Abstract
The response of mice genetically unable to up-regulate GATA-1 expression (GATA-1low mice) to acute (phenylhydrazine [PHZ]–induced anemia) and chronic (in vivo treatment for 5 days with 10 U erythropoietin [EPO] per mouse) erythroid stimuli was investigated. Adult GATA-1low mice are profoundly thrombocytopenic (platelet counts [× 109/L] 82.0 ± 28.0 vs 840 ± 170.0 of their control littermates, P < .001) but have a normal hematocrit (Hct) (approximately .47 proportion of 1.0 [47%]). The spleens of these mutants are 2.5-fold larger than normal and contain 5-fold more megakaryocytic (4A5+), erythroid (TER-119+), and bipotent (erythroid/megakaryocytic, TER-119+/4A5+) precursor cells. Both the marrow and the spleen of these animals contain higher frequencies of burst-forming units–erythroid (BFU-E)– and colony-forming units–erythroid (CFU-E)–derived colonies (2-fold and 6-fold, respectively) than their normal littermates. The GATA-1low mice recover 2 days faster from the PHZ-induced anemia than their normal littermates (P < .01). In response to EPO, the Hct of the GATA-1low mice raised to .68 proportion of 1.0 (68%) vs the .55 proportion of 1.0 (55%) reached by the controls (P < .01). Both the GATA-1low and the normal mice respond to PHZ and EPO with similar (2- to 3-fold) increases in size and cellularity of the spleen (increases are limited mostly to cells, both progenitor and precursor, of the erythroid lineage). However, in spite of the similar relative cellular increases, the increases of all these cell populations are significantly higher, in absolute cell numbers, in the mutant than in the wild-type mice. In conclusion, the GATA-1low mutation increases the magnitude of the response to erythroid stimuli as a consequence of the expansion of the erythroid progenitor cells in their spleen.
Introduction
GATA-1 is a member of a highly conserved family of transcription factors whose expression is restricted to the Sertoli cells in the testis and to hemopoietic cells.1 In the hemopoietic system, expression of GATA-1 is activated at the level of multilineage progenitor cells and is maintained in cells maturing toward all the myeloid lineages.1,2 In most of those lineages, GATA-1 expression decreases with maturation, with the exception of the erythroid lineage, in which its expression actually increases with progression toward differentiation.1 The important role of GATA-1 in the regulation of erythroid differentiation is strongly suggested by the fact that GATA-1 cognate sequences are present in the regulatory regions of all the erythroid genes identified to date,1 including the erythropoietin receptor (EpoR)3,4 and GATA-1 itself.5,6Furthermore, mice whose expression of either GATA-1,7 or its partner, Friend of GATA (Fog),8has been impaired by gene disruption, die prenatally owing to severe anemia.
How GATA-1 specifically regulates erythroid differentiation has been the subject of intensive investigation and is still unclear. Some evidence has suggested that the levels of GATA-1 expression are responsible for the establishment of this differentiation program. In fact, avian myelomonocytic cell lines that have been transfected with GATA-1 differentiate into hemopoietic cells whose phenotype is linked to the levels of GATA-1 ectopic expression. Only those cell lines that express the highest levels are erythroid.9 Similarly, when murine stem cells are transfected with a GATA-1–containing retrovirus and used to reconstitute hematopoiesis in sublethally irradiated mice, the mice engrafted with those cells express lower white blood cell counts and higher red blood cell counts than animals reconstituted with normal stem cells.10 Furthermore, the GATA-1–transduced stem cell recipients are partially resistant to induction of anemia by phlebotomy and, when they finally become anemic, recover faster than controls.10
To directly prove that the levels of GATA-1 expression determine erythroid differentiation, genetically modified mice lacking upstream regions have been generated by homologous recombination.11The mutants lacking the first enhancer (DNA hypersensitive site I) and the distal promoter are born both thrombocytopenic12 and anemic.13 In the case of one of these mutants, the GATA-1low (neoδHS) mouse, in which the DNA hypersensitive site I has been disrupted with a neo cassette, the few animals (fewer than 5%) that survive recover from their anemia at 3 to 4 weeks of age.13 The apparent lack of an erythroid phenotype in the few surviving adults is intriguing but could be the result of trivial reasons, eg, that only animals whose red cell precursors express, by in vivo selection, higher GATA-1 (or GATA-2) levels and/or whose erythroid progenitors are more sensitive to erythropoietin (EPO) survive until adulthood.
To clarify the mechanism of erythroid compensation in the adult GATA-1low mice, we have characterized their hemopoiesis under steady-state conditions (anatomic site of cell production, size and growth factor sensitivity of the progenitor cells, levels of GATA-1 and GATA-2 expression, and apoptosis within the erythroblast compartment, etc) and have measured their response to both acute (phenylhydrazine [PHZ]–induced anemia) and chronic (prolonged exposure to EPO) erythroid stimulation. The 2 stimuli operate through at least partially different mechanisms: the response to PHZ is mediated primarily by the glucocorticoid receptor,14 a nonspecific receptor involved in the response to stress that stimulates erythropoiesis by favoring proliferation over differentiation at the levels of late erythroid progenitors.15 On the other hand, EPO specifically induces production of red cells by promoting commitment, proliferation, and survival of erythroid cells of all types.16 The results presented indicate that the GATA-1low mice have a normal hematocrit thanks to a massive expansion of the early erythroid progenitors (colony-forming units–erythroid [CFU-E]) in the spleen that compensate for the increased apoptosis observed at the erythroblast level. Furthermore, because of such an expansion, they have an accelerated response to both acute and chronic erythroid stimuli, compared with their normal littermates.
Materials and methods
Mice
Two GATA-1low (neoδHS) mice13 (one female and one male of mixed C57 Bl/6-SV 129 background) were kindly provided for this study by Dr S. Orkin. The mice were crossed with CD1 mice (Charles River, Calco, Italy) to generate, according to standard genetic protocols, a GATA-1low colony that was kept at the animal facilities of the Istituto Superiore di Sanità. The mice were all genotyped by polymerase chain reaction (PCR) at birth for the absence of the deleted GATA-1 sequences (negative genotyping) and for the presence of the neo cassette (positive genotyping). Littermates whose genotype contained the GATA-1 region and did not contain the neo cassette were considered to be wild-type and used as negative controls. All the experiments were performed with sex- and age-matched mice under protocols approved by the institutional animal care committee.
In vivo stimulation of erythropoiesis
Anemia was induced with PHZ (60 mg/kg body weight) (Sigma Chemical, St Louis, MO) injected intraperitoneally for 2 consecutive days.17 18 On the first day after the second PHZ injection, mice were killed by cervical dislocation, and their bones and spleen removed under sterile conditions for further analysis. Polycythemia was induced with human recombinant EPO (10/U per mouse, corresponding to 300 U/kg body weight) (Dompè Biotech, Milan, Italy) injected subcutaneously for 5 consecutive days. After 6 days from the first injection, the animals were killed for further analysis.
Hematological blood parameters
Blood was collected from the retro-orbital plexus into EDTA-coated microcapillaries (20 to 40 μL per sampling). Hematocrit (Hct) and platelet counts were determined manually. Reticulocyte counts were done on smears of blood that had been stained with methylene blue according to standard protocols. At least 1000 red blood cells were counted in each determination.
Immunohistochemical analysis
Samples of spleen and bone marrow were routinely fixed in phosphate-buffered formalin (10%, vol/vol), paraffin embedded, and sectioned (2.5 to 3 μM) for hematoxilin-eosin staining and immunostaining with a GATA-1–specific antibody (N6) (Santa Cruz Biotechnology, CA). In some experiments, the spleens were quickly frozen in liquid nitrogen, and cryostated sections (3 μm) were labeled with 4A5 (a gift of Dr S. Burstein19). Immunohistochemical staining was performed according to the commercial 3-step alkaline phosphatase developing system (APAAP) (Dako, Carpinteria, CA) or with an indirect peroxidase method (Sigma).
Flow cytometry analysis
Marrow and spleen cells were suspended in Ca++-free, Mg++-free phosphate-buffered saline (PBS) supplemented with 1% (vol/vol) bovine serum albumin, 2 mM EDTA, and 0.1% NaN3 and labeled with the erythroid-specific phycoerythrin (PE)–conjugated TER-11920 (Ly-76) (Pharmingen, San Diego, CA) monoclonal antibody and the megakaryocytic-specific fluorescein isothiocyanate (FITC)–conjugated 4A5 (approximately 1 μg/106 cells) antibody for 30 minutes on ice. The cell fluorescence was analyzed with the FACScan flow cytometer (Becton Dickinson, San Jose, CA). Cells incubated with appropriately labeled isotype controls (Pharmingen) were used to gate nonspecific fluorescence signal. Mature red cells were depleted by hypotonic lysis (0.87% ammonium chloride for 15 minutes on ice), and dead cells were excluded by propidium iodide staining (5 μg/mL) (Sigma).
Progenitor cell counts
The frequency of progenitor cells in the light-density (fewer than 0.080) fractions (0.25 to 1.0 × 105 cells per plate) of marrow, isolated from either normal or GATA-1lowmice as described,21 was determined in standard methylcellulose culture (0.9% wt/vol) in the presence of fetal bovine serum (30% vol/vol) (Sigma) and of a combination of recombinant growth factors, including rat stem cell factor (SCF) (100 ng/mL), mouse interleukin 3 (10 ng/mL) (both from Sigma), and either human EPO (2 U/mL) (Boehringer Mannheim, Germany) for burst-forming unit–erythroid (BFU-E) growth or mouse granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (50 ng/mL each) for granulocyte-macrophage colony-forming unit (CFU-GM) growth (G-CSF and GM-CSF were purchased from Sigma).21 The growth of CFU-E–derived colonies was stimulated with EPO alone (2 U/mL).22 The cultures were incubated at 37°C in a humidified incubator containing 5% CO2 in air and scored either 3 days (for CFU-E–derived colonies) or 7 days (for CFU-GM– and BFU-E–derived colonies) following initiation of culture.
RNA isolation and semiquantitative reverse transcriptase PCR analysis
Total RNA was prepared with a commercial guanidine thiocyanate/phenol method (Trizol) (Gibco BRL, Paisley, United Kingdom) as described by the manufacturer. Glycogen (20 μg) (Boehringer Mannheim) was added to each sample as a carrier. Total RNA (1 μg) was reverse transcribed at 42°C for 30 minutes in 20 μL of 10 mM Tris-HCl, pH 8.3, containing 5 mM MgCl2, 1 U RNAse inhibitor, 2.5 U Moloney murine leukemia virus reverse-transcriptase, and 2.5 μM random hexamers (all from Perkin-Elmer, Norwalk, CT). The expression of β-globin and of the total, proximal, and distal GATA-1 transcripts was analyzed by amplifying reverse-transcribed complementary DNA (cDNA) (2.5 μL) in the presence of the specific sense and antisense primers (100 nM each) described elsewhere.23 The following primers were used for the amplification of GATA-2: sense 5′TGCAA-CACACCACCCGATACC3′, antisense 5′CAATTTGCACAACAGGTGCCC3′. These primers generated an expected amplification fragment of 336 base pairs. The reaction was performed in 100 μL of 10 mM Tris-HCl, pH 8.3, containing MgCl2 (2 mM), dNTP (200 μM each), 0.1 μCi [α32P]–deoxycytidine triphosphate (specific activity 3000 Ci/mmol) (Amersham Italia, Cologno Monzese, Italy), and 2 U AmpliTaq DNA polymerase. Primers specific for β2-microglobulin (50 nM each) were added to each amplification after the first 10 cycles as a control for the amount of cDNA used in the reaction.23 PCR conditions were as follows: 60 seconds at 95°C, 60 seconds at 60°C, and 60 seconds at 72°C. All of the reactions were done by means of a GeneAmp 2400 Perkin-Elmer thermocycler and were analyzed in the linear range of amplification defined by preliminary experiments to be between 20 and 35 cycles for GATA-1 and GATA-2; 32 and 38 cycles for the distal and proximal GATA-1 transcripts; 18 and 24 cycles for β-globin; and 20 and 30 cycles for β2-microglobulin. Positive (RNA from adult marrow) and negative (mock cDNA) controls were included in each experiment. Aliquots (20 μL) were removed from the PCR mixture after amplification, and the amplified bands separated by electrophoresis on 4% polyacrylamide gel. Gels were dried by means of a Biorad (Hercules, CA) apparatus and exposed to Hyperfilm-MP (Amersham) for 2 hours at −70°C. All procedures were done according to standard protocols.24
Purification of erythroblast precursors and terminal deoxy transferase uridine triphosphate nick end labeling reaction
Erythroid precursors (TER-119+ cells) were purified from the spleens of wild-type and GATA-1low mice by immunoselection on magnetic beads (Miltenyi Biotec, Bologna, Italy) coated with the TER-119 antibody as described.26 The cells were either lysed in Trizol for gene expression analysis or cytocentrifuged for the detection of apoptotic cells by terminal deoxy transferase uridine triphosphate nich end-labeling (TUNEL). In this last case, the cytospin preparations were fixed with paraformaldehyde (4% vol/vol in PBS, pH = 7.4) for 30 minutes at room temperature and incubated in a permeabilizing solution (0.1% Triton, 0.1% sodium citrate) for 2 minutes on ice. The DNA strand breaks that are characteristic of apoptotic cells were identified by labeling the free 3′-OH nucleotide termini with fluorescein–deoxyuridine triphosphate with the In Situ Cell Death Detection Kit (Boehringer Mannheim) as described by the manufacturer. The cells were counterstained with propidium iodide, mounted in glycerol, and analyzed under a fluorescent microscope (Leica Microscopy System, Heidelberg, Germany).
Statistical analysis
Statistical analysis was performed by analysis of variance by means of Origin 3.5 software for Windows (Microcal Software, Northampton, MA).
Results
Comparative analysis of the erythroid compartments in adult GATA-1low and wild-type mice
The general hemopoietic features of adult (6 to 12 months of age) GATA-1low and wild-type mice are compared in Table1 and Figure1. As already reported,11,12the blood of adult GATA-1low mice contained normal levels of red (Hct) and white cells but contained 10-fold fewer platelets than controls (P < .001) (Table 1). Furthermore, the few platelets detectable on the blood smears from GATA-1lowmice appeared morphologically abnormal and bigger than normal platelets25 (also in data not shown).
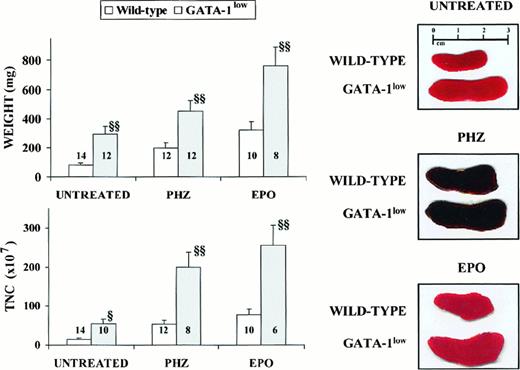
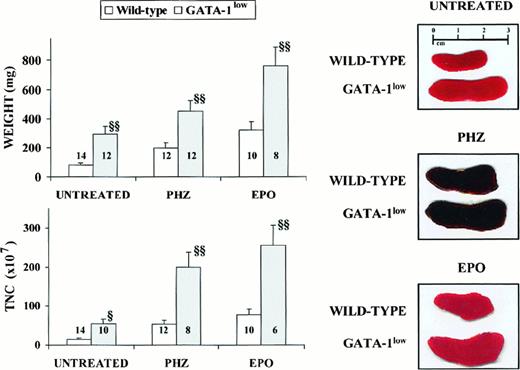
General features of the spleens from adult (6 to 12 months of age) GATA-1low and wild-type mice.
Photographic picture (on the right), weight (top on the left) and total number of cells (TNC, bottom on the left) of spleens from untreated (intact), day-3 PHZ-treated, and day-6 EPO-treated wild-type (white bars) and GATA-1low (gray bars) mice. The values are reported as the mean ( ± SD) of replicate measures. The number on each bar indicates the number of mice analyzed for that experimental group. The values observed in the GATA-1low mice are statistically different from those observed in the corresponding wild-type animals. §P < .05; §§P < .01.
General features of the spleens from adult (6 to 12 months of age) GATA-1low and wild-type mice.
Photographic picture (on the right), weight (top on the left) and total number of cells (TNC, bottom on the left) of spleens from untreated (intact), day-3 PHZ-treated, and day-6 EPO-treated wild-type (white bars) and GATA-1low (gray bars) mice. The values are reported as the mean ( ± SD) of replicate measures. The number on each bar indicates the number of mice analyzed for that experimental group. The values observed in the GATA-1low mice are statistically different from those observed in the corresponding wild-type animals. §P < .05; §§P < .01.
The cellularity of the marrow and spleen from the GATA-1lowanimals was profoundly different from that of the controls: the marrow from the mutants contained 3 times fewer cells, whereas their spleen contained 3 times more cells than the corresponding tissues from the normal littermates (Table 1). The abnormal size, weight, and cellularity of the spleen from the mutants are also evident from the data presented in Figure 1.
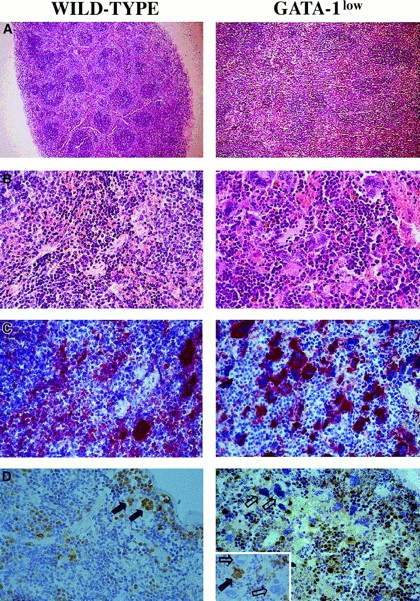
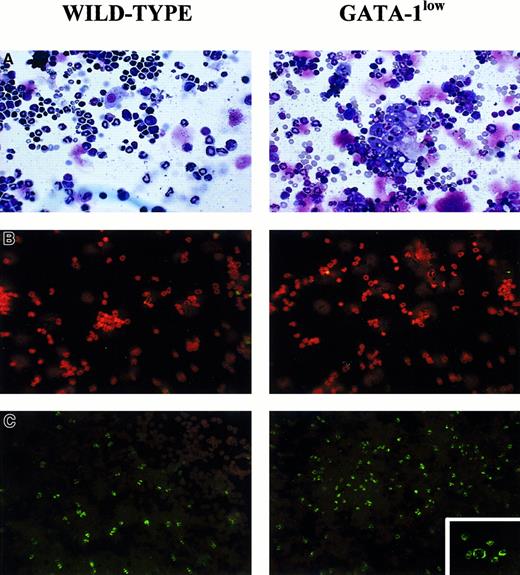
May-Grünwald staining of cytospin marrow preparations showed that the tissue from the mutant mouse contains larger-than-normal red cells and cells with clear morphological signs of apoptosis (see the picnotic nuclei in the erythroblasts in Figure 2). The higher percentage of apoptotic cells in the GATA-1lowmarrow preparations was confirmed by TUNEL staining (Figure 2C): TUNEL-positive cells are 3 times more frequent (18% vs 5% of the cells labeled by propidium iodide) in cytocentrifuged preparations from GATA-1low mice than from the normal littermates. By fluorescence-activated cell sorting (FACS) analysis (not shown), the marrow from the GATA-1low mice contained slightly higher percentages of megakaryocytic (1.8% ± 0.4% vs 0.4% ± 0.1% 4A5+ cells) and bipotent (1.5% ± 0.5% vs 0.7% ± 0.2% 4A5+/TER-119+ cells) precursors and similar percentages of erythroid precursors (11% ± 2.8% vs 12% ± 3.3% TER-119+ cells) than the marrow from the controls. However, because of the poor cellularity of the marrow from the mutants (Table 1), its overall erythroid precursor cell content was lower than that of the wild-type marrow (Table 2).
Staining of cytocentrifuged preparations of marrow cells harvested from GATA-1low (right) and normal (left) littermates.
(A) May-Grünwald staining; 25 × magnification. (B) Propidium iodide staining (negative controls); 25 × magnification. (C) TUNEL staining; 25 × magnification except for the small insert of the GATA-1low mice, which is 40 × to show the prevalent perinuclear localization of the DNA breaks in the TUNEL-positive cells.
Staining of cytocentrifuged preparations of marrow cells harvested from GATA-1low (right) and normal (left) littermates.
(A) May-Grünwald staining; 25 × magnification. (B) Propidium iodide staining (negative controls); 25 × magnification. (C) TUNEL staining; 25 × magnification except for the small insert of the GATA-1low mice, which is 40 × to show the prevalent perinuclear localization of the DNA breaks in the TUNEL-positive cells.
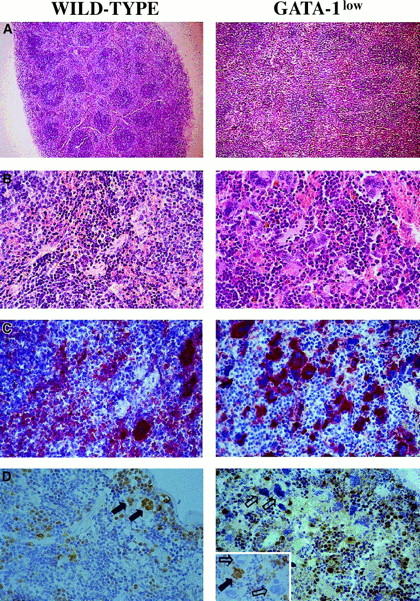
The increased cellularity of the spleen from the GATA-1lowmice is reflected by deep alterations in its whole structural organization (Figure 3A,B): the interfollicular space of the red pulp was filled with abnormally large and dysplastic megakaryocytes recognized by the 4A5 antibody (Figure3B,C). The spleens' red pulp and subcapsulary space was filled with lymphocyte-sized cells resembling immature erythroid cells (Figure3A,B) that could not be positively identified as erythroid in these sections because the TER-119 antibody reacted poorly in immunohistochemistry (data not shown). The anti–GATA-1 antibody labeled all of the erythroidlike blasts but failed to recognize the megakaryocytes within the spleens of the GATA-1low animals (Figure 3D). Interestingly, it labeled all the megakaryocytes present in the spleens of normal mice but only a portion (variable from specimen to specimen) of the megakaryocytes in the splenic sections from heterozygote females25 (Figure 3D, insert).
Immunohistochemical analysis of spleens obtained from wild-type (left panels) and GATA-1low (right panels) mice.
(A) (B) Hematoxilin-eosin staining of paraffin-embedded sections; 10 × and 40 × magnification. (C) (D) 4A5-specific immunostaining (panel C). GATA-1–specific immunostaining (panel D). The black and white arrows identify representative GATA-1+ or GATA-1− megakaryocytes, respectively, while the small arrows identify representative GATA-1+ erythroblasts. The small insert in the GATA-1low panel shows the GATA-1 staining of a splenic section from a representative heterozygote female, as a control. There is 40 × magnification in all cases.
Immunohistochemical analysis of spleens obtained from wild-type (left panels) and GATA-1low (right panels) mice.
(A) (B) Hematoxilin-eosin staining of paraffin-embedded sections; 10 × and 40 × magnification. (C) (D) 4A5-specific immunostaining (panel C). GATA-1–specific immunostaining (panel D). The black and white arrows identify representative GATA-1+ or GATA-1− megakaryocytes, respectively, while the small arrows identify representative GATA-1+ erythroblasts. The small insert in the GATA-1low panel shows the GATA-1 staining of a splenic section from a representative heterozygote female, as a control. There is 40 × magnification in all cases.
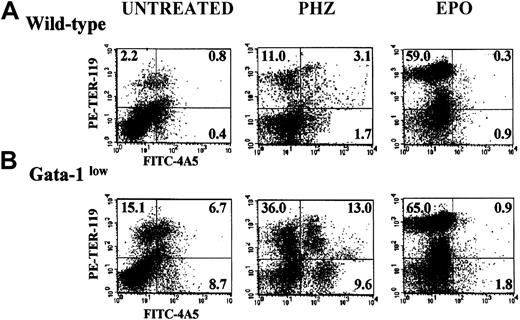
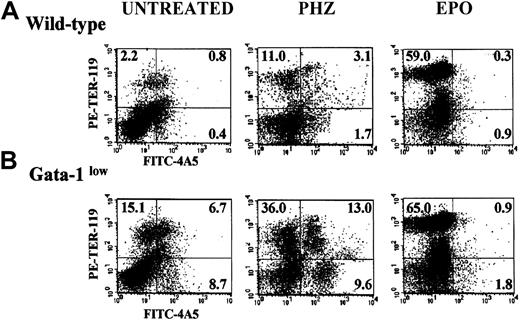
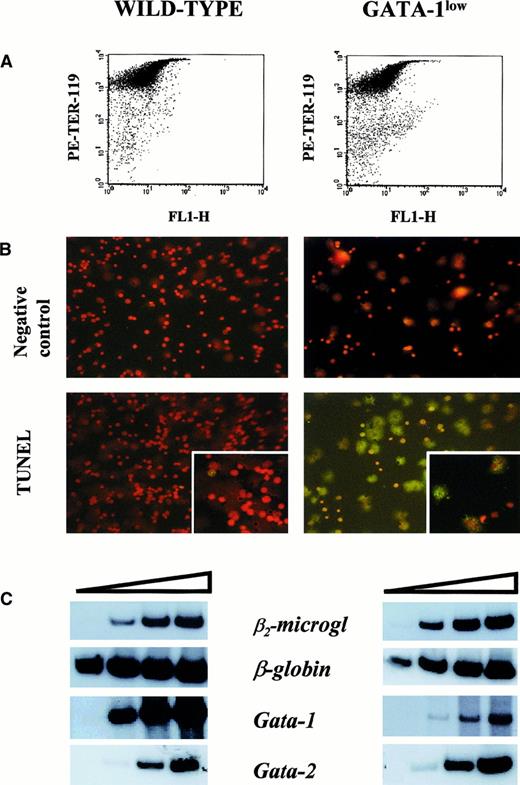
The FACS analyses of the erythroid and megakaryocytic precursor cell content of the spleens from GATA-1low and normal mice are compared in Figure 4. The spleens of the GATA-1low mice contained significantly higher numbers (7- to 20-fold, P < .01) of megakaryocytic (single 4A5+), erythroid (single TER-119+), and bipotent (erythroid and megakaryocytic, 4A5+/TER-119+)26 precursors than the spleens from normal mice (Figure 4).
Expression of TER-119 and 4A5 in the light-density cells of the spleen.
(A) (B) Representative flow cytometric analysis for the expression of TER-119 (y-axes) and 4A5 (x-axes) in the light-density cells of the spleens from untreated (steady-state), day-1 PHZ-treated, and day-6 EPO-treated wild-type (panel A, top panels) and GATA-1low(panel B, bottom panels) mice. The numbers in the quadrants of each dot plot indicate the frequency of cells with the corresponding phenotype. Similar results were obtained in 3 additional experiments. Each experiment included negative controls represented by PE- and FITC-conjugated isotype-matched irrelevant antibodies, which are not presented because they are identical to those already published.26
Expression of TER-119 and 4A5 in the light-density cells of the spleen.
(A) (B) Representative flow cytometric analysis for the expression of TER-119 (y-axes) and 4A5 (x-axes) in the light-density cells of the spleens from untreated (steady-state), day-1 PHZ-treated, and day-6 EPO-treated wild-type (panel A, top panels) and GATA-1low(panel B, bottom panels) mice. The numbers in the quadrants of each dot plot indicate the frequency of cells with the corresponding phenotype. Similar results were obtained in 3 additional experiments. Each experiment included negative controls represented by PE- and FITC-conjugated isotype-matched irrelevant antibodies, which are not presented because they are identical to those already published.26
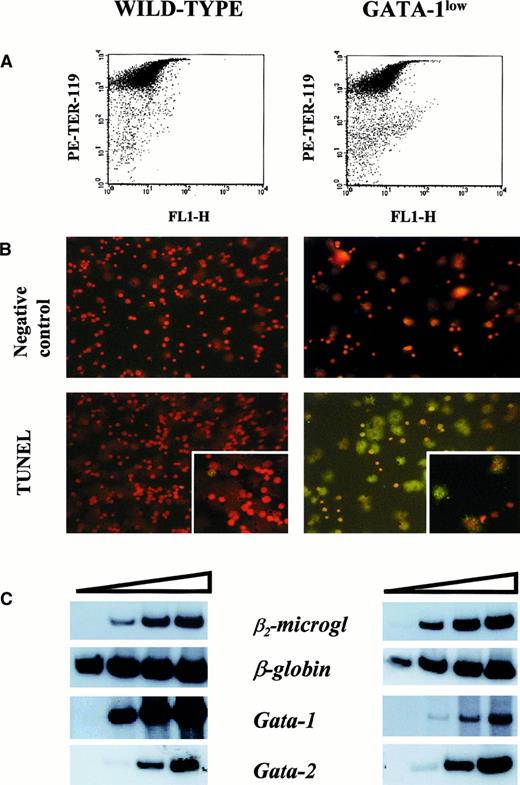
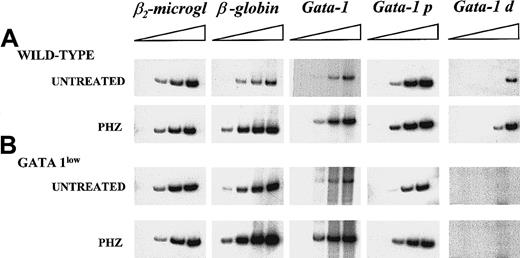
The higher erythroid cell content of the spleen from the GATA-1low mice was also reflected by the levels of expression of the β-globin gene in this tissue (Figure5). The β-globin transcripts were detectable after only 15 cycles of reverse transcriptase (RT) PCR by means of cDNA prepared from GATA-1low spleen, while at least 18 cycles were necessary to begin the detection of these fragments by means of cDNA from normal spleen. In contrast, GATA-1 transcripts were amplified with comparable kinetics by means of cDNA prepared from the spleens of either one of these animals. The only cells expressing GATA-1 in the GATA-1low spleens are the erythroid cells (Figure 3). Therefore, the fact that the spleens from the GATA-1low mice, in spite of their higher erythroid cell content (by morphological, FACS, and β-globin gene expression analysis), expressed levels of GATA-1 similar to those expressed by the spleens from the wild-type animals, suggests that the GATA-1 expression per cell is lower in GATA-1low erythroblasts than in the normal ones. To clarify this point, TER-119+ cells were purified (higher than 95% pure) by immunomagnetic selection from the spleens of the mutant and normal littermates (Figure6A), and gene expression was analyzed by RT-PCR (Figure 6C). The GATA-1low TER-119+cells expressed comparable levels of β-globin, GATA-2, and β2-microglobulin, but lower levels of GATA-1 than their normal counterparts. The purified cells were also used to determine the percentages of apoptotic cells at the erythroblast level by TUNEL (Figure 6B). TUNEL-positive nuclei were very rare among normal TER-119+ cells (2% or lower) but represented greater than 60% of the cells purified from the GATA-1low spleens.
Semiquantitative RT-PCR analysis of the expression of β2-microglobulin (as control), of β-globin, and of the total (GATA-1), proximal (GATA-1p), and distal (GATA-1d) GATA-1 transcripts in the spleens of untreated or day-1 PHZ-treated wild-type and GATA-1low mice.
Each product was analyzed for increasing numbers of cycles as indicated by a triangle on the top of the panels. In particular, the products were analyzed after 18, 21, 24, and 27 cycles for β2-microglobulin; 15, 18, 21, and 24 cycles for β-globin; 20, 24, 26, and 30 cycles for the total GATA-1 transcripts; and 20, 25, 30, and 35 cycles for the GATA-1p and GATA-1d transcripts. The failure of the amplification of transcripts originating from the distal (or testis) GATA-1 promoter28 with the use of cDNA prepared from the GATA-1low spleens represents an indirect control of the genotype of the mutant mice analyzed in this study. Similar results were obtained in 3 additional experiments.
Semiquantitative RT-PCR analysis of the expression of β2-microglobulin (as control), of β-globin, and of the total (GATA-1), proximal (GATA-1p), and distal (GATA-1d) GATA-1 transcripts in the spleens of untreated or day-1 PHZ-treated wild-type and GATA-1low mice.
Each product was analyzed for increasing numbers of cycles as indicated by a triangle on the top of the panels. In particular, the products were analyzed after 18, 21, 24, and 27 cycles for β2-microglobulin; 15, 18, 21, and 24 cycles for β-globin; 20, 24, 26, and 30 cycles for the total GATA-1 transcripts; and 20, 25, 30, and 35 cycles for the GATA-1p and GATA-1d transcripts. The failure of the amplification of transcripts originating from the distal (or testis) GATA-1 promoter28 with the use of cDNA prepared from the GATA-1low spleens represents an indirect control of the genotype of the mutant mice analyzed in this study. Similar results were obtained in 3 additional experiments.
Apoptosis and gene expression of TER-119+ cells.
(A) Forward-light scatter (FL1-H) vs PE-TER-119 staining of single TER-119+ cells purified from wild-type and GATA-1low mice by immunomagnetic adsorption. The purity of the TER-119+ cells was 97% and 95%, respectively. (B) Propidium iodide (top) and propidium iodide–TUNEL double staining (bottom) of the TER-119+ cells purified in panel A (25 × magnification except for the small quadrants in the bottom panel, which are 40 × magnification to show the details of the apoptotic nuclei). (C) Semiquantitative RT-PCR analysis of the expression of β2-microglobulin (as control), β-globin, GATA-1, and GATA-2 in the TER-119+ cells purified from the spleens of wild-type (left) and GATA-1low (right) mice. Each product was analyzed for increasing numbers of cycles as indicated by a triangle on the top of the panels. In particular, the products were analyzed after 18, 21, 24, and 27 cycles for β2-microglobulin; 15, 18, 21, and 24 cycles for β-globin; and 20, 24, 26, and 30 cycles for the total GATA-1 and GATA-2 transcripts.
Apoptosis and gene expression of TER-119+ cells.
(A) Forward-light scatter (FL1-H) vs PE-TER-119 staining of single TER-119+ cells purified from wild-type and GATA-1low mice by immunomagnetic adsorption. The purity of the TER-119+ cells was 97% and 95%, respectively. (B) Propidium iodide (top) and propidium iodide–TUNEL double staining (bottom) of the TER-119+ cells purified in panel A (25 × magnification except for the small quadrants in the bottom panel, which are 40 × magnification to show the details of the apoptotic nuclei). (C) Semiquantitative RT-PCR analysis of the expression of β2-microglobulin (as control), β-globin, GATA-1, and GATA-2 in the TER-119+ cells purified from the spleens of wild-type (left) and GATA-1low (right) mice. Each product was analyzed for increasing numbers of cycles as indicated by a triangle on the top of the panels. In particular, the products were analyzed after 18, 21, 24, and 27 cycles for β2-microglobulin; 15, 18, 21, and 24 cycles for β-globin; and 20, 24, 26, and 30 cycles for the total GATA-1 and GATA-2 transcripts.
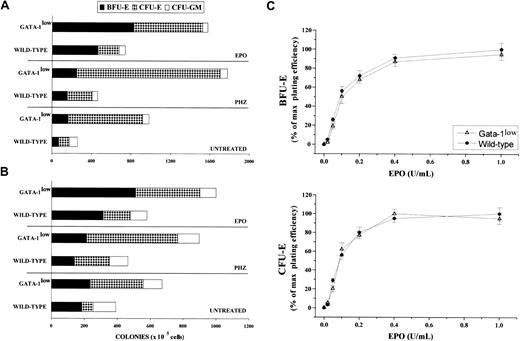
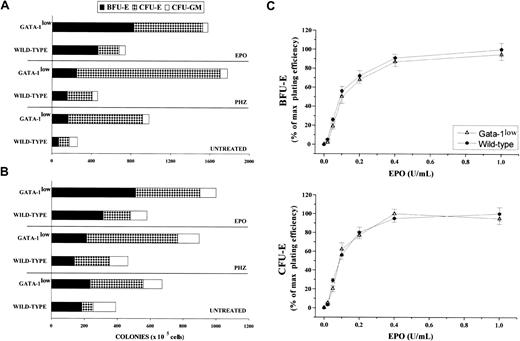
The frequency of progenitor cells in the spleen and marrow from normal and GATA-1low mice is compared in Figure7. The tissues of the GATA-1low mice displayed frequencies of myelomonocytic progenitors (CFU-GM) similar to those found in the corresponding tissues from their normal counterparts. In contrast, they contained 2-fold more early (BFU-E, P < .05) and 5- to 7-fold more late (CFU-E, P < .001) erythroid progenitor cells than the normal tissues.
Frequency of progenitor cells in the spleen and marrow from normal and GATA-1low mice.
(A) (B) Frequency of progenitor cells (BFU-E, dark bars; CFU-E, dotted bars; and CFU-GM, white bars) in the spleen (panel A) and the bone marrow (panel B) of untreated (steady-state), day-1 PHZ-treated, and day-6 EPO-treated wild-type and GATA-1low mice. The results are presented as the mean ( ± SD) of 4 separate experiments. Four wild-type and 4 GATA-1low mice were analyzed for each experimental point (4 untreated, 4 EPO-treated, and 4 PHZ-treated animals for a total of 12 wild-type and 12 GATA-1lowmice). (C) EPO concentration/response curve on the growth of CFU-E–derived (bottom) and BFU-E–derived (top) colonies in serum-deprived cultures of light-density spleen cells from wild-type (closed circles) and GATA-1low (open triangles) mice. The results are presented as a percentage of the maximal colony growth and are the mean ( ± SD) of 4 separate experiments. Maximal colony efficiency (100%) was as follows: wild-type BFU-E = 68 ± 22; wild-type CFU-E = 110 ± 30; GATA-1lowBFU-E = 166 ± 30; GATA-1low CFU-E = 750 ± 175. Identical EPO concentration/response curves were observed in cultures of marrow cells (not shown).
Frequency of progenitor cells in the spleen and marrow from normal and GATA-1low mice.
(A) (B) Frequency of progenitor cells (BFU-E, dark bars; CFU-E, dotted bars; and CFU-GM, white bars) in the spleen (panel A) and the bone marrow (panel B) of untreated (steady-state), day-1 PHZ-treated, and day-6 EPO-treated wild-type and GATA-1low mice. The results are presented as the mean ( ± SD) of 4 separate experiments. Four wild-type and 4 GATA-1low mice were analyzed for each experimental point (4 untreated, 4 EPO-treated, and 4 PHZ-treated animals for a total of 12 wild-type and 12 GATA-1lowmice). (C) EPO concentration/response curve on the growth of CFU-E–derived (bottom) and BFU-E–derived (top) colonies in serum-deprived cultures of light-density spleen cells from wild-type (closed circles) and GATA-1low (open triangles) mice. The results are presented as a percentage of the maximal colony growth and are the mean ( ± SD) of 4 separate experiments. Maximal colony efficiency (100%) was as follows: wild-type BFU-E = 68 ± 22; wild-type CFU-E = 110 ± 30; GATA-1lowBFU-E = 166 ± 30; GATA-1low CFU-E = 750 ± 175. Identical EPO concentration/response curves were observed in cultures of marrow cells (not shown).
To clarify the reason for the higher erythroid progenitor cell content in the tissues from the GATA-1low mice, we compared the effect of increasing EPO concentrations on the growth of erythroid colonies from the mutant or normal marrow and spleen cells in semisolid cultures (Figure 7C). The EPO growth/response curves for BFU-E– and CFU-E–derived colonies obtained in cultures of GATA-1lowand wild-type spleen cells were identical. In both cases, 50% of maximal colony growth was sustained by a concentration of 0.1 U/mL EPO, and maximal colony formation was observed with 0.4 EPO U/mL.
Response of the GATA-1low mice to in vivo stimulation of erythropoiesis
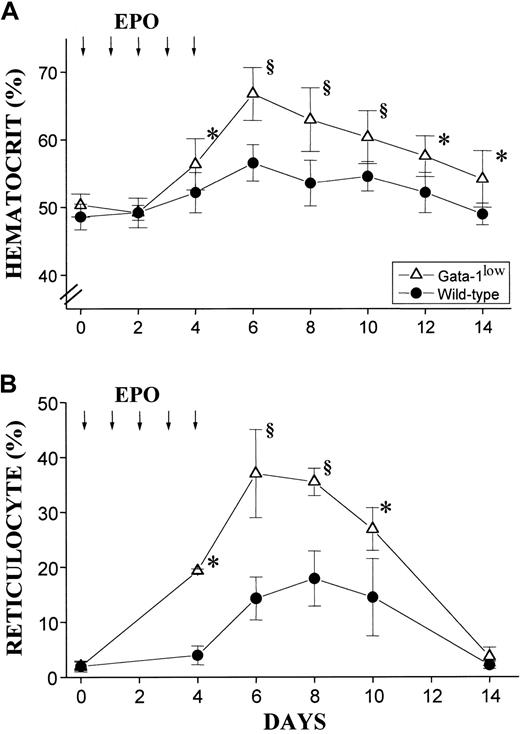
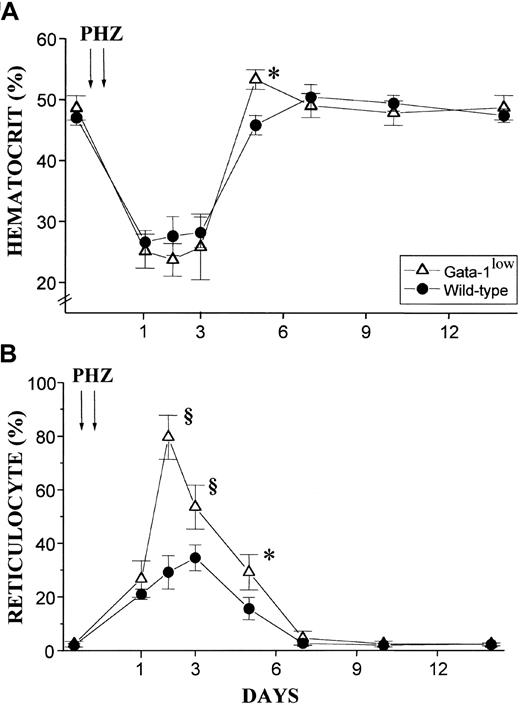
The red cell recovery after PHZ treatment of normal and GATA-1low animals is compared in Figure8. At 5 days after the PHZ treatment, the Hct of GATA-1low mice had exceeded .5 proportion of 1.0 (50%) (higher than the prebleeding level) while the wild-type mice had mostly, but not completely, recovered from their anemia17 (Figure 8A). Furthermore, at day 2 of recovery from the anemia, reticulocytes represented greater than 80% of the total red cells in the blood from the GATA-1low mice, as compared with fewer than 30% found in blood from the corresponding controls (Figure 8B). In the blood of the mutant mice, the percentages of reticulocytes remained significantly higher than in the blood of the controls until day 5 after PHZ treatment (Figure 8B).
Hematocrit and reticulocyte counts after PHZ treatment of normal and GATA-1low animals.
(A) (B) Hematocrit (panel A) and reticulocyte counts (in percentage of total red cells) (panel B) in the blood of wild-type (closed circles) and GATA-1low (open triangles) mice during recovery from anemia induced by PHZ (2 consecutive injections as indicated by the arrows). The day of the second PHZ injection was considered to be day 0. The results are presented as the mean ( ± SD) of the values observed in 18 wild-type and 12 GATA-1low mice. Values are statistically different. *P < .05. §P < .01.
Hematocrit and reticulocyte counts after PHZ treatment of normal and GATA-1low animals.
(A) (B) Hematocrit (panel A) and reticulocyte counts (in percentage of total red cells) (panel B) in the blood of wild-type (closed circles) and GATA-1low (open triangles) mice during recovery from anemia induced by PHZ (2 consecutive injections as indicated by the arrows). The day of the second PHZ injection was considered to be day 0. The results are presented as the mean ( ± SD) of the values observed in 18 wild-type and 12 GATA-1low mice. Values are statistically different. *P < .05. §P < .01.
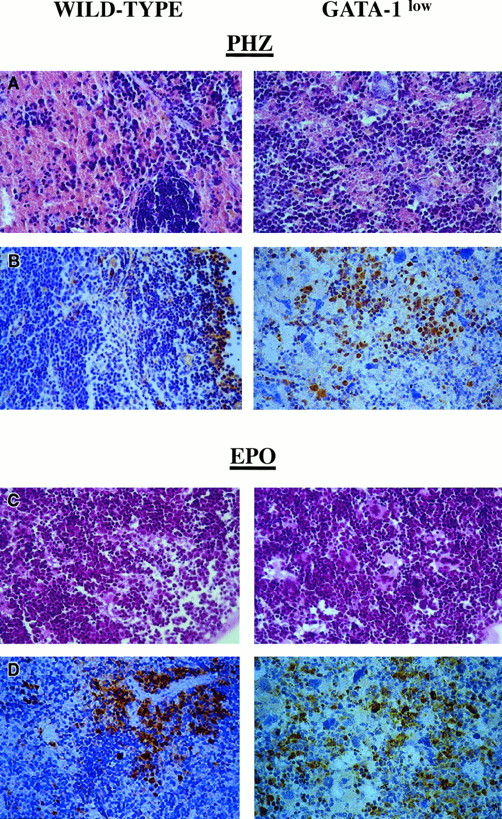
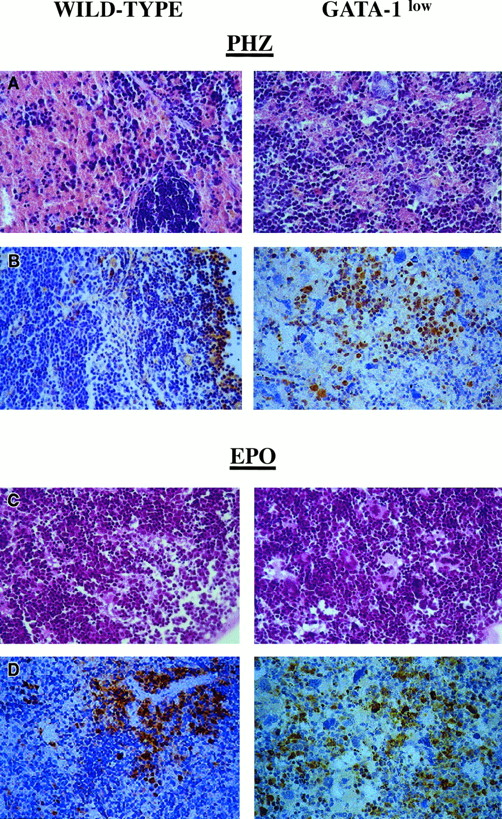
Although the relative increases in size (by 2-fold) and cellularity (by 3.5-fold) of the spleens from the GATA-1low and wild-type mice were similar, the spleens of the PHZ-treated mutants were significantly (P < .01) bigger and contained more cells than their PHZ-treated controls (Figure 1). At day 1 from the PHZ treatment, the spleens from both GATA-1low and wild-type animals were engulfed with erythroblasts expressing GATA-1 by immunostaining (Figure 7). The sinusoid spaces were markedly enlarged owing to the presence of red cells in the process of being lysed locally after having been damaged by PHZ (Figure9). The presence of this massive red cell lysis was also evident by the dark red color of these spleens in gross morphological examination (Figure 1).
Immunohistochemical analysis of spleens obtained from day-1 PHZ-treated (top panels) or day-6 EPO-treated (bottom panels) wild-type and GATA-1low mice, as indicated.
Hematoxilin-eosin staining and anti–GATA-1–specific immunostaining are presented in the left and right panels, respectively (40 × magnification).
Immunohistochemical analysis of spleens obtained from day-1 PHZ-treated (top panels) or day-6 EPO-treated (bottom panels) wild-type and GATA-1low mice, as indicated.
Hematoxilin-eosin staining and anti–GATA-1–specific immunostaining are presented in the left and right panels, respectively (40 × magnification).
At day 1, the PHZ treatment increased the frequency of erythroid (TER-119+), megakaryocytic (4A5+), and bipotent (TER-119+/4A5+) precursors, not only in the spleens of the wild-type mice as reported,26 but also in the spleens of the mutant animals (Figure 4). The spleens of the GATA-1low mice contained 2 to 3 times (P < .001) more erythroid/megakaryocytic precursors than the spleens from the wild-type mice although, in comparison with the corresponding baseline levels, the increases observed in the spleens of the normal mice were 2- to 3-fold higher than those observed in the GATA-1low mice (Figure 4).
The increases of the erythroid precursors in the spleens of PHZ-treated animals were paralleled by increases in the expression of β-globin and GATA-1, as determined by the kinetics of the RT-PCR amplification by means of cDNA prepared from the spleens of PHZ-treated GATA-1low and wild-type animals (Figure 5).
At day 1 of PHZ treatment, the frequency of late erythroid (CFU-E) progenitor cells, but not those of early erythroid (BFU-E) or myelomonocytic (CFU-GM) progenitors, increased in the marrow and spleen from the mutants as well as from the wild-type mice (Figure 7A,B). Particularly high was the frequency reached by the CFU-E in the hemopoietic tissues of the GATA-1low mice (greater than 600 and 1300 colonies per 105 marrow and spleen cells, respectively).
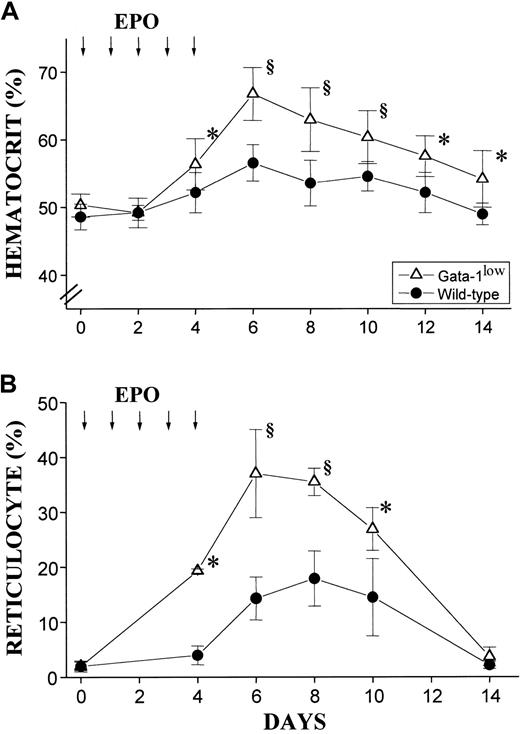
In vivo administration of EPO (10 U per mouse for 5 days) induced a statistically significant rise in the Hct of both the mutant and the normal mice (Figure 10A). The peak of the Hct increase was reached 6 days after the first EPO injection. In the case of the mutant mice, the Hct increased sooner (the first statistically different Hct value was observed at day 4 instead of day 6); more (up to .67 ± .05 proportion of 1.0 [67% ± 4.9%] as compared with .57 ± .03 proportion of 1.0 [56.5% ± 3.4%],P < .001); and for a longer period of time (until day 14 instead of day 12) than the Hct increases observed in their normal littermates (Figure 10A).
Hematocrit and reticulocyte counts in wild-type and GATA-1low mice during the EPO-induced polycythemia.
(A) (B) Hematocrit (panel A) and reticulocyte counts (in percentage of total red cells) (panel B) in the blood of wild-type (closed circles) and GATA-1low (open triangles) mice. Polycythemia was induced with 5 consecutive EPO intraperitoneal injections, as indicated by the arrows. The day of the first EPO injection was considered to be day 0. The results are presented as the mean ( ± SD) of the values observed in 12 wild-type and 8 GATA-1low mice. Values are statistically different. *P < .05. §P < .01.
Hematocrit and reticulocyte counts in wild-type and GATA-1low mice during the EPO-induced polycythemia.
(A) (B) Hematocrit (panel A) and reticulocyte counts (in percentage of total red cells) (panel B) in the blood of wild-type (closed circles) and GATA-1low (open triangles) mice. Polycythemia was induced with 5 consecutive EPO intraperitoneal injections, as indicated by the arrows. The day of the first EPO injection was considered to be day 0. The results are presented as the mean ( ± SD) of the values observed in 12 wild-type and 8 GATA-1low mice. Values are statistically different. *P < .05. §P < .01.
The changes in Hct values were reflected by symmetrical increases in the percentage of circulating reticulocytes that were also higher in the blood of the mutants than in the blood of normal mice. At day 6 of the EPO treatment, reticulocytes represented up to 40% of the circulating red cells in the GATA-1low mice and only 20% of the red blood cells in the wild-type mice (Figure 10B).
The cellular compartments in the marrow and spleen of some of the animals treated with EPO were analyzed on day 6 of the treatment (ie, when the highest Hct and reticulocyte increases had been observed). The spleens of both the mutant and the normal mice became enormously enlarged after EPO treatment and reached a weight of 780 ± 50 mg in the GATA-1low mice and 365 ± 38 mg in the wild-type animals (Figure 1). The total nucleated cells per organ increased accordingly, and up to 2.8 ± 0.3 × 109 cells were detected per spleen in the GATA-1low mice. The immunohistochemistry revealed massive spleen infiltration of GATA-1+ erythroblasts at all stages of maturation that disrupted all the architecture of the organ both in the wild-type and in the GATA-1low mice (Figure 9). After EPO treatment, the spleens of the GATA-1low mice still contained a relatively high number of GATA-1− megakaryocytes dispersed among the vast areas containing maturing erythroid cells (Figure 9).
The results of the histologic analysis were confirmed by FACS analysis (Figure 3). At day 6 post–EPO treatment, the frequency of single TER-119+ cells increased up to 59.0% in the normal spleen and to 65% in the spleen of the GATA-1low mice (P < .01). The percentage of single 4A5+ and of double TER-119+/4A5+ cells decreased after EPO stimulation both in the mutant and in the wild-type mice (Figure3), although when total spleen cellularity is taken into account, their total numbers either increased or remained constant (Table3).
In contrast with the PHZ treatment, which increased the frequency of only CFU-E, EPO induced a significant rise in the frequency of both early (BFU-E) and late (CFU-E) erythroid progenitor cells in the marrow and spleen from the mutant and the normal mice (Figure 7). Once again, in absolute numbers, the increases in the erythroid progenitors in the mutant mice were statistically higher than those observed in the wild-type mice (Figure 7). No changes were detected after EPO treatment in the frequencies of myeloid progenitor cells (Figure 7).
That the frequency of early (BFU-E) erythroid progenitors increased after EPO stimulation but not after PHZ stimulation (Figure 7) while the percentage of bipotent (erythroid/megakaryocytic) precursor cells increased after PHZ stimulation but decreased after EPO stimulation (Figure 3) supports the notion that at least partially different mechanisms are responsible for the erythroid stimulation exerted by EPO and PHZ.
Discussion
We show that the phenotype of the GATA-1low mutants that survive until adulthood involves not only, as reported,12,25 accumulation of dysplastic megakaryocytes arrested at terminal stages of differentiation but also (1) massive expansion of erythroid progenitors (BFU-E and CFU-E) (Figure 7A,B) and bipotent (erythroid/megakaryocytic) precursors (Figure 4) and (2) the transition of the major hemopoietic site from the marrow to the spleen (Tables 2, 3). Because of their lower levels of GATA-1 expression, the GATA-1low erythroblasts should have an increased apoptotic rate.29 This suggests to us that the progenitor cell expansion is the reason the Hct levels in the adult mutants are normal and have an accelerated response to both acute (PHZ treatment) (Figure8) and chronic (EPO administration) (Figure 10) erythroid stimulation. In fact, normal adult mice produce a constant number (64) of reticulocytes for every CFU-E, most of which (90%) egress and mature into red cells in the blood where they circulate with a half-life of 40 to 50 days. Since the Hct and the percentages of circulating reticulocytes in the GATA-1low mice were normal (Table 1, Figures 8, 10) but their CFU-E and bipotent precursor cell compartments were expanded (Tables 2, 3), the number of their erythroblasts actually maturing into reticulocytes had to be lower than normal. In agreement with this conclusion, the proportion of GATA-1lowerythroblasts that stained positive for apoptosis was found to be higher than normal (60% vs 2%) (Figure 6B).
In apparent contrast with these data, Shivdasani et al12and McDevitt et al13 had reported that the liver from the GATA-1low fetuses (when the mutants are anemic) contained normal numbers of progenitor cells. Since these authors have not reported the number of progenitor cells in the adult tissues (when the mutants have a normal Hct), it is not possible to assess whether the different results obtained are due to the slightly different genetic background (mixed C57 Bl/6-SV 129 vs mixed C57 Bl/6-SV 129 plus CD 1) of the mice used in the 2 sets of studies or an ontogenetic switch in the control of the erythroid differentiation program.
The high perinatal mortality rate of the GATA-1low mutants had suggested that the normal Hct in the adults was due to natural selection of those few animals whose erythroid cells either were more responsive to EPO30 or expressed higher levels of GATA-1 (or other members of the GATA family). In fact, in the case of targeted deletions of GATA-1,31 the GATA-1nullerythroblasts capable of differentiating in vitro from the embryonic stem cells express 50-fold higher-than-normal levels of GATA-2, a factor whose functions are at least partially redundant with GATA-1.1 Furthermore, ectopic expression of GATA-3 rescues the GATA-1null mutation.32 However, the EPO concentration/response curves of the mutant BFU-E and CFU-E, both from the marrow (not shown) and the spleen (Figure 7C), were normal, and highly purified erythroblasts isolated from the GATA-1lowspleens expressed very low GATA-1 levels while their level of expression of GATA-2 was comparable to that expressed by their counterpart isolated from normal spleens (Figure 6).
It is known that the physiological functions of certain genes are not always unveiled by ablation/overexpression studies in genetic mouse models because the phenotype could be masked by a homeostatic compensatory loop.33 This was the case, for example, of the Stat5a−/−Stat5b−/−mice.34,35 Since the Jak2/Stat5 pathway is the major EPO signaling pathway,36 Stat5 ablation should result, as in the case of Jak2 deletion,37,38 in fatal anemia. In contrast, Stat5a−/−Stat5b−/− mice are viable and have normal Hct levels.34 A careful analysis of their phenotype revealed, however, that, as is the case in the GATA-1low mice,13 the Stat5a−/−Stat5b−/− fetuses are anemic but the mice recover from the anemia after birth.35 In this case, normal levels of Hct were achieved by a 2-fold expansion of the size of the BFU-E compartment that compensated for the decreased CFU-E/erythroblast survival consequential to the Stat5 deletion, maintaining the CFU-E numbers within the normal range.34
The compensatory mechanism underlying the normal Hct of the GATA-1low mice was found to be very similar to that of the Stat5a−/−Stat5b−/− mice. In fact, the normal Hct observed in the adult GATA-1low mice was also associated with increased frequencies of early (BFU-E, 2-fold) and late (CFU-E, 7-fold) erythroid progenitors (Figure 7A,B) and of bipotent (greater than 20-fold) precursors (Figure 4) in their spleen. However, differences were observed between the compensatory mechanisms of the GATA-1low and the Stat5a−/−Stat5b−/− mutation in terms of types of cells involved and magnitude of expansion. In fact, in the Stat5a−/− Stat5b−/− mutants, 2-fold BFU-E expansion was sufficient to sustain normal Hct while, in the GATA-1low mutants, the expansion required was higher (2- to 20-fold depending on the cell type) and involved mainly the CFU-E compartment. The lower magnitude of the expansion that compensates for the Stat5a−/−Stat5b−/−mutation compared with that observed in the GATA-1low mice indicates that erythropoiesis is affected with more severity by the GATA-1low than by the Stat5a−/−Stat5b−/− mutation. Furthermore, the 2 mutations were, at least partially, compensated for by different mechanisms, restricted to the BFU-E compartment in one case and involving cells from BFU-E down to the bipotent cell precursor in the other.
That the GATA-1low mice have an accelerated response to both acute (Figure 8) and chronic (Figure 10) erythroid stimulation suggests that the mechanism that compensates for their erythroid defect does not saturate either the signaling pathway related to the response to stress (PHZ anemia) or the EPO pathway. It is unlikely, then, that it involves control elements extrinsic to the erythroid differentiation program,39 and it could be either extrinsic or intrinsic to the GATA-1 mutation itself.
One possible extrinsic mechanism could result from the fact that the chronic thrombocytopenia stimulates growth factor production in vivo. Although the serum from the GATA-1low mice contains normal levels of EPO and SCF (data not shown), thrombocytopenia stimulates thrombopoietin (TPO) production,40 which has been shown in vivo to increase the number of megakaryocytic as well as erythroid progenitor cells.41 However, the frequency of megakaryocytic progenitors was normal in the tissues of the mutant mice (32.5 ± 7.5 vs 26.0 ± 6.5 per 105 cells). Furthermore, their marrow and spleen contained high numbers of immature megakaryocytes interspersed with the erythroid cells (Figure 2, 3; also results not shown). Since megakaryocytes are responsible for binding and degrading TPO in vivo,40 their high numbers make it unlikely that TPO could accumulate in the mutant tissues at levels above normal. Other growth factors, produced as a consequence of the thrombocytopenia, might prime erythroid cells to be more sensitive to EPO. However, as mentioned earlier, the EPO concentration/response curves of the mutant erythroid progenitors were normal (Figure7C).
Alternatively, the compensatory mechanism of the GATA-1lowmutation in adult mice could be intrinsic to the mutation itself. In fact, it has been shown that GATA-2 expression favors proliferation over differentiation in experimental models32,42 and is suppressed, either directly or indirectly, by GATA-1 itself.43 It has been, therefore, suggested that the ratio between the levels of GATA-2 and GATA-1 expression determines whether a progenitor cell will proliferate (high GATA-2) or differentiate (high GATA-1).1 According to this model, the lower-than-normal levels of GATA-1 expression in the GATA-1low cells would allow the erythroid progenitors to express GATA-2 for a longer period of time, favoring their proliferation over differentiation. Eventually, because of the long GATA-1 messenger RNA and protein half-life, progenitor cells would accumulate enough GATA-1 to suppress GATA-2 expression and to begin erythroid differentiation.
It remains to be explained why the phenotype of the adult mutants involves the transition of the major hemopoietic site from the marrow to the spleen. Similar increases in the percentage of erythroid cells were observed in the marrow and spleen from the GATA-1lowmice (Figure 7 and results in the text). However, because the marrow from the mutants had poor cellularity (Table 1), it contained absolute numbers of erythroid progenitor and precursor cells similar to, if not lower than, normal (Table 2). It is possible that this transition is pleiotropic to the mutation itself and is linked to the different accessory cell populations present in the marrow and spleen and how they respond to growth factors released by the megakaryocytes. The marrow, but to a much lesser extent the spleen, contains fibroblasts, endothelial cells, and osteoblasts, all of which are exquisitely responsive to growth factors, such as platelet-derived growth factor, released by the megakaryocytes. Therefore, stromal cells within the GATA-1low marrow might be continuously stimulated, and their consequent proliferation might reduce, over time, the space available for the hemopoietic cells, forcing them to migrate in the spleen. In agreement with this hypothesis, marrow sections from adult GATA-1low mice show gross accumulation of collagen fibers and a myelofibrosis-like morphology. We are currently investigating whether the phenotype of the adult GATA-1low mutants involves development of myelofibrosis and how the mutation is eventually responsible for this disease.
The data presented here might have some clinical relevance; in fact, 2 GATA-1 mutations have been recently described in humans that result in partially defective protein function and thrombocytopenia associated with either dyserythropoietic anemia44 or thalassemia.45 The fact that the GATA-1lowmice respond better than the normal mice to erythroid stimulation (Figures 8 and 10) suggests that treatment with EPO may correct, at least in part, the erythroid defect in some of these patients.
We thank Dr Stuart Orkin for providing the GATA-1low mouse model, for continuous support, and for critical reading of the manuscript; Professor Giuliano D'Agnolo for encouragement; Luca Marsili for assistance with the breeding program; and Margaret Oppenheimer for editorial assistance.
Supported by Ministero per la Ricerca Scientifica e Tecnologica; Progetti di Ricerca di Interesse Nazionale 2000; grant 1172 from the Telethon Foundation, Fondi Ricerca Corrente, Ministry of Health; and institutional funds from Istituto Superiore di Sanità, Rome, Italy. C. Cellai was the recipient of a fellowship from Fondazione Italiana per la Ricerca sul Cancro (Milan, Italy).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anna Rita Migliaccio, Laboratorio di Biochimica Clinica, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy; e-mail: migliar@iss.it.