from clearance by α2M*. In addition, our studies indicate that FGF-2 may also interact directly with other important components of the basement membrane, such as collagen, again enabling FGF-2 to evade α2M*-mediated inhibition.
We show here that the regulation of FGF activity depends not only on the presence of fluid-phase inhibitors such as α2M but also, critically, on the presence of ECM. In the absence of ECM, α2M*, and to some extent α2M, can dramatically inhibit the activity of FGF-2. However, the ECM is able to protect FGF-2 from inhibition by α2M and α2M*. These findings have implications for the proposed regulation in vivo by α2M and α2M* of other growth factors and cytokines that have been demonstrated to bind to α2M or α2M* in vitro. We suggest that the activities of growth factors or cytokines that bind to α2M or α2M* must be examined in an assay system that includes other important contributors to cellular function and activity. We show here, employing an in vitro model of the ECM, that regulation of FGF activity by α2M is dependent on the extracellular environment. Importantly, this work suggests that, in vivo, mechanisms exist to allow growth factors to evade inhibition by α2M and α2M* in the presence of the ECM. Knowledge of the detailed kinetics of the interactions between α2M, proteinases, growth factors, and the ECM may further elucidate the role that α2M plays in these processes.
The ability of α2M to affect cellular growth and differentiation through the binding and regulation of growth factors and cytokines may depend critically on its well-known regulatory role in proteinase activity. Inhibition of proteinase activity by α2M, therefore, may permit α2M to localize and modulate the activity of the many cytokines and growth factors and link proteinase activity, required for tissue remodeling, to the activity of growth factors that control these processes.
We thank Dr George Cianciolo for his critical reading of the manuscript and Marie Thomas for her assistance in preparation of the manuscript. We also gratefully acknowledge Susan Heffelfinger (Department of Pathology and Laboratory Medicine, University of Cincinnati, OH) for her expert advice concerning collagen gels and endothelial tubule formation.
Supported by National Heart, Lung, and Blood Institute grant HL-24066.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Salvatore V. Pizzo, Dept of Pathology, Box 3712, Duke University Medical Center, Durham, NC 27710; e-mail:pizzo001@mc.duke.edu.


![Fig. 3. Effect of binding to α2M or α2M* on FGF-2–induced endothelial cell proliferation. / FGF-2, 0.5 mg/mL, was incubated with the indicated concentrations of α2M or α2M* for 2 hours at 37°C. This mixture was then added to quiescent FBHE cells, and cell proliferation was determined by [methyl-3H]thymidine incorporation as described in “Materials and methods.” FGF-2 activity in the absence of α2M or α2M* was defined as 100%. Data presented are the mean ± SD from 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/11/10.1182_blood.v97.11.3450/6/m_h81111134003.jpeg?Expires=1767900799&Signature=LJOCx9oypdg~eLV3a-RjS7r9tShbbia~ijF19x6YcvrB7vqAQRaW2ZNQArd85b6lmq6h6MZ5-o1mqwwIAD3YEevvd~hf-lUO-OFJRoYD27MhtYEK~XzdVLxxTmrLE-xO8hMaIzm~iB8H-ca5KZxL8rQYcLtmGA2rugxuCAtbWgLNCMDg3MVn6Ty0eZtpvgvHBX-n-uzmjx3UkcuIgoa3Lih~h3~zuVTPCQquIyrhJUetH1N4iW6hUXU7OkF~r-LIq9p0a0FxHBVqdAPg81xXZDIrhxOkVemLTJ6oT4uhXkMeUJ9wFKw2gwPSgFfQiWfH4gy3HfOl6M6-1VyefUihCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Fig. 3. Effect of binding to α2M or α2M* on FGF-2–induced endothelial cell proliferation. / FGF-2, 0.5 mg/mL, was incubated with the indicated concentrations of α2M or α2M* for 2 hours at 37°C. This mixture was then added to quiescent FBHE cells, and cell proliferation was determined by [methyl-3H]thymidine incorporation as described in “Materials and methods.” FGF-2 activity in the absence of α2M or α2M* was defined as 100%. Data presented are the mean ± SD from 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/11/10.1182_blood.v97.11.3450/6/m_h81111134003.jpeg?Expires=1767900800&Signature=w2XEgk9g8PfyhMU~qgmcjos4zRpzXXouBfxwbhmMid59wzN4P62l-H8YICfbpN9SkB3OTPnW37kVxIBPrXm0cPvNmZI-WmZVXYjcLfTMNqTZboGe4SawM1a84guaEMzc5hN46dxlWt1WZHFcW57fGM0IIHRXXtH8HWceX9suQK04XjHy2ptEmn7e~5E0bVcpf5FEETEah8h9adhoC6H264NkaIWdnnVHSD5gu3VgoSDdB1vA8QKOv1txAIcyHiXtkQMf2mebfGKZ8ZZGh0Z~ocot1rkyD-S9xxG-Kas0QpZlpkd2yr144N8ASjpoGgqUtnpMPphWTQxacwSvBuowWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)