Abstract
Human thioredoxin (Trx) is the major 12-kd cellular disulfide-reductase that on secretion acts as a cocytokine with several interleukins. Truncated Trx with the 80 N-terminal residues (Trx80), also present in plasma, was by itself a mitogenic cytokine for human peripheral blood mononuclear cells (PBMC). This study investigated which cells in PBMC are targets of recombinant Trx80. Purified human CD14+ monocytes, but not B or T cells, in a synthetic medium were activated to differentiation by Trx80 as measured by flow cytometry of surface antigens because exposure to 100 nM Trx80 increased expression of CD14, CD40, CD54, and CD86. Proliferation of the monocytes was increased in a dose-dependent manner by Trx80 in concentrations ranging from 10 nM to 1 μM. Trx or interleukin (IL) 2 did not induce proliferation or expression of surface antigens on monocytes. Trx80 alone induced secretion of IL-12 from CD40+ monocytes in the PBMC cultures and this effect was enhanced by IL-2. Trx80 and IL-2 together were strongly synergistic to induce secretion of interferon-γ in PBMC cultures. The results showed that Trx80 is a potent cytokine for normal human monocytes and directs the immune system in favor of a Th1 response via IL-12 production.
Introduction
Thioredoxin (Trx) is a ubiquitous 12-kd redox enzyme that catalyzes thiol-disulfide exchange reactions via 2 cysteine residues in the conserved active site sequence -C-G-P-C-1-3 (single-letter amino acid codes). The 3-dimensional structure of thioredoxins (the thioredoxin fold) is characterized by a central 5-stranded sheet surrounded by 4 alpha helices.1-6 The active site disulfide in the oxidized Trx is reduced to a dithiol by NADPH and thioredoxin reductase (TrxR).1,2,7,8 Human T-cell leukemia virus I (HTLV-I)-transformed human T cells produce a factor previously named ADF, which is identical to human Trx.9 Trx is also secreted from several other cell types including activated normal B lymphocytes, B lymphocytes from B-type chronic lymphocytic leukemia, liver cells, fibroblasts, and T lymphocytes.10,11 Among its regulatory functions, Trx is involved in redox control of DNA binding of transcription factors like nuclear factor-κB (NF-κB) and AP-1,12-14 the latter by a direct association with Ref-1.15 Trx up-regulates IL-2 receptors in leukemic T cells.16 Moreover, Trx promotes cell growth of several malignant cell types, specifically, liver cell lines (PLC/PRF/5), Burkitt lymphoma cell lines (BL41), lymphoblastoid cell lines (CRAG8, CRB 95, 1G8), or chronic lymphocytic leukemia (CLL) cells,17-19 and prevents apoptosis via direct binding interaction of the reduced form with apoptosis signal-regulating kinase (ASK) 1. ASK1 is a kinase that is required for apoptosis induced by tumor necrosis factor-α (TNF-α).20 Chemokine activity of Trx has also recently been discovered in vivo and in vitro and this function appears not to be mediated via known chemokine receptors.21 An active site mutant with the cysteine residues substituted to serines is not active as a chemokine, suggesting that the redox function of Trx is required for its chemokine activity.21
Apart from full-length Trx, a fragment of Trx, containing the 80 to 84 first N-terminal amino acids, is known. This truncated protein, Trx80, is secreted from various cells, namely, U937 monocytes, cytotrophoblasts, CD4+ T cells,17,22-24 and is present on the cell surface of the monocytic cell lines U937 and THP-1.25 Trx80 is present in normal human plasma26 and we recently discovered that recombinant Trx80 alone stimulates proliferation of PBMC in a synthetic medium.26 Truncated Trx is elevated in plasma from patients with schistosomiasis and enhances eosinophilic cytotoxicity in vitro.27-30 Trx80 is a dimer in solution and is devoid of catalytic oxidoreductase activity, but Trx can reduce disulfides in Trx80.26 It has also been reported that Trx80 as a fusion protein increased human immunodeficiency virus (HIV) viral replication in infected macrophages, whereas Trx decreased the same.31
Interleukin 12 is a disulfide-linked cytokine composed of a 35-kd light chain (p35) and a 40-kd heavy chain (p40).32,33 It was first purified from conditioned media of an Epstein-Barr virus (EBV)-transformed human B cell line.34-36 However, the major producers of IL-12 in PBMC are monocytes or monocytic-derived macrophages, although B cells may also produce IL-12.37There are few known endogenous inducers of IL-12. Bacteria, bacterial products, and intracellular parasites are strong inducers of IL-12 expression37; however, the initial events that induce the production of IL-12 are not known. IL-12 stimulates proliferation of hematopoietic progenitor cells in response to other growth factors, such as IL-3 and IL-11.38 Moreover, it induces proliferation of preactivated T cells and natural killer (NK) cells, and the induction of proliferation of T cells is IL-2 independent.39 IL-12 also stimulates production of Th1 cytokines such as interferon-γ (IFN-γ), TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-8, and IL-2 from peripheral T cells and NK cells.39-41 IL-12 can induce differentiation of naive CD4+ T cells to a Th1 subset. This occurs rapidly in cell culture and the Th1 response is not weakened by addition of IL-4.42 In contrast Th2 subsets of naive CD4+ T cells induced by IL-4 will change subset to Th0 or Th1 after one single stimulation of IL-12.42 IL-2 can act in synergy with IL-12 in triggering the expression of IFN-γ by T cells.43
To understand which cells in human PBMC are stimulated by Trx80, we have purified human monocytes and T and B cells from healthy blood donors. We found that Trx80 activates monocytes to proliferate and express elevated levels of CD14, CD40, CD54, and CD86. We also observed that Trx80 by itself induces a Th1 response in PBMC by inducing secretion of IL-12 and IFN-γ and that Trx80 acts in synergy with IL-2 when inducing these Th1 cytokines.
Materials and methods
Materials
Recombinant human Trx80 and full-length Trx were prepared and purified from possible lipopolysaccharide (LPS) contamination as described previously8 26; AIM V and RPMI 1640 cell culture medium and l-glutamine were purchased from Gibco BRL, Life Technologies, Paisley, Scotland; Ficoll-Paque from Pharmacia Biotec, Uppsala, Sweden; polymyxin B (PMB) sulfate, ultralow attachment cell culture plates, and LPS from Sigma Chemical, St Louis, MO; 5-3H-thymidine and human recombinant IL-2 from Amersham Pharmacia Biotech, Uppsala, Sweden; penicillin/streptomycin from Bio-Whittaker Europe, Verviers, Belgium; CD14+ MACS from Miltenyi Biotec, Bergisch Gladbach, Germany; CD2+ Dynabeads from Dynal, Oslo, Norway; IL-12, IL-4, and IFN-γ enzyme-linked immunosorbent assay (ELISA) antibodies from Mabtech, Nacka, Sweden; IL-5 ELISA antibodies, Brefaldin A, and human recombinant IL-5 from Pharmingen, San Diego, CA; human recombinant IL-4 and IFN-γ from Preprotech, London, England; mouse monoclonal fluorescein isothiocyanate (FITC)-conjugated antibodies for flow cytometry were anti-CD14 (Leu-M3) and anti-CD19 (Leu-12) from Becton Dickinson, San Jose, CA; FITC-conjugated anti-CD40(5C3) from Pharmingen; mouse monoclonal phycoerythrin (PE)-conjugated antibodies for flow cytometry were PE-conjugated anti-CD3 (Leu-4) and anti-CD54 (Leu-54) from Becton Dickinson; mouse monoclonal allophycocyanin (APC)-conjugated antibodies were anti-IL-12 (C11.5) and isotype matched control from Pharmingen; FITC- and PE-IgG1 isotype-matched control antibodies were from Becton Dickinson.
Purification of CD14+, CD14−, and CD3+ cells
Human PBMC were prepared by standard Ficoll-Paque centrifugation of blood from healthy donors (Blood Bank, Karolinska Hospital). The resulting cells were counted and resuspended in 3 mL cold phosphate-buffered saline (PBS) and 100 μL CD14+ MACS was added per 100 × 106 PBMC and incubated one hour at 4°C, gently mixing every 10 minutes. The incubated cells were then applied to a LS/VS column (Miltenyi Biotec) pre-equilibrated with cold RPMI 1640 and attached to a magnet. The column was washed 3 times with 5 mL cold RPMI 1640 and CD14− cells were collected. Subsequently the column was removed from the magnet and flushed 5 times with 5 mL cold RPMI 1640 and CD14+ cells were collected. CD14+/− cells were centrifuged 400g for 8 minutes and resuspended in 10 mL AIM V medium supplemented with 2 mMl-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin and counted. Purity of CD14+ purified monocytes was more than 94% in all experiments measured by flow cytometry. The contamination of CD14+ cells in the CD14−cells was less than 5% in all experiments. In some experiments T cells were purified from CD14− cells with CD2+Dynabeads according to instructions from the manufacturer. Purity of cultured CD3+ T cells was more than 95% in all experiments measured by flow cytometry. All cell experiments were performed with a concentration of 1 × 106 cells/mL. The study was approved by the Ethics Committee of the Karolinska Institutet and the Karolinska Hospital.
Cell proliferation assay of purified CD14+, CD14−, and CD3+ cells
All 3H-thymidine assays were performed in 96-well flat-bottomed plates in a final volume of 200 μL with all treatments incubated in triplicate wells at 37°C and 5% CO2 in humidified cell incubators for 3 days. For determination of3H-thymidine uptake, each well was pulsed with 0.5 μCi3H-thymidine 12 hours prior to harvesting onto fiberglass filters using a multichannel cell harvester (Tomtec, Wallac, Sweden). Filters were counted with a MicroBeta Plus Scintillation counter (Tomtec). All data reported are mean counts per minute of triplicate wells. Cells were stimulated with Trx or Trx80 at concentrations ranging from 10 nM to 1 μM, IL-2 at 20 U/mL, IL-2 at 20 U/mL with either 100 nM or 1 μM Trx or Trx80 and also with 10 ng/mL of LPS. To ascertain that no endotoxin contamination in the preparation of Trx80 gave the observed effects, the endotoxin inhibitor PMB was used. Trx80 and LPS were added alone or together with PMB to cell cultures and the effect of the endotoxin inhibitor PMB on thymidine incorporation of the purified CD14+ monocytes was recorded.
Flow cytometry analysis of surface markers
The CD14+, CD14−, or CD3+cells were grown in AIM V medium supplemented with 2 mMl-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin for 3 days at the same conditions as described above in ultralow attachment wells pretreated with AIM V medium according to manufacturer's instruction. Cells were stimulated with either Trx80 or Trx at 100 nM or 1 μM, IL-2 at 20 U/mL, and with IL-2 at 20 U/mL with either 100 nM or 1 μM Trx or Trx80. Cells were harvested by adding cold PBS to cell cultures, incubated on ice for 10 minutes, and resuspended with a Pasteur pipette. Cells were then incubated for 45 minutes on ice with FITC-conjugated CD14, CD40, and CD86 antibodies and isotype-matched control antibodies and PE-conjugated CD3 and CD54 antibodies and isotype-matched control antibodies, washed once with 1 mL PBS and finally resuspended in 500 μL PBS. The cells were analyzed in a FACSCalibur flow cytometer (Becton Dickinson) using Cell Quest software (Becton Dickinson). In each sample 5000 cells were analyzed. Dead cells were excluded by cell size or by staining with propidium iodide.
Detection of cytokines secreted to cell medium
The PBMC, CD14+, CD14−, or CD3+ cells, respectively, were cultured in 24-well flat-bottomed wells for 24, 48, or 72 hours under the same conditions described above. Cells were stimulated with 10 nM to 1 μM Trx80 or Trx with or without 20 U/mL IL-2, with IL-2 at 20 U/mL alone, with 10 ng/mL LPS and with 1 μg/mL PMB. At defined times, cells were harvested and centrifuged and supernatants were collected in 2 Eppendorf tubes, which were frozen at −20°C and analyzed in duplicate wells using sandwich ELISA for IL-4, IL-5, IL-12, or IFN-γ according to the manufacturer's instructions. The sandwich ELISA for IL-12 detected both IL-12 heavy chain (p40) and the heterodimer (p75). The detection limit of the IL-12 ELISA was 0.3 ng/mL and for the IL-4, IL-5, and IFN-γ ELISA the detection limits were 0.03 ng/mL.
Detection of intracellular cytokines
The PBMC were cultured in 6-well ultralow attachment wells for 24, 48 or 72 hours as above. At a time point 22 hours before terminating cultures, Brefaldin A was added, to inhibit export via the Golgi pathway, to a final concentration of 0.1 ng/mL cell culture. Cells were then harvested, fixed with 4% paraformaldehyde for 45 minutes on ice, washed twice with PBS, and permeabilized with washing buffer (Pharmingen) containing saponin. Subsequently, PBMC were incubated for 45 minutes on ice with APC-conjugated anti-IL-12 antibody and isotype-matched control antibody and FITC-conjugated CD14, CD40, and CD19 antibodies and isotype-matched control antibodies. The APC-conjugated anti-IL-12 antibody detected both IL-12 heavy chain (p40) and IL-12 heterodimer. Cells were then washed twice with washing buffer containing saponin and resuspended in 500 μL PBS. The cells were analyzed in a FACSCalibur. In each sample 5000 cells from the large cell population, that is, monocytes, and 5000 cells from the small cell population, that is, B and T cells, were analyzed.
Statistics
Thymidine incorporation and flow cytometry data of the CD14+ monocytes were compared using the nonparametric Wilcoxon matched pairs test. A P value of less than .05 was considered statistically significant.
Results
Trx80 induces proliferation of monocytes
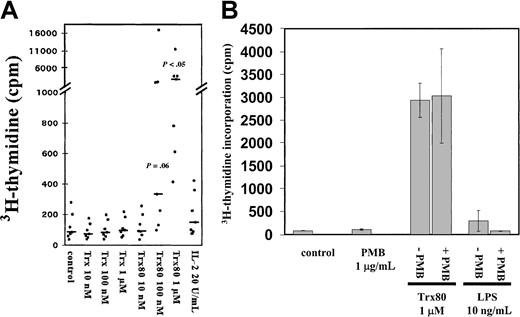
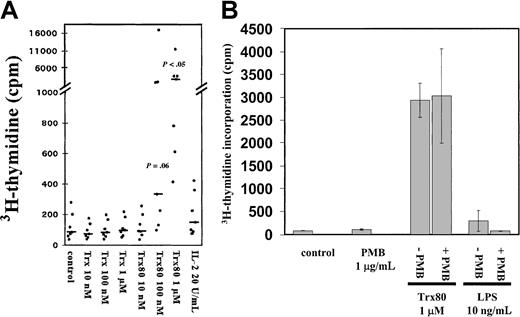
Purified CD14+ monocytes were incubated in the synthetic AIM V medium and exposed to Trx80 for 3 days with3H-thymidine added during the last 12 hours of incubation. Trx80 stimulated DNA synthesis in a dose-dependent manner, whereas Trx or IL-2 alone (Figure 1A) or added together (data not shown) gave no increase in thymidine incorporation. With 1 μM Trx80, a 35-fold increase of thymidine incorporation was observed compared to unstimulated monocytes (n = 7,P < .05). Trx80 at 10 nM gave no increase in thymidine incorporation, whereas Trx80 at 100 nM gave a 4-fold increase (n = 7,P = .06) (Figure 1A). There were no synergistic effects between Trx80 and IL-2 or between Trx and IL-2 on cell proliferation (data not shown). When a mixture of B and T cells or when pure T cells were treated with Trx80, no increase in cell proliferation was seen (data not shown). The background levels of 3H-thymidine incorporation differed from donor to donor, although all cultures had more than 94% CD14+ cells. The response to Trx80 also varied among the different donors. However, in 7 of 7 donors Trx80 gave an increase in 3H-thymidine incorporation in the purified monocytes, and this increase differed from 7- to 80-fold among the donors when 1 μM Trx80 was used. In 5 of the 7 donors this increase was more than 19-fold when the monocytes were stimulated with 1 μM Trx80 (Figure 1A). The viability of the purified monocytes after 3 days in culture also varied among the different donors. However, Trx and Trx80 increased the viability of the cultured monocytes from all 7 donors (data not shown).
Trx80 induces proliferation of CD14+monocytes.
(A) Purified CD14+ monocytes (> 94% purity) were stimulated with Trx, Trx80, or IL-2 and grown in AIM V medium in triplicate wells for 3 days. Twelve hours before harvesting, 0.5 μCi3H-thymidine was added. The mean 3H-thymidine incorporation from 7 donors is shown with black dots and the median values are indicated with black bars. There was a significant increase in thymidine incorporation in cultures stimulated with 100 nM and 1 μM Trx80 compared to the unstimulated cells, which is indicated withP values in the figure. (B) Purified CD14+monocytes were grown in AIM V medium and stimulated with 1 μg/mL PMB, Trx80, and LPS alone, or PMB together with either 1 μM Trx80 or 10 ng/mL LPS for 3 days. Twelve hours before harvesting 0.5 μCi3H-thymidine was added. Error bars indicate SDs of triplicate cultures. The figure depicts one representative experiment of 3.
Trx80 induces proliferation of CD14+monocytes.
(A) Purified CD14+ monocytes (> 94% purity) were stimulated with Trx, Trx80, or IL-2 and grown in AIM V medium in triplicate wells for 3 days. Twelve hours before harvesting, 0.5 μCi3H-thymidine was added. The mean 3H-thymidine incorporation from 7 donors is shown with black dots and the median values are indicated with black bars. There was a significant increase in thymidine incorporation in cultures stimulated with 100 nM and 1 μM Trx80 compared to the unstimulated cells, which is indicated withP values in the figure. (B) Purified CD14+monocytes were grown in AIM V medium and stimulated with 1 μg/mL PMB, Trx80, and LPS alone, or PMB together with either 1 μM Trx80 or 10 ng/mL LPS for 3 days. Twelve hours before harvesting 0.5 μCi3H-thymidine was added. Error bars indicate SDs of triplicate cultures. The figure depicts one representative experiment of 3.
To further ascertain that the effects seen were truly generated by Trx80, control experiments with PMB and LPS were performed. Addition of 10 ng/mL LPS slightly induced proliferation of monocytes. This effect was totally abolished when 10 ng/mL LPS and 1 μg/mL endotoxin inhibitor PMB were added together (Figure 1B). When PMB was added to cultured monocytes stimulated with Trx80 no difference in proliferation was seen compared to when only Trx80 was added (Figure 1B).
Trx80 enhances the expression of CD14, CD40, CD54, and CD86 in monocytes
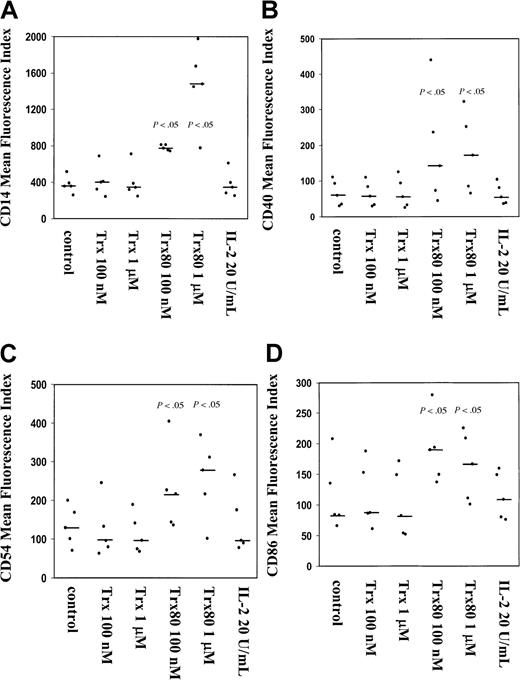
The CD14+ monocytes isolated from PBMC up-regulated the expression of several surface antigens after stimulation with Trx80 for 3 days; however, this was not the case when CD14+monocytes were treated with Trx and IL-2 alone or in synergy (Figure2). When a mixture of T and B cells or purified T cells were stimulated with 100 nM or 1 μM Trx80, no increase in expression of surface antigens was seen (data not shown).
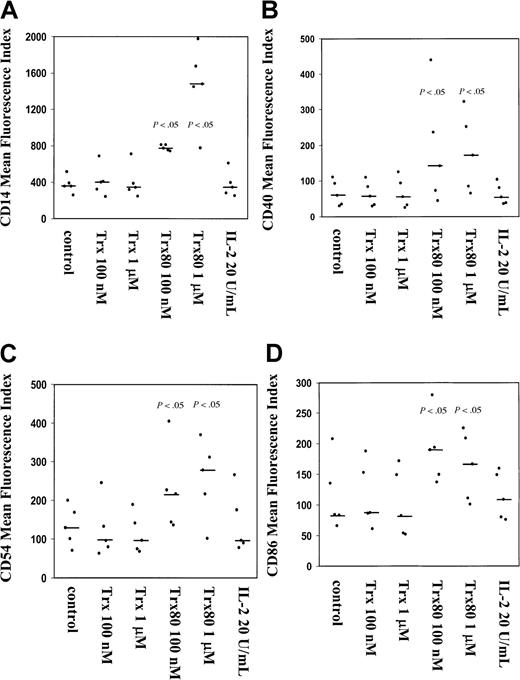
Trx80 induces expression of CD14, CD40, CD54, and CD86 on purified human CD14+ monocytes.
Purified CD14+ monocytes were stimulated with either Trx, Trx80, or IL-2 and grown for 3 days in ultralow attachment plates. Harvested cells were analyzed for expression of CD14 (A), CD40 (B), CD54 (C), and CD86 (D). The MFI from 5 different donors is shown in black dots with the median value indicated with a black bar. There was a significant increase in the expression of these markers when monocytes were stimulated with 100 nM and 1 μM Trx80 compared to unstimulated cells, which is indicated with P values in the figure.
Trx80 induces expression of CD14, CD40, CD54, and CD86 on purified human CD14+ monocytes.
Purified CD14+ monocytes were stimulated with either Trx, Trx80, or IL-2 and grown for 3 days in ultralow attachment plates. Harvested cells were analyzed for expression of CD14 (A), CD40 (B), CD54 (C), and CD86 (D). The MFI from 5 different donors is shown in black dots with the median value indicated with a black bar. There was a significant increase in the expression of these markers when monocytes were stimulated with 100 nM and 1 μM Trx80 compared to unstimulated cells, which is indicated with P values in the figure.
The surface expression of the monocyte marker CD14 increased significantly in a dose-dependent fashion when cells were stimulated with Trx80. At the starting point of each experiment there were more than 94% CD14+ cells. Trx80 at 100 nM gave a 2-fold increase of the mean fluorescence index (MFI) of CD14, whereas 1 μM Trx80 gave a 4- to 5-fold increase in MFI compared to unstimulated cells (P < .05, n = 5; Figure 2A). When monocytes were treated with Trx or IL-2, no change in MFI for CD14 was seen (Figure2A). No synergy with Trx or Trx80 together with IL-2 was seen when CD14 expression was analyzed (data not shown).
The expression of the activation marker CD40, the adhesion molecule CD54, and the costimulatory CD86 was increased significantly by Trx80 compared to unstimulated cells (P < .05, n = 5). When the experiment started there were between 40% and 90% CD40+ cells in the different subjects, apart from one donor who had only 7% CD40+ cells. No increased expression was seen with IL-2 or Trx; however, 100 nM or 1 μM Trx80 increased the MFI of CD40 expression by 2- to 3-fold compared to unstimulated cells (Figure 2B). No synergistic effects on CD40 expression with IL-2 and Trx or Trx80 was seen (data not shown). More than 88% of the CD14+ monocytes in all 5 subjects tested expressed CD54. The proportion of CD86+ monocytes was between 30% to 95% in the different subjects. No change in the proportion of CD54+ and CD86+ cells was seen during the culture time. However, the MFI of CD54 and CD86 increased 2- to 3-fold compared to unstimulated cells when cells were stimulated with 100 nM and 1 μM Trx80 (P < .05, n = 5) (Figure 2C,D). No synergy was seen with IL-2 and Trx80 (data not shown).
Trx80 induces secretion of IL-12 and IFN-γ in PBMC
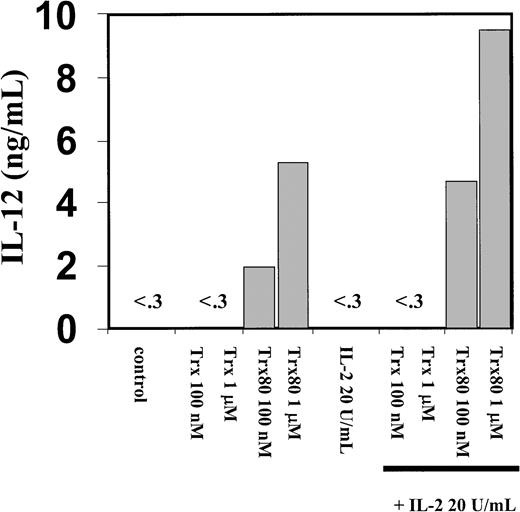
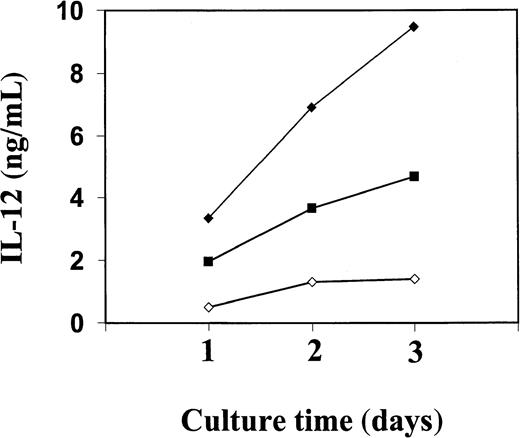
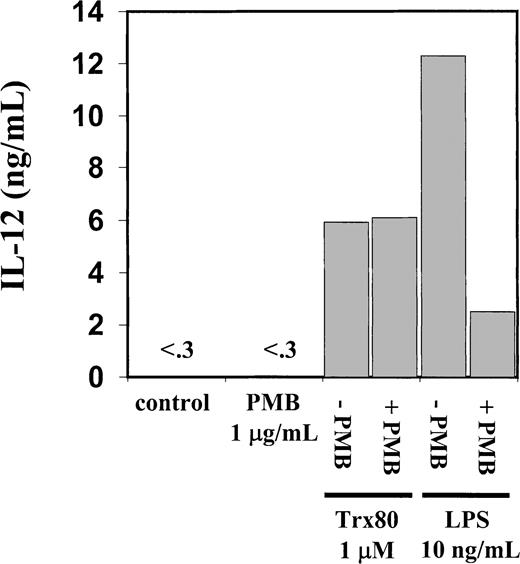
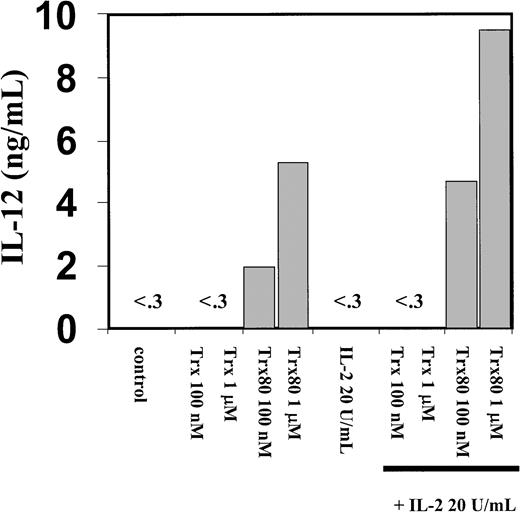
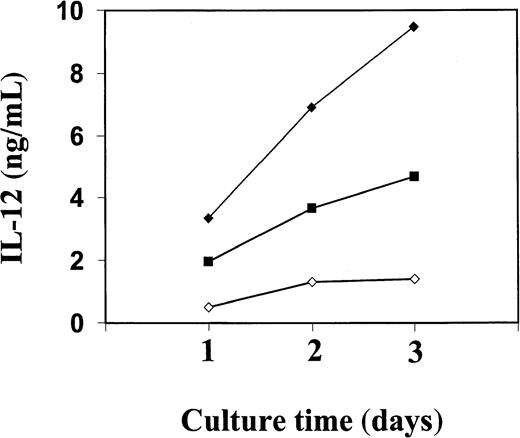
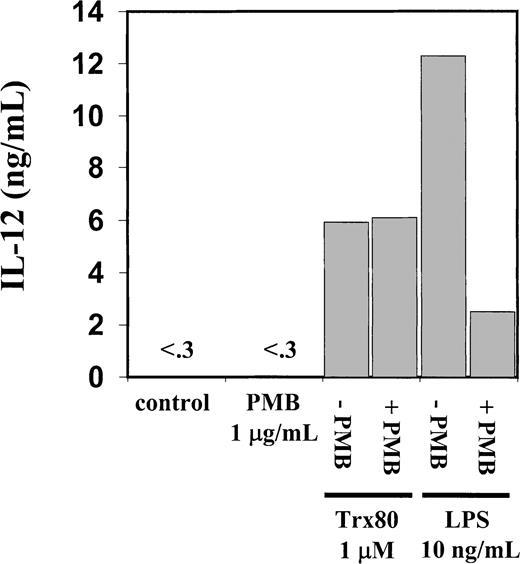
The PBMC or the corresponding isolated monocytes, T cells, and B cells were exposed to Trx80, Trx, or IL-2 and analyzed for the production of the Th1 cytokines IL-12 and IFN-γ and the Th2 cytokines IL-4 and IL-5. No Th2 cytokines were detected when PBMC, CD14+, CD14− or T cells were stimulated with Trx80, Trx, or IL-2 either alone or in synergy for 24 to 72 hours in culture (data not shown). In PBMC cultured for 3 days with no stimulus, IL-12 was undetectable in the cell culture medium. However, when PBMC were stimulated with 100 nM Trx80, the IL-12 levels were between 0.5 and 2 ng/mL and with 1 μM Trx80 the levels were between 1 and 9 ng/mL (n = 9). With IL-2 alone or in combination with Trx, no secretion of IL-12 was seen. However, the stimulation of IL-12 secretion caused by Trx80 was enhanced by IL-2. Thus, PBMC stimulated with 20 U/mL IL-2 and 1 μM Trx80 together had IL-12 levels between 5 and 12 ng/mL (n = 9; Figure 3). IL-12 levels in the cell culture medium increased over time when PBMC were stimulated with Trx80 or Trx80 and IL-2. The highest levels of IL-12 was seen after 3 days (Figure 4). LPS at 10 ng/mL gave approximately double the amount of IL-12 in PBMC supernatants compared to 1 μM Trx80 (12 ng/mL and 6 ng/mL, respectively). When 10 ng/mL LPS and 1 μg/mL PMB were added to PBMC cultures, the effect of LPS on secretion of IL-12 by PBMC was reduced, whereas when 1 μM Trx80 and 1 μg/mL PMB were used the effect of Trx80 was unchanged (Figure5).
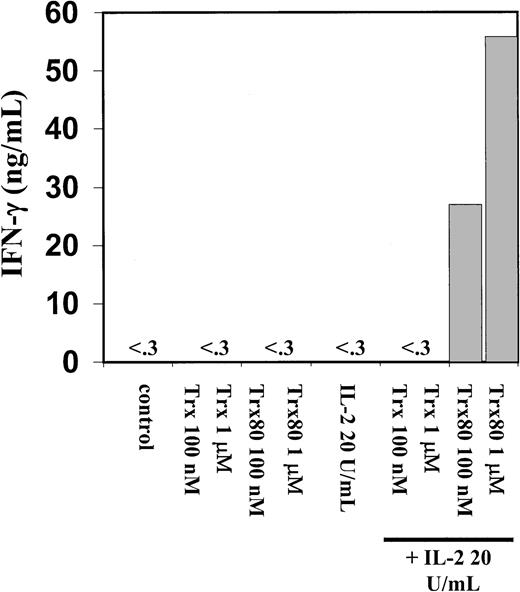
Trx80 induces secretion of IL-12 from human PBMC.
Human PBMC were stimulated with Trx, Trx80, and IL-2, alone and together. PBMC were stimulated for 3 days in AIM V medium and subsequently cell medium was collected and analyzed for IL-12 content with sandwich ELISA, which detects both p40 and IL-12 heterodimer with a detection limit of 0.3 ng/mL. The figure shows one representative experiment of 9 and the bars depict the mean of duplicate measurements in sandwich ELISA.
Trx80 induces secretion of IL-12 from human PBMC.
Human PBMC were stimulated with Trx, Trx80, and IL-2, alone and together. PBMC were stimulated for 3 days in AIM V medium and subsequently cell medium was collected and analyzed for IL-12 content with sandwich ELISA, which detects both p40 and IL-12 heterodimer with a detection limit of 0.3 ng/mL. The figure shows one representative experiment of 9 and the bars depict the mean of duplicate measurements in sandwich ELISA.
Kinetics of IL-12 secretion from purified human PBMC stimulated with Trx80 and IL-2.
Human PBMC were stimulated with Trx80 alone or together with IL-2 in AIM V medium. Cell medium was collected after 1, 2, and 3 days and subsequently analyzed for IL-12 content using a sandwich ELISA that detects both p40 and IL-12 heterodimer with a detection limit of 0.3 ng/mL. Trx80 at 1 μM is indicated in open diamonds, 100 nM Trx80 together with 20 U/ml IL-2 in black squares, and 1 μM Trx80 together with 20 U/ml IL-2 in black diamonds. The values shown are means from duplicate measurements in sandwich ELISA. The figure shows one representative experiment of 3.
Kinetics of IL-12 secretion from purified human PBMC stimulated with Trx80 and IL-2.
Human PBMC were stimulated with Trx80 alone or together with IL-2 in AIM V medium. Cell medium was collected after 1, 2, and 3 days and subsequently analyzed for IL-12 content using a sandwich ELISA that detects both p40 and IL-12 heterodimer with a detection limit of 0.3 ng/mL. Trx80 at 1 μM is indicated in open diamonds, 100 nM Trx80 together with 20 U/ml IL-2 in black squares, and 1 μM Trx80 together with 20 U/ml IL-2 in black diamonds. The values shown are means from duplicate measurements in sandwich ELISA. The figure shows one representative experiment of 3.
Endotoxin inhibitor PMB inhibits IL-12 secretion induced by LPS but not by Trx80.
Human PBMC were stimulated for 3 days with Trx80, LPS, or PMB alone and together in AIM V medium. Subsequently cell medium was collected and analyzed for IL-12 content with sandwich ELISA, which detects both p40 and IL-12 heterodimer with a detection limit of 0.3 ng/mL. Bars indicate means from duplicate measurements in sandwich ELISA. Figure shows one representative experiment of 3.
Endotoxin inhibitor PMB inhibits IL-12 secretion induced by LPS but not by Trx80.
Human PBMC were stimulated for 3 days with Trx80, LPS, or PMB alone and together in AIM V medium. Subsequently cell medium was collected and analyzed for IL-12 content with sandwich ELISA, which detects both p40 and IL-12 heterodimer with a detection limit of 0.3 ng/mL. Bars indicate means from duplicate measurements in sandwich ELISA. Figure shows one representative experiment of 3.
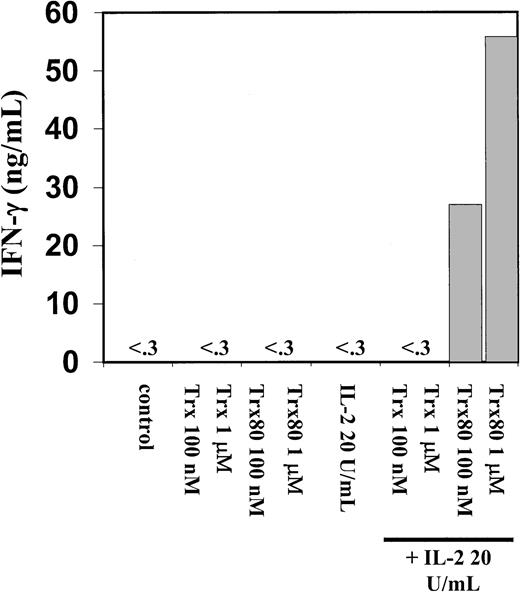
A dramatic increase in IFN-γ secretion was seen when 100 nM or 1 μM Trx80 and 20 U/mL IL-2 were added together to the PBMC cultures (Figure6). IFN-γ secretion from PBMC was not detected when IL-2, Trx, or Trx80 was used alone to stimulate PBMC cultures (Figure 6). When pure monocytes, T cells, or a mixture of B and T cells were stimulated with Trx80, Trx, and IL-2, respectively, or in synergy no secretion of IL-12 or IFN-γ was detected (data not shown).
Trx80 in synergy with IL-2 induce secretion of IFN-γ from human PBMC.
Human PBMC were stimulated for 3 days in AIM V medium with Trx, Trx80, and IL-2, alone and together and subsequently cell medium was collected and analyzed for IFN-γ content with a sandwich ELISA with detection limit 0.03 ng/mL. Bars indicate means from duplicate measurements in sandwich ELISA. The figure shows one representative experiment of 5.
Trx80 in synergy with IL-2 induce secretion of IFN-γ from human PBMC.
Human PBMC were stimulated for 3 days in AIM V medium with Trx, Trx80, and IL-2, alone and together and subsequently cell medium was collected and analyzed for IFN-γ content with a sandwich ELISA with detection limit 0.03 ng/mL. Bars indicate means from duplicate measurements in sandwich ELISA. The figure shows one representative experiment of 5.
Trx80 stimulates production of IL-12 from CD40+ monocytes in PBMC
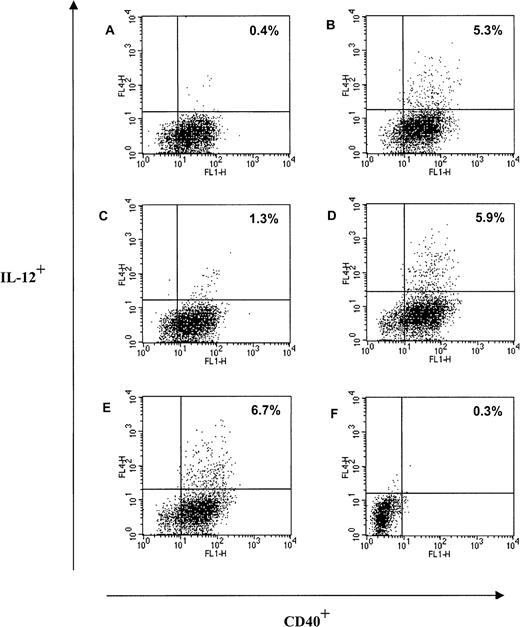
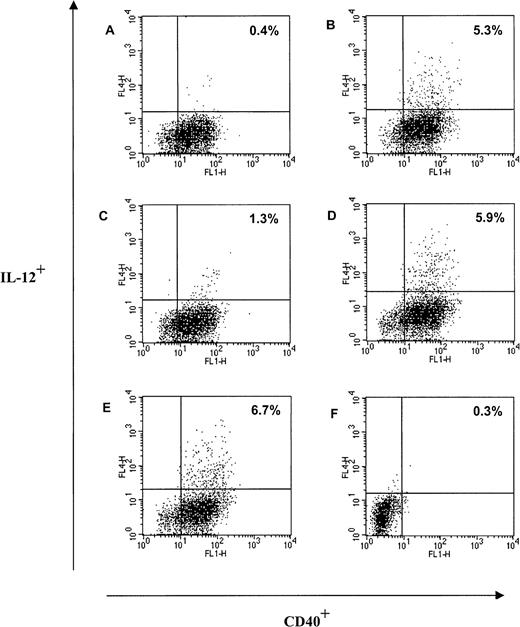
When PBMC were stimulated with 1 μM Trx80 or Trx80 in combination with 20 U/mL IL-2 for 48 hours from 5% to 6% of the CD40+ cells in the large cell population in PBMC became IL-12+ as analyzed by FACS analysis (Figure7B,D). There were no difference in IL-12+ cells between PBMC stimulated with only Trx80 at 1 μM and PBMC stimulated with Trx80 and IL-2 (Figure 7B,D). All cells in the large population expressing IL-12 were CD40+ (Figure7). Approximately 50% of the large cells expressing IL-12 were CD14+ (data not shown). However, Brefaldin A, which was used to inhibit the export of IL-12 via the Golgi pathway, seemed to have a negative effect on the CD14 expression in the large cell population; when PBMC were stimulated with 1 μM Trx80 for 2 days, 78% of the cells in the large cell population in PBMC were CD14+. If Brefaldin A was added 22 hours before harvesting, the CD14+ cells in the large cell population in the PBMC culture had decreased to 38% (data not shown). No cells in the small population, which consisted of CD3+ T cells and CD19+ B cells, expressed IL-12 when cultured unstimulated, with Trx80 or IL-2 alone or in combination or with LPS at 100 ng/mL (data not shown). When PBMC were cultured with 20 U/mL IL-2 there were 1.3% of the CD40+ cells in the large cell population that expressed IL-12 (Figure 7C). No unstimulated cells in the large population expressed IL-12 (Figure 7A). LPS at 100 ng/mL induced IL-12 expression in the large cells to a similar extent as Trx80 (Figure 7E).
Trx80 induces production of IL-12 from CD40+monocytes in PBMC.
Human PBMC were cultured in AIM V medium in ultralow attachment plates for 48 hours. Twenty-two hours before terminating the cultures, Brefaldin A was added to each culture. After harvesting, the large cell population in PBMC was analyzed for intracellular expression of IL-12 and expression of CD40. The culture conditions were unstimulated cells (A), Trx80 1 μM (B), IL-2 at 20 U/mL (C), Trx80 1 μM and IL-2 at 20 U/mL (D), LPS 100 ng/mL (E), and unstimulated cells with isotype-matched antibodies for IL-12 and CD40 (F). One representative experiment of 4 is shown. The proportion of cells positive for both CD40 and IL-12 is indicated in upper right corner.
Trx80 induces production of IL-12 from CD40+monocytes in PBMC.
Human PBMC were cultured in AIM V medium in ultralow attachment plates for 48 hours. Twenty-two hours before terminating the cultures, Brefaldin A was added to each culture. After harvesting, the large cell population in PBMC was analyzed for intracellular expression of IL-12 and expression of CD40. The culture conditions were unstimulated cells (A), Trx80 1 μM (B), IL-2 at 20 U/mL (C), Trx80 1 μM and IL-2 at 20 U/mL (D), LPS 100 ng/mL (E), and unstimulated cells with isotype-matched antibodies for IL-12 and CD40 (F). One representative experiment of 4 is shown. The proportion of cells positive for both CD40 and IL-12 is indicated in upper right corner.
Discussion
Trx80 is an extracellular natural cleavage product of human cytosolic thioredoxin24,30,31 lacking the c-terminal βα motif of the thioredoxin fold.5,6 It has distinct properties lacking the disulfide oxidoreductase activity of thioredoxin and is a folded dimer.26 We previously discovered that recombinant Trx80 alone acts as a mitogenic cytokine for human PBMC prepared from healthy blood donors and that Trx80 is present in plasma.26
In this study we demonstrate that the primary target cell in PBMC for the mitogenic effects of Trx80 is the monocyte. Addition of Trx80 induced proliferation and differentiation of isolated monocytes as measured by thymidine incorporation and enhanced expression of the surface markers CD14, CD40, CD54, and CD86. Trx80, by itself, and in combination with IL-2, induced secretion of the Th1 inducer IL-12 from normal resting human PBMC. In addition, Trx80 in synergy with IL-2 induced secretion of IFN-γ from the same PBMC. No direct effect of Trx80 was seen on either the purified B or T cells. The concentrations of Trx80 used in this study are physiologic because the levels of truncated thioredoxin in human plasma from blood donors are up to 20 nM,26 and local concentrations at cell-cell interactions should be much higher.
When purified monocytes were stimulated for 3 days with Trx80, CD14 expression on the cell surface was increased 5-fold. One of the major biologic significances of the enhanced CD14 expression by Trx80 should be in the defense against intracellular and extracellular parasites by innate immunity, because CD14 is a receptor for LPS, with a broad substrate specificity.44 In addition, CD14 is also important for recognition and removal of apoptotic cells.45 Moreover, CD14 expression promotes survival of cells and antagonizes apoptosis.44 Th2 cell-derived cytokines such as IL-4 are able to induce monocyte apoptosis. This process is preceded by down-regulation of the CD14 surface receptor.44
Baldini and colleagues have shown a genetic polymorphism in the 5′ flanking region of the CD14 gene, which can be one cause of the variation among different donors in CD14 expression. In their study, 50% in the population were heterozygotes and 21% were homozygotes for this mutation.46 The homozygotes had higher levels of circulating soluble CD14, which most probably arises from membrane-bound CD14.46
In addition, our results show that Trx80 induces phenotypic changes on monocytes that will influence T-cell activation. Trx80 enhanced expression of CD40 on monocytes 3-fold measured as MFI. Moreover, in one donor in which only 7% of the CD14+ monocytes expressed CD40, Trx80 turned CD40− to CD40+. After 3 days of culture with 100 nM Trx80 70% of the CD14+monocytes expressed CD40, whereas the unstimulated monocytes or monocytes stimulated with IL-2 or Trx still had only 7% CD40+ cells. Furthermore, Trx80 alone increased the expression of the costimulatory molecule CD86 and of the adhesion molecule CD54 (ICAM-1). Previous reports have shown that stimulation of human peripheral monocytes by GM-CSF, IFN-γ, or soluble CD40L induces up-regulation of several molecules important for antigen- presenting cell functions, including MHC class ΙΙ, CD86, and CD40.47,48 Moreover, ligation of CD40 on monocytes enhances survival of these cells and CD40-CD40L interaction is crucial for a sustained Th1 response and CD40-CD40L interaction synergizes with IL-12 in enhancing IFN-γ production from T cells.48 49
Trx80 at concentrations between 100 nM and 1 μM induced secretion of IL-12 in a dose-dependent fashion from human resting PBMC. Trx or IL-2 alone did not have this property. Moreover, we found that the cells in PBMC cultures expressing IL-12 were the large CD40+monocytes. It is clear that monocytes require, in addition to Trx80, costimulatory signals to be able to induce IL-12 production because purified CD14+ monocytes or CD19+ B cells did not increase secretion of IL-12 when stimulated with Trx80. It is probable that the required costimulatory signals are given by T cells. In support of this Shu and coworkers found that monocytes cultured for 48 hours with GM-CSF and IFN-γ produce low levels of IL-12 (50-100 pg/mL IL-12 p75) after stimulation with CD40L.50 In addition, Cella and associates reported that monocytes produce small amounts of IL-12 p75 only when they were triggered by CD40L in the presence of IFN-γ.51 In both of these experimental systems, signaling via CD40 and additional cytokines were needed to induce secretion of IL-12 from monocytes. Our results confirm these data because all cells expressing IL-12 in our experiments were CD40+.
One of the most important properties of IL-12 is its ability to induce the production of IFN-γ from activated T and NK cells.40All donor cells in our study showed an increase in IFN-γ levels when incubated with IL-2 and Trx80, whereas all untreated cultured PBMC had no secretion of IFN-γ. Previous reports have shown IFN-γ production when PBMC were stimulated with polyclonal mitogenic activators such as phytohemagglutinin (PHA), Staphylococcus aureus, and phorbol diesters.37,52 Moreover, IFN-γ production was enhanced when preactivated T cells (by PHA or anti-CD3) were costimulated with CD28 or CD40L in the presence of IL-12.48,52 53 It is possible that in the culture conditions that we use (Trx80 plus IL-2), the activated monocytes expressing higher levels of the costimulatory molecules CD40 and CD86 can bind CD40L and CD28 expressed on T cells. Engagement of their respective receptors is going to further activate both types of cells, inducing the monocytes to produce IL-12 and the T cells to produce IFN-γ.
Full-length Trx is known to be secreted from cells and have cocytokine activities extracellularly.10,11,17 Among other functions Trx up-regulates the IL-2 receptor on T cells.16,54,55 Trx levels are increased in plasma from HIV-infected individuals and also in inflammatory diseases.56-58 The exact redox state of secreted Trx is unknown, but it should be kept oxidized by the oxidizing extracellular environment. Secretion of Trx and Trx80 occur in conditions of oxidative stress. Our results suggest a novel mechanism for extracellular Trx, namely, to act as a reservoir for Trx80, which can be generated by cleavage of full-length Trx by macrophages.31 Trx80, which is devoid of redox activity,26 could then activate monocytes, as shown herein, and eosinophilic cells.26,29 59
Our results clearly demonstrate that Trx80 is a potent inducer of a Th1 response in normal resting human PBMC and that the monocyte is the primary target cell for the molecule. Moreover, our data suggest that Trx80-activated monocytes could be more efficient in phagocytosing apoptotic cells, invasive bacteria, or parasites via the CD14 receptor. The increased CD14 expression may also confer survival advantages for monocytes.
Medical Nobel Institute for Biochemistry, Department of Biochemistry and Biophysics, Karolinska Institutet, and the Department of Medicine, Unit of Clinical Allergy Research, Karolinska Institutet and Hospital, Stockholm, Sweden.
Submitted October 30, 2000; accepted January 24, 2001.
Supported by grants from the Swedish Cancer Society (961), the Swedish Medical Research Council (grants 03X-3529, 03XS-13005, and 16X-7924), the Karolinska Institute, the Swedish Asthma and Allergy Association, the Swedish Council for Work Life Research, and the Swedish Foundation for Health Care Sciences and Allergy Research. J. A.-C. is the recipient of a fellowship from AMF-Sjukförsäkrings Jubilee Foundation for Research in National Diseases, and R. G. is the recipient of a fellowship from the Wenner-Gren Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arne Holmgren, Medical Nobel Institute for Biochemistry, Dept of Biochemistry and Biophysics, Karolinska Institutet, S-171 77 Stockholm, Sweden; e-mail:Arne.Holmgren@mbb.ki.se.