Abstract
The regulator of G-protein signaling (RGS) negatively regulates the α subunit of G proteins by accelerating their intrinsic guanosine triphosphatase (GTPase) activity. Here are reported the isolation and characterization of a novel mouse RGS, termed RGS18, which is a new member of RGS subfamily B. Northern blot analysis showed that RGS18 messenger RNA was detected predominantly in spleen and hematopoietic cells, and immunohistochemical studies demonstrated that RGS18 was expressed in megakaryocytes, platelets, granulocytes/monocytes, and, weakly, in hematopoietic stem cells, but not in lymphocytes or erythrocytes. Although various subcellular localizations of RGS have been reported, RGS18 was found to be localized in cytoplasm in megakaryocytes. In vitro binding assays of RGS18 with megakaryocyte cell lysates with or without AlF4− treatment demonstrated that RGS18 specifically binds to 2 α subunits of the G protein, Gαi and Gαq. Furthermore, RGS18 clearly exhibited GTPase-activating protein (GAP) activity for Gαi and Gαq but not for Gαs or Gα12. In addition, chemokine stromal-derived factor 1 (SDF-1), which has been reported to stimulate megakaryocyte colony formation in the presence of thrombopoietin, affected the binding of RGS18 to Gαi but not to Gαq. Therefore, the newly isolated RGS18 turned out to be a new member of the RGS family bearing GAP activity for Gαi, which might be stimulated by SDF-1 in megakaryocytes, as well as for Gαq. Thus, RGS18 may play an important role in proliferation, differentiation, and/or migration of megakaryocytes.
Introduction
The guanosine triphosphate (GTP)–binding protein (G-protein) signaling pathway is one of the most important signaling cascades used to relay extracellular signals and sensory stimuli to eukaryotic cells.1 Heterotrimeric G proteins, which couple heptahelical receptors to effectors in the signal transduction pathway, are composed of α, β, and γ subunits.2,3 Each subunit has multiple isoforms; the α subunit isoforms are grouped into 4 subfamilies: Gαi, Gαs, Gαq, and Gα12.4 The guanosine diphosphate (GDP)–bound heterotrimer is an inactive form of the G protein. Ligand-bound activated receptors catalyze the exchange of GDP by GTP on the α subunit, leading to dissociation of the α from the βγ dimer and signal transduction by the separated G-protein subunits (Gα and Gβγ). The large body of research examining G-protein–mediated signaling pathways has focused mainly on the involvement of receptor phosphorylation and sequestration in the desensitization process.
Recently, a new family of proteins, the regulator of the G-protein–signaling (RGS) family, has been identified.5-7Genetic screenings for negative regulators for the pheromone response pathway in yeast identified a protein, Sst2.8 Further analyses revealed that Sst2 interacted directly with the G-protein α subunit.9 To date, at least 26 different RGS proteins have been described in mammals. They all contain a conserved, characteristic domain (termed RGS domain) that interacts specifically with the activated form of Gα subunits. Biochemical studies have demonstrated that RGS proteins function primarily as guanosine triphosphatase (GTPase)–activating proteins (GAPs) for heterotrimeric G-protein Gα subunits, accelerating the inactivation rate of GTP-bound Gα subunits.7 Most of the RGS proteins inhibit signaling pathways that use Gαi and/or Gαq as signal transducers. Recently, p115 RhoGEF has been shown to have an RGS-like domain that has GAP activity for Gα12/13.10 However, no RGS protein with GAP activity for Gαs has been identified.
Outside the RGS domain, members of the family are structurally diverse and can contain additional motifs that could mediate subcellular targeting or assembly of signaling complexes or both. Additionally, certain RGS family members exhibit highly restricted patterns of expression, implying cell-specific functions in embryogenesis and cell differentiation. An Axin family, containing Axin and Axil, was identified from mammals, and the proteins in this family containing the RGS domain were shown to regulate an early step in embryonic axis formation by RNA injection into Xenopus embryos.11,12Moreover, the loco gene was identified in Drosophila, and its mutants revealed a severe glial cell differentiation defect. The loco encodes 2 RGS domain proteins, and these were found to show a significant similarity to rat RGS12. The interaction and the coexpression of LOCO and Gαi demonstrated a function of G-protein signaling for glial cell development.13 Furthermore, the expression of RGS messenger RNAs (mRNAs) in the central nervous system has recently been examined, and one family member, RGS9, was found to be expressed almost exclusively in brain tissues that receive dense dopaminergic innervation.14-16 Thus, it has been shown that RGS proteins are also involved in regulation of embryogenesis and cell differentiation.
Production of blood cells is regulated by the interplay of various cytokines and bone marrow stromal cells.17-21 Platelets are originally derived from pluripotent hematopoietic stem cells. The stem cells differentiate into committed megakaryocyte progenitors, and finally the matured megakaryocytes release a number of platelets. Past studies on megakaryocyte differentiation and platelet production have been hampered because of the rarity of megakaryocytes in bone marrow; the delay of identification of megakaryocyte-specific cytokine, thrombopoietin (TPO); and the lack of a useful platelet-inducible megakaryocytic cell line. Since TPO was identified in 1994, it has been reported to regulate the proliferation and maturation of megakaryocytes22-27 and to actually stimulate polyploidization of primary immature megakaryocytes.28-31The availability of TPO and its capacity to induce the proliferation and differentiation of megakaryocyte progenitor cells have resulted in the expansion of megakaryocyte numbers in vitro. This culture system has allowed us to investigate the molecular and cellular mechanisms of megakaryocyte maturation and platelet formation.
During the isolation of TPO-inducible transcripts in primary megakaryocytes, a complementary (cDNA) encoding a novel member of the RGS family, termed RGS18, was incidentally isolated. We found that RGS18 was expressed predominantly in megakaryocytes and that it had GAP activity for Gαi and Gαq but not for Gαs or Gα12 subunits of G protein. Furthermore, a chemokine, stromal-derived factor 1 (SDF-1), which has been reported to stimulate megakaryocyte colony formation in the presence of TPO,32 affected the binding capacity of RGS18 to Gαi but not to Gαq. We discuss the possible role of this newly isolated RGS member in megakaryocyte growth, differentiation, and migration.
Materials and methods
Preparation of megakaryocytes and cell culture
Bone marrow cells were freshly prepared from BDF1 mice (7-week-old females) by flushing marrow cavities with Iscove's modified Dulbecco's medium through 26-gauge needles. Embryonic stem (ES) cells (D3) were cocultured with OP9 stromal cells for 3 days in α-minimum essential medium (MEM) containing 20% fetal calf serum (FCS) and cocultured for a further 9 days in the presence of recombinant mouse TPO (50 U/mL) with medium changes. Recombinant mouse TPO was prepared from the supernatants of COS-7 cells transfected with mouse TPO cDNA in pME18.27Megakaryocytes were purified by a modified 2-step separation technique.33 Mixtures of cells were sedimented at unit gravity in a 2% to 16% gradient of bovine serum albumin (BSA) solution. Interleukin (IL) 3–dependent mouse pro-B cells Ba/F3, TPO-dependent mouse hematopoietic progenitor cells FD-TPO,27 mouse myeloid cells WEHI-3B, and IL-2–dependent mouse killer T cells CTLL-2 were grown in RPMI 1640 supplemented with 10% FCS and appropriate cytokines. SKT6 cells were maintained as described.34
Isolation of RGS18 cDNA
Megakaryocytes purified from mouse bone marrow were incubated in S-clone (Sanko, Tokyo, Japan) containing 1% BSA with or without TPO for 6 hours, and each total RNA was isolated by means of the RNeasy Mini Kit (Qiagen, Valencia, CA). The PCR-Select cDNA subtraction kit (Clontech, Palo Alto, CA) was used for cDNA synthesis and suppressive subtractive hybridization, according to the recommended protocol. The cDNA from megakaryocytes incubated in the presence of TPO was used as the tester sample, and that in the absence of TPO as the driver sample. The full-length RGS18 cDNA was isolated by screening the mouse megakaryocyte cDNA library by means of a newly isolated RGS cDNA fragment, and its complete nucleotide sequence was determined by ABI 310 sequencer (Applied Biosystems, Foster City, CA). The 5′ end of RGS18 was confirmed by the rapid amplification of the cDNA ends (5′ RACE) method. This sequence has been deposited in the DNA Data Bank of Japan database and assigned accession number AB042807.
Northern blot analysis
A mouse multiple-tissue blot filter was purchased from OriGene (Rockville, MD). We separated 5 μg polyA+ mRNA of mouse hematopoietic cell lines on a 1% agarose gel and then transferred it to a Hybond-N+ membrane (Amersham Pharmacia Biotech, Little Chalfont, England). The filters were hybridized with the digoxigenin (DIG)–labeled RGS18 cDNA probe (nucleotides 350 to 750) at 42°C for 16 hours in DIG Easy Hyb solution (Roche Diagnostics, Indianapolis, IN). After washing at 68°C for 30 minutes in 1 × SSC containing 0.2% SDS, the hybridized bands were detected by chemiluminescent detection by means of CDP-Star substrate (Roche Diagnostics).
Reverse transcriptase–polymerase chain reaction analysis
Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed by means of Multiple Tissue cDNA Panels (Clontech), following the manufacturer's protocol. We used 1 ng cDNA in 50 μL PCR mixture. The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (G3PDH), was used as endogenous mRNA standard. Annealing temperature was 50°C for RGS18 and 60°C for G3PDH. The following RGS18 primers were used: 5′-TACAGAGGCCTGACTTCCAT-3′ and 5′-TTCCATGAGCTGGTACACTC-3′, and the following G3PDH primers were used: 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ and 5′-C ATGTAGGCCATGAGGTCGTCCACCAC-3′.
Preparation of antibody
The peptide (CESKEKTFFKLMHGS) of RGS18 (amino acids 14 to 28) was synthesized (Sawady, Tokyo, Japan), and conjugated to multiple antigen peptide. The conjugated peptide (100 μg) was injected into 8-week-old female WKY/NCrj rats (Charles River Japan, Yokohama, Japan) 3 times at 2-week intervals. Polyclonal RGS18 antiserum was purified by GST-RGS18 affinity chromatography.
Indirect immunofluorescence microscopic analysis
Smear samples of bone marrow cells and purified megakaryocytes were fixed with 100% methanol for 10 seconds at room temperature and then washed with phosphate-buffered saline (PBS) for 10 minutes at room temperature. For the sections of mouse spleen, adult BL6 mice (SLC, Shizuoka, Japan) were perfused with 4% paraformaldehyde in 100 mM PBS (pH 7.4) for 20 minutes. The removed spleens were fixed with 4% paraformaldehyde in 100 mM PBS for 20 minutes and washed with PBS 5 times. After that, the spleens were cryoprotected overnight in PBS containing 30% sucrose and embedded in O.C.T. compound (Miles, Elkhart, IN) and then cut on a freezing, sliding microtome at 6 μm. Various lineages of hematopoietic progenitors were purified from mouse bone marrow by FACS Vantage (BD Pharmingen, San Diego, CA), by means of anti-CD45R/B220 (RA3-6B2), anti-CD3e (145-2C11), anti–Ly-6G/Gr-1 (RB6-8C5), anti-CD11b/Mac-1 (M1/70), and anti–TER-119 (TER-119) antibodies (BD Pharmingen).
Coverslips of fixed suspension cells and fixed mouse spleen sections were blocked for 10 minutes in blocking buffer (PBS containing 5% FCS), and then incubated in blocking buffer containing anti-RGS18 rat antibody or fluorescein isothiocyanate (FITC)–labeled anti-RGS18 antibody for 2 hours at 37°C and overnight at room temperature, respectively. After rinsing with PBS 5 times, blocking buffer containing FITC-conjugated F(ab′)2 fragment donkey or Cy3-conjugated F(ab′)2 fragment goat antirat antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was applied for 1 hour at 37°C. The coverslips were washed with PBS 5 times, and mounted in 90% glycerol/PBS containing 0.1% p-phenylenediamine and 0.2 μg/mL 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI). To assess the specificity of the antibody, parallel series of cells and sections were incubated in blocking buffer without primary antibody or in blocking buffer with rat preimmune antiserum of primary antibody. The coverslips were observed under a fluorescence microscope (Olympus BX60-34-FLBD1, Olympus, Tokyo, Japan) at a final magnification of 600 × or 1500 ×.
In vitro binding assay
The RGS18 cDNA (708 base pairs [bp]) in pGEX4T-1 was expressed in Escherichia coli BL21. The binding of endogenous G proteins with GST-RGS18 was performed as follows: The purified megakaryocytes were starved in S-Clone containing 1% BSA for 6 hours. FD-TPO cells were starved in RPMI 1640 containing 0.4% FCS, 0.125 mg/mL transferrin, and 0.01% BSA without TPO for 7 hours. The starved cells were restimulated with or without 50 ng/mL SDF-1α (R&D Systems, Minneapolis, MN) and/or 1 U/mL mouse recombinant TPO for up to 1 hour. Cells were then lysed in lysis buffer A (50 mM Hepes, pH 7.5, 300 mM NaCl, 1 mM dithiothreitol (DTT), 6 mM MgCl2, 1% Triton X-100, 2 mM Pefabloc, 10 ng/mL leupeptin, and 10 ng/mL aprotinin). Cell lysates were activated with GDP (30 μM) or GDP plus 30 μM AlCl3 and 100 mM NaF for 30 minutes at 30°C. We then incubated 1 mg of the cell lysates with GST-RGS18 (40 μg) bound to glutathione Sepharose 4B (Amersham Pharmacia Biotech) (25 μL) for 2 hours at 4°C. After washing 3 times with lysis buffer A containing 0.025% C12E10 (Sigma, St Louis, MO) instead of Triton X-100, the bound proteins were eluted with Laemmli buffer and boiled for 10 minutes. Samples were fractionated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to ECL membrane (Amersham Pharmacia Biotech). The membrane was blocked with 5% milk in 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.5% Tween 20 (TBS-T), and incubated with anti–Gαi-3 (C-20) or anti-Gαq/11 (C-19) antibody (Santa Cruz Biotechnology, CA) for 2 hours. After washing 3 times with TBS-T, the membrane was incubated with antirabbit immunoglobulin G–conjugated horseradish peroxidase antibody, and the antibody complexes were visualized by an ECL system (Amersham Pharmacia Biotech).
Gα and GAP assays
Gαs and Gαi were expressed in and purified from E coli.35 Gα12 and Gαq were expressed in Sf9 cells and purified as described.36 Gαi, Gαs, and Gα12 (50 pmol) were loaded with 5 to 20 μM [γ-32P]GTP (approximately 5000 Ci/mmol) at 20°C (for Gαs) or 30°C (for Gαi and Gα12) for 20 to 30 minutes in the presence of 5 mM EDTA. Samples were then gel-filtered at 4°C through a Sephadex G-50 spin column (Amersham Pharmacia Biotech) equilibrated with buffer B (25 mM Hepes, pH 8.0, 1 mM DTT, 5 mM EDTA, 0.05% C12E10) to remove free [γ-32P]GTP and [32P]O. Hydrolysis of GTP was initiated by adding Gα loaded with [γ-32P]GTP to buffer B containing 8 mM MgSO4 and 1 mM GTP with the indicated amount of RGS proteins. The reaction mixture was incubated on ice (for Gαi and Gαs) or 15°C (for G12). Aliquots (50 μL) were removed at the indicated times and mixed with 750 μL of 5% (wt/vol) activated charcoal in 50 mM NaH2PO4. The mixture was centrifuged at 2000 rpm for 10 minutes, and 400 μL supernatant containing [32P]O was counted by liquid scintillation spectrometry. Direct measurement of the kcat for GTPase activity of Gαq was assayed with the use of mutant GαqR183C.37 The slow GTPase activity of GαqR183C made it possible to load [γ-32P]GTP on Gαq without accelerating GDP-GTP exchange by agonist-bound receptor. GαqR183C was loaded with 10 μM [γ-32P]GTP in the presence of 50 mM Hepes, pH 7.4, 0.1 mg/mL BSA, 1 mM DTT, 1 mM EDTA, 0.9 mM MgSO4, 30 mM (NH4)2SO4, 4% glycerol, and 5.5 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) at 20°C for 2 hours. The reaction mixture was gel-filtered through a Sephadex G50 spin column equilibrated with 50 mM Hepes, pH 7.4, 1 mM DTT, 1 mM EDTA, 0.9 mM MgSO4, 0.1 mg/mL BSA, and 1 mM CHAPS. GTPase assays were initiated by addition of 1 mM GTP and the indicated amount of RGS proteins followed by incubation at 20°C. Aliquots (50 μL) were removed and processed as described above.
Results
Isolation of a novel RGS
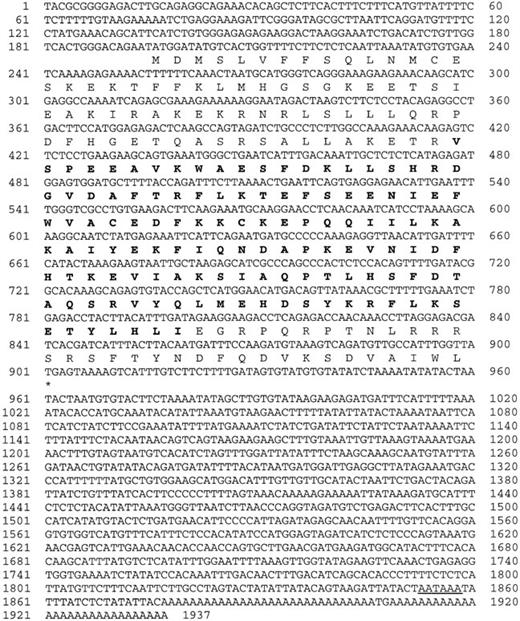
To study the megakaryocyte-specific differentiation/maturation process, we isolated the genes whose transcription is induced by the stimulation of megakaryocyte growth and differentiation factor TPO. Subtraction of mRNAs of primary mouse bone marrow megakaryocytes before and after TPO stimulation led to the isolation of a number of novel cDNA fragments encoding factors related to the megakaryocyte-specific differentiation/maturation events. We focused on a novel mouse RGS, termed RGS18, although this gene expression turned out not to be up-regulated by TPO stimulation (data not shown). Figure1 shows the nucleotide sequence of the isolated RGS18 cDNA (1937 bp) and the amino acid sequence of the encoded polypeptide. The cDNA contained an open reading frame, which encoded a highly basic polypeptide of 235 amino acids with a calculated molecular mass of 27 500 d. By a BLAST search of the NCBI database, mouse RGS18 cDNA was found to be highly homologous to a human RGS13 (GenBank AAF80227), which was different from the previously reported human RGS13 (GenBank AF030107).38 To date, at least 26 different RGS proteins have been described in mammals and designated from RGS1 to RGS17. Therefore, we used the term RGS18 for this novel mouse RGS (accession number, AB042807) and human RGS13 (GenBankAAF80227). Alignment of the deduced amino acid sequences of mouse and human RGS18 (previously described as RGS13 for the human) shows that they have 83% identity and 91% similarity in amino acid sequences. Like other members of the RGS family, RGS18 contain a highly conserved RGS domain, which mediates G-protein interactions,39 40 and several potential phosphorylation sites for protein kinase A, protein kinase C, and casein kinase II.
Structure of mouse RGS18.
The deduced amino acid sequence of mouse RGS18 cDNA is shown in single-letter code under the respective codons. The bold amino acid sequence corresponds to the putative RGS domain. The polyadenylation signal is underlined. Accession number of this sequence was assigned as AB042807.
Structure of mouse RGS18.
The deduced amino acid sequence of mouse RGS18 cDNA is shown in single-letter code under the respective codons. The bold amino acid sequence corresponds to the putative RGS domain. The polyadenylation signal is underlined. Accession number of this sequence was assigned as AB042807.
RGS18 in subfamily B
To examine the relationship of RGS18 to other RGS family members, a phylogenetic tree was constructed by using the amino acid sequences of RGS domains of all known mammalian RGS family members (Figure 2A). It was proposed that the mammalian RGS family consists of at least 6 distinct subfamilies.38 As shown in Figure 2A, RGS18 turned out to be a member of subfamily B. Figure 2B shows an alignment of RGS domains of RGS18 with those of other subfamily B members. The RGS domain of mouse RGS18 has 85% identity with and 89% similarity to that of human RGS18 (previously described as RGS13) and has a significant homology (between 45% and 60% identity and between 65% and 75% similarity) to that of other subfamily B members. The essential amino acid residues, especially N152 (single-letter amino acid code), constituted with RGS domains are conserved in RGS18. The S152 is a characteristic residue that exists only in subfamilies B, C, and D, and the N residue at this position is believed to be crucial for the GAP activity and might be involved in stabilization of the transition state of Gα subunits.41 However, S127 (single-letter amino acid code), which has heretofore been viewed as a subfamily B–specific residue, was not found in either mouse or human RGS18 or in mouse RGS1, indicating that it is no longer a unique residue in this subfamily.
RGS18 in RGS subfamily B.
(A) Phylogenetic tree of the RGS domains of all RGS family members. The primary amino acid sequences of RGS domains from 48 mammalian RGS proteins were aligned by means of Clustal W1.7. The phylogenetic tree was constructed by the neighbor-joining method by means of Clustal W1.7 on the basis of this alignment and visualized with the Treeview program 1.5. All known RGS proteins (except RGS10 and D-AKAP2) are grouped into 6 subfamilies A to F. Species abbreviations: h, Homo sapiens; m, Mus musculus; r, Rattus norvegicus; b, Bos taurus. The accession numbers for the sequences used in this analysis are as follows: hRGS18, AAF80227; hRGS1, NP_002913; mRGS1, NM_015811; hRGS2, NP_002914; mRGS2, O08849; rRGS2, AF279918; hRGS3,P49796; mRGS3, AF215670; hRGS4, P49798; mRGS4, O08899; rRGS4, P49799; hRGS5, NP_003608; mRGS5, O08850; rRGS5, NM_019341; hRGS6, NP_004287; mRGS6, AAC70011; hRGS7, AAD34290; mRGS7, O54829; rRGS7, BAA75635; bRGS7, O46470; rRGS8, BAA23680; hRGS9, AAC64040; mRGS9, O54828; rRGS9,AAC01959; hRGS10, NP_002916; hRGS11, AAC69175; hRGS12, O14924; rRGS12,O08774; hRGS13, AF030107; mRGS14, P97492; rRGS14, O08773; hRGS16,O15492; mRGS16, P97428; rRGS16, P56700; bRGS16, O46471; hRGS17,AAF08978; hAxin, AAC51624; mAxin, AAC53285; rAxin, AAC40066; bRET-RGS1,P79348; hRGS-GAIP, P49795; hRGSZ1, NP_003693; mRGSZ1, AF191554; mConductin, AAC26047; hp115-RhoGEF, NP_004697; mLsc, AAC52693; hPDZ-RhoGEF, BAA20834; mD-AKAP2, AAC61898. (B) Phylogenetic tree of all RGS subfamily B members constructed with the use of full-length amino acid sequences. (C) Alignment of RGS domains of all RGS subfamily B members. The RGS domain of mouse RGS18 is compared with that of other RGS subfamily B members. The regions of RGS domains are defined according to Tesmer et al.41 The conserved amino acids are shaded. The identical residues in all proteins in the alignment are displayed at the top. An asterisk indicates subfamily B–specific residue (S127 [single-letter amino acid code]). Residue numbers shown at the top correspond with mRGS18. Sources of primary sequences are the same as in panel A.
RGS18 in RGS subfamily B.
(A) Phylogenetic tree of the RGS domains of all RGS family members. The primary amino acid sequences of RGS domains from 48 mammalian RGS proteins were aligned by means of Clustal W1.7. The phylogenetic tree was constructed by the neighbor-joining method by means of Clustal W1.7 on the basis of this alignment and visualized with the Treeview program 1.5. All known RGS proteins (except RGS10 and D-AKAP2) are grouped into 6 subfamilies A to F. Species abbreviations: h, Homo sapiens; m, Mus musculus; r, Rattus norvegicus; b, Bos taurus. The accession numbers for the sequences used in this analysis are as follows: hRGS18, AAF80227; hRGS1, NP_002913; mRGS1, NM_015811; hRGS2, NP_002914; mRGS2, O08849; rRGS2, AF279918; hRGS3,P49796; mRGS3, AF215670; hRGS4, P49798; mRGS4, O08899; rRGS4, P49799; hRGS5, NP_003608; mRGS5, O08850; rRGS5, NM_019341; hRGS6, NP_004287; mRGS6, AAC70011; hRGS7, AAD34290; mRGS7, O54829; rRGS7, BAA75635; bRGS7, O46470; rRGS8, BAA23680; hRGS9, AAC64040; mRGS9, O54828; rRGS9,AAC01959; hRGS10, NP_002916; hRGS11, AAC69175; hRGS12, O14924; rRGS12,O08774; hRGS13, AF030107; mRGS14, P97492; rRGS14, O08773; hRGS16,O15492; mRGS16, P97428; rRGS16, P56700; bRGS16, O46471; hRGS17,AAF08978; hAxin, AAC51624; mAxin, AAC53285; rAxin, AAC40066; bRET-RGS1,P79348; hRGS-GAIP, P49795; hRGSZ1, NP_003693; mRGSZ1, AF191554; mConductin, AAC26047; hp115-RhoGEF, NP_004697; mLsc, AAC52693; hPDZ-RhoGEF, BAA20834; mD-AKAP2, AAC61898. (B) Phylogenetic tree of all RGS subfamily B members constructed with the use of full-length amino acid sequences. (C) Alignment of RGS domains of all RGS subfamily B members. The RGS domain of mouse RGS18 is compared with that of other RGS subfamily B members. The regions of RGS domains are defined according to Tesmer et al.41 The conserved amino acids are shaded. The identical residues in all proteins in the alignment are displayed at the top. An asterisk indicates subfamily B–specific residue (S127 [single-letter amino acid code]). Residue numbers shown at the top correspond with mRGS18. Sources of primary sequences are the same as in panel A.
Figure 2C shows the phylogenetic tree of RGS subfamily B, which was constructed with the use of full-length amino acid sequences of all subfamily B members. When outside sequences of RGS domains were included in this assay, RGS18 turned out to be closest to RGS5, which was not clear when we compared RGS18 with other RGS members within RGS domains.
RGS18 is expressed in spleen and hematopoietic cells
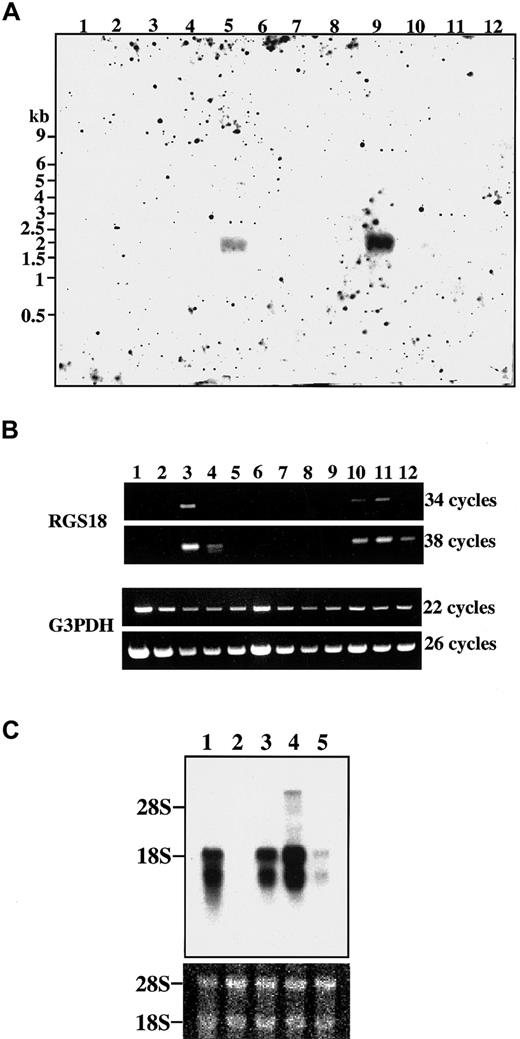
Expression of RGS18 in various mouse tissues was examined by Northern blot analyses. As shown in Figure3A, a single hybridized band of about 2 kilobases (kb) was clearly detected in spleen (Figure 3A, lane 9) and a very faint band was detectable in lung (Figure 3A, lane 5), suggesting that RGS18 is expressed predominantly in hematopoietic cells.
Expression of RGS18 mRNA in mouse tissues and hematopoietic cells.
The filters were hybridized with the DIG-labeled RGS18 cDNA probe. (A) Northern blot analysis of RGS18 in various mouse tissues. Two micrograms of polyA+ mRNAs from the mouse tissues were loaded on the blots: brain (lane 1), heart (lane 2), kidney (lane 3), liver (lane 4), lung (lane 5), skeletal muscle (lane 6), skin (lane 7), small intestine (lane 8), spleen (lane 9), stomach (lane 10), testis (lane 11), and thymus (lane 12). Sizes of mRNA standards in kilobases (kb) are shown at left. (B) Detection of RGS18 by RT-PCR in various mouse tissues. The cDNAs prepared from various mouse tissues were used as templates for PCR. Upper panel: RGS18; lower panel: G3PDH. PCR was performed in 34 and 38 cycles for RGS18, and 22 and 26 cycles for G3PDH. Heart (lane 1), brain (lane 2), spleen (lane 3), lung (lane 4), liver (lane 5), skeletal muscle (lane 6), kidney (lane 7), testis (lane 8), 7-day embryo (lane 9), 11-day embryo (lane 10), 15-day embryo (lane 11), and 17-day embryo (lane 12). (C) Northern blot analysis of various mouse hematopoietic cell lines. Five micrograms of polyA+mRNAs were loaded on the blots: Ba/F3 cells (lane 1), CTLL-2 cells (lane 2), WEHI-3B cells (lane 3), FD-TPO cells (lane 4), and SKT6 cells (lane 5). Equal loading and integrity of the mRNA samples was confirmed by ethidium bromide staining of the ribosomal RNA bands (bottom panel).
Expression of RGS18 mRNA in mouse tissues and hematopoietic cells.
The filters were hybridized with the DIG-labeled RGS18 cDNA probe. (A) Northern blot analysis of RGS18 in various mouse tissues. Two micrograms of polyA+ mRNAs from the mouse tissues were loaded on the blots: brain (lane 1), heart (lane 2), kidney (lane 3), liver (lane 4), lung (lane 5), skeletal muscle (lane 6), skin (lane 7), small intestine (lane 8), spleen (lane 9), stomach (lane 10), testis (lane 11), and thymus (lane 12). Sizes of mRNA standards in kilobases (kb) are shown at left. (B) Detection of RGS18 by RT-PCR in various mouse tissues. The cDNAs prepared from various mouse tissues were used as templates for PCR. Upper panel: RGS18; lower panel: G3PDH. PCR was performed in 34 and 38 cycles for RGS18, and 22 and 26 cycles for G3PDH. Heart (lane 1), brain (lane 2), spleen (lane 3), lung (lane 4), liver (lane 5), skeletal muscle (lane 6), kidney (lane 7), testis (lane 8), 7-day embryo (lane 9), 11-day embryo (lane 10), 15-day embryo (lane 11), and 17-day embryo (lane 12). (C) Northern blot analysis of various mouse hematopoietic cell lines. Five micrograms of polyA+mRNAs were loaded on the blots: Ba/F3 cells (lane 1), CTLL-2 cells (lane 2), WEHI-3B cells (lane 3), FD-TPO cells (lane 4), and SKT6 cells (lane 5). Equal loading and integrity of the mRNA samples was confirmed by ethidium bromide staining of the ribosomal RNA bands (bottom panel).
In order to detect rare RGS18 mRNA in various tissues, RT-PCR at 2 different cycles was performed in whole mouse embryos at different developmental stages as well as in several mouse tissues (Figure 3B). G3PDH was used as an internal control (Figure 3B, lower panel). It was found that RGS18 expression began in day-11 embryos, reached the maximum at day 15, and decreased thereafter (Figure 3C, lanes 9-12), suggesting that RGS18 was expressed in embryonic hematopoietic cells in fetal liver. It was also confirmed that RGS18 was predominantly expressed in spleen (Figure 3B, lane 3), weakly expressed in lung (Figure 3B, lane 4), but not expressed in other tissues examined (Figure 3B. lanes 1, 2, 5-9).
We next analyzed RGS18 expression in various mouse hematopoietic cell lines (Figure 3C); erythroid cells SKT6; TPO-dependent hematopoietic progenitor cells FD-TPO, which has megakaryocytic characters27; IL-3–dependent pro-B cells Ba/F3; myeloid cells WEHI-3B; and IL-2–dependent killer T cells CTLL-2. RGS18 transcripts were found most abundantly in FD-TPO cells (Figure 3C, lane 4) and less abundantly in Ba/F3 cells and WEHI-3B cells (Figure 3C, lanes 1 and 3). The transcripts were weakly seen in SKT6 cells (Figure3C, lane 5), but no transcript was detected in CTLL-2 cells (Figure 3C, lane 2). These results indicate that RGS18 is expressed predominantly in hematopoietic cells.
RGS18 expression in megakaryocytes
The expression of RGS18 in the protein level of hematopoietic cells was examined by preparing anti-RGS18 rat antiserum by injecting a peptide (CESKEKTFFKLMHGS) encoding outside of the RGS domain; this avoided cross-reaction with other RGS family members, and the antibody was purified by RGS18 affinity chromatography. This antibody clearly detected mouse RGS18 of 27.5 kd in ES cell–derived megakaryocytes in immunoblot analysis as well as immunoprecipitation (data not shown).
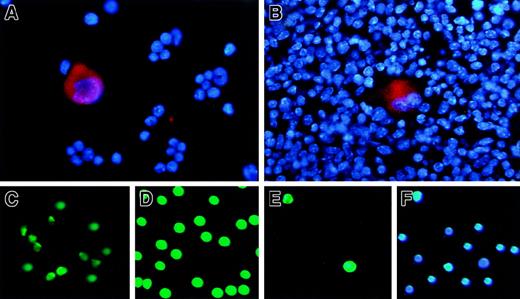
The immunohistochemical staining of the cells isolated from adult mouse bone marrow (Figure 4A) and spleen (Figure 4B) was performed with the purified anti–GST18-specific antibody. The megakaryocytes with a large cytoplasmic region, which was stained with anti-RGS18 antibody in red, as well as with large polyploid chromosomes stained with DAPI in blue, were clearly observed (Figure 4A-B). However, RGS18 was not detectable in other hematopoietic cells such as erythroid progenitors in bone marrow (Figure 4A) or spleen (Figure 4B), which were seen as a number of small cells stained only with DAPI in blue.
RGS18 expression in hematopoietic cells.
The fixed suspension cells isolated from mouse bone marrow (A) and the fixed section of mouse spleen (B) were stained with anti-RGS18 rat antibody followed by Cy3-conjugated F(ab′)2 fragment goat antirat antibody, together with DAPI. RGS18 is seen in red and chromosomes are seen in blue. The Hoechst−Rhodamine− cells/SP cells as hematopoietic stem cells (C), Ly-6G/Gr-1+ or CD11b/Mac-1+ cells as granulocyte and monocyte mix (D), and TER-119+ cells as erythrocyte and megakaryocyte mix (E, F) were isolated from mouse bone marrow by fluorescence-activated cell sorting and were immunostained with a purified FITC-labeled anti-RGS18 antibody (C-E). The TER-119+ cells were stained with FITC-labeled anti-RGS18 antibody (E) and with DAPI (F).
RGS18 expression in hematopoietic cells.
The fixed suspension cells isolated from mouse bone marrow (A) and the fixed section of mouse spleen (B) were stained with anti-RGS18 rat antibody followed by Cy3-conjugated F(ab′)2 fragment goat antirat antibody, together with DAPI. RGS18 is seen in red and chromosomes are seen in blue. The Hoechst−Rhodamine− cells/SP cells as hematopoietic stem cells (C), Ly-6G/Gr-1+ or CD11b/Mac-1+ cells as granulocyte and monocyte mix (D), and TER-119+ cells as erythrocyte and megakaryocyte mix (E, F) were isolated from mouse bone marrow by fluorescence-activated cell sorting and were immunostained with a purified FITC-labeled anti-RGS18 antibody (C-E). The TER-119+ cells were stained with FITC-labeled anti-RGS18 antibody (E) and with DAPI (F).
We further performed the immunofluorescence stainings of the other lineages of the hematopoietic progenitor cells in mouse bone marrows. We isolated Hoechst−Rhodamine− cells/SP cells as hematopoietic stem cells (Figure 4C), CD45R/B220+ or CD3e+ cells as T and B lymphocyte mix (not shown), Ly-6G/Gr-1+ or CD11b/Mac-1+ cells as granulocyte and monocyte mix (Figure 4D), and TER-119+cells as erythrocyte and megakaryocyte mix (Figure 4E-F), from mouse bone marrow by fluorescence-activated cell sorting. The cells were immunostained with a purified FITC-labeled anti-RGS18 antibody. It was found that RGS18 was expressed in granulocytes/monocytes (Figure 4D) as well as in relatively mature megakaryocytes (Figure 4A) and in megakaryocyte progenitors (Figure 4E, see below). It was weakly but clearly expressed in hematopoietic stem cells (Figure 4C), but not expressed in lymphocytes (data not shown) or erythroids (Figure 4E-F). Most of the TER-119+ cells, which were stained with DAPI shown in Figure 4F, were not stained with anti-GST18 antibody (Figure4E), but a small fraction of TER-119+ cells were clearly stained (Figure 4E). We found that these positive cells were megakaryocyte progenitors, since they could be stained with megakaryocyte-specific anti-CD41 antibody (data not shown).
RGS18 is localized in cytoplasm of megakaryocytes
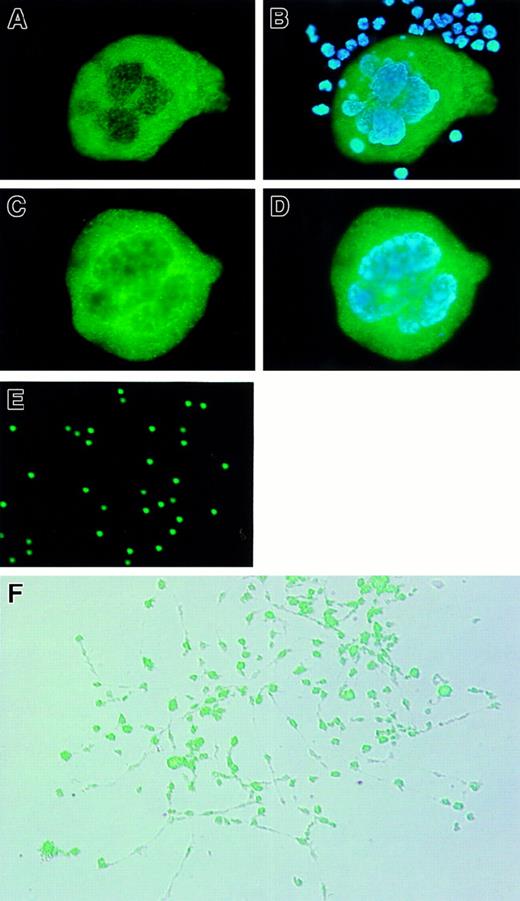
Subcellular localization of RGS18 in primary megakaryocytes was determined by indirect immunofluorescence microscopic analysis (Figure5). We prepared 2 kinds of primary megakaryocytes: those from mouse bone marrow (Figure 5A-B) and those isolated from in vitro culture of ES cells on OP9 cells with TPO (Figure 5C-D). The endogenous RGS18 were clearly stained in green and found to be localized in cytoplasm but not in nucleus of either of these primary megakaryocytes (Figures 5A-C). The polyploid chromosomes were stained with DAPI in blue in both megakaryocytes (Figure 5B,D). A number of other hematopoietic cells in bone marrow were stained only with DAPI (Figure 5B). These results clearly demonstrated that RGS18 is localized predominantly in cytoplasm of megakaryocytes.
Expression and subcellular localization of RGS18 in megakaryocytes and platelets.
Fixed megakaryocytes and some hematopoietic suspension cells prepared from mouse bone marrow (A, B) and the fixed megakaryocytes derived from mouse ES cells (C, D) were stained with anti-RGS18 rat antibody followed by FITC-conjugated F(ab′)2 fragment donkey antirat, together with DAPI (B, D). RGS18 is stained in green and polyploid chromosomes in blue. The platelets isolated from mouse peripheral blood (E) and megakaryocytes during proplatelet formation (PPF) (F) were immunostained with purified FITC-labeled anti-RGS18 rat antibody. The fluorescent image of PPF (F) was overlaid on a difference-inteference contrast image.
Expression and subcellular localization of RGS18 in megakaryocytes and platelets.
Fixed megakaryocytes and some hematopoietic suspension cells prepared from mouse bone marrow (A, B) and the fixed megakaryocytes derived from mouse ES cells (C, D) were stained with anti-RGS18 rat antibody followed by FITC-conjugated F(ab′)2 fragment donkey antirat, together with DAPI (B, D). RGS18 is stained in green and polyploid chromosomes in blue. The platelets isolated from mouse peripheral blood (E) and megakaryocytes during proplatelet formation (PPF) (F) were immunostained with purified FITC-labeled anti-RGS18 rat antibody. The fluorescent image of PPF (F) was overlaid on a difference-inteference contrast image.
RGS18 acts as GAP for Gαi and Gαq but not for Gαs or Gα12
The α subunit of G proteins is divided into 4 subfamilies, Gαi, Gαq, Gαs, and Gα12,4 and each RGS directly interacts with a subset of Gα subunits. To directly test the effects of RGS18 on the GTPase activity of various Gα subunits, we measured the catalytic activity of purified recombinant Gα subunits during a single GTPase cycle in the presence or absence of recombinant RGS18 (Figure 6). RGS4 or p115RhoGEF was used as a positive control for GAP activity for Gαi and Gαq, or for Gα12, respectively.10 42 RGS18 was found to be a nearly as effective Gαi GAP as RGS4 (Figure 6A), although at equal molar concentrations RGS4 was slightly superior. To measure the GAP activity of RGS18 for Gαq, we employed a mutant Gαq, GαqR183C, since slow GTPase activity of GαqR183C made it a suitable target for testing potential Gαq GAPs. RGS18 showed good GAP activity for Gαq exactly as did RGS4 (Figure 6B). RGS18 did not show GAP activity for Gαs or for Gα12 (Figure 6C-D). These results indicate that RGS18 acts as GAP for Gαi and Gαq but not for Gαs or Gα12.
GAP activity of RGS18 for specific Gα subunits.
Different concentrations of recombinant RGS18 or recombinant RGS4 were tested for their ability to accelerate the GTPase activity of Gαi (A), GαqR183C (B), Gαs (C), and Gα12 (D). Hydrolysis of GTP was initiated by adding the Gα subunit loaded with γ-32P]GTP to a buffer containing MgSO4 with the indicated amount of RGS protein. The reaction mixture was incubated, and aliquots were removed at the indicated times and processed, and the amount of 32Pi was counted by liquid scintillation spectrometry. HED is buffer without RGS protein. The GAP assays were performed twice with similar results.
GAP activity of RGS18 for specific Gα subunits.
Different concentrations of recombinant RGS18 or recombinant RGS4 were tested for their ability to accelerate the GTPase activity of Gαi (A), GαqR183C (B), Gαs (C), and Gα12 (D). Hydrolysis of GTP was initiated by adding the Gα subunit loaded with γ-32P]GTP to a buffer containing MgSO4 with the indicated amount of RGS protein. The reaction mixture was incubated, and aliquots were removed at the indicated times and processed, and the amount of 32Pi was counted by liquid scintillation spectrometry. HED is buffer without RGS protein. The GAP assays were performed twice with similar results.
Specific binding of RGS18 to Gαi and Gαq in vitro
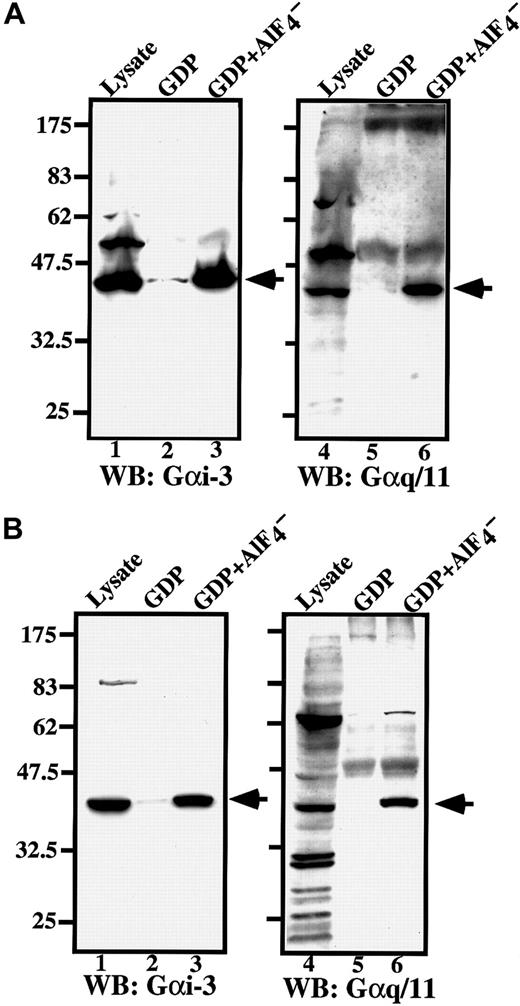
RGS does not bind to GDP-bound Gα, which is an inactive form, but does bind with high affinity to the Gα-GDP–AlF4− complex, which mimics the transition states of GTP hydrolysis and is the active form.5 Because we found that RGS18 acts as GAP for Gαi and Gαq but not for Gαs or Gα12, we performed in vitro binding assay of RGS18 to Gαi and Gαq as follows: The primary megakaryocytes prepared from ES cells (Figure7A) and FD-TPO cells (Figure 7B) were pretreated with excess GDP to inactivate Gα, or with GDP and AlF4− to activate Gα, and the cell lysates were incubated with recombinant GST-RGS18–bound beads. The bound proteins were separated by SDS-PAGE, and immunoblotted with antibodies specific to Gαi-3 or Gαq/11. Anti–Gαi-3 rabbit antibody (C-10) specifically reacts with Gαi-1, Gαi-2, and Gαi-3, but does not cross-react with other Gα subunits. Anti-Gαq/11 rabbit antibody (C-19) specifically reacts with Gαq and Gα11, but does not react with other Gα subunits.
RGS18 binds to Gαi and Gαq in megakaryocytes.
ES cell–derived primary megakaryocytes (A) or FD-TPO cells (B) were treated with GDP (lanes 2 and 5) or GDP and AlF4− (lanes 3 and 6), and the cell lysates were incubated with GST-RGS18 immobilized on glutathione Sepharose beads. Bound proteins were separated by 10% SDS-PAGE and detected with anti–Gαi-3 antibody or anti-Gαq/11 antibody. Arrowheads indicate the bound Gα of 42 kd. Lanes 1 and 4 show the immunoblots of total cell lysates.
RGS18 binds to Gαi and Gαq in megakaryocytes.
ES cell–derived primary megakaryocytes (A) or FD-TPO cells (B) were treated with GDP (lanes 2 and 5) or GDP and AlF4− (lanes 3 and 6), and the cell lysates were incubated with GST-RGS18 immobilized on glutathione Sepharose beads. Bound proteins were separated by 10% SDS-PAGE and detected with anti–Gαi-3 antibody or anti-Gαq/11 antibody. Arrowheads indicate the bound Gα of 42 kd. Lanes 1 and 4 show the immunoblots of total cell lysates.
None of Gα bound to the beads themselves or to the beads coupled to GST alone (data not shown). Both anti–Gαi-3 and anti-Gαq/11 rabbit antibodies reacted with corresponding Gα of 42 kd in whole cell lysates (Figure 7A-B, lanes 1 and 4, indicated by arrowheads). The results of in vitro binding assays clearly demonstrated that RGS18 bound to activated Gαi and Gαq and/or Gα11 in primary megakaryocytes (Figure 7A, lanes 3 and 6) and in FD-TPO cells (Figure7B, lanes 3 and 6), but not to inactive Gαi, Gαq, or Gα11 in megakaryocytes (Figure 7A, lanes 2 and 5) or FD-TPO cells (Figure 7B, lanes 2 and 5). Weak interactions of RGS18 with Gαi were observed in both megakaryocytes and FD-TPO cells even without AlF4− stimulation, but little or no binding of RGS18 to Gαq or Gα11 was detected when these cell lysates were not treated with A lF4−.
SDF-1 affects the binding of RGS18 to Gαi
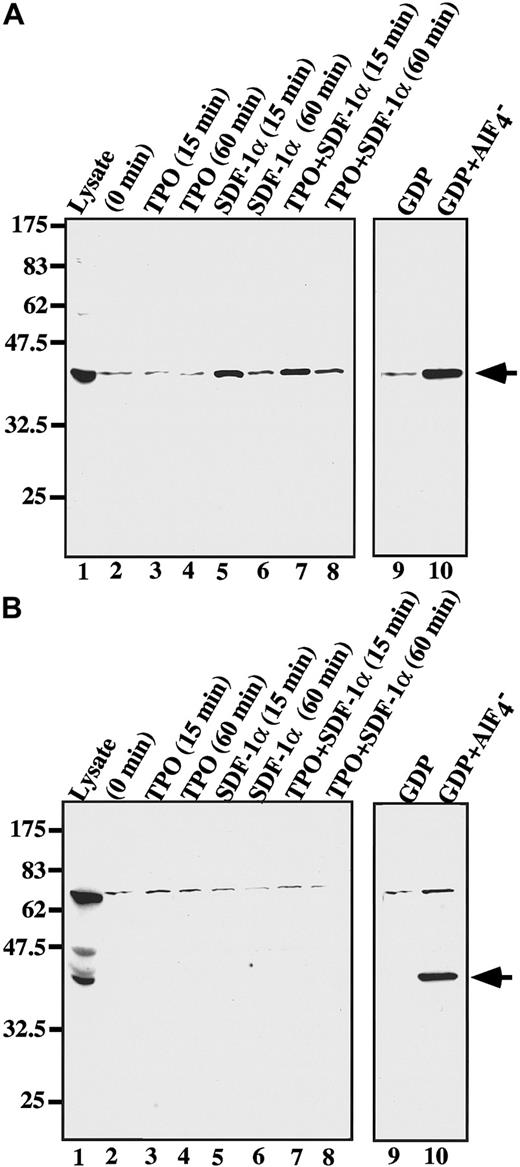
It has been stated that a chemokine SDF-1 stimulates megakaryocyte colony formation in cooperation with TPO.32 To examine the possible effects of SDF-1 in Gα binding to RGS18, we performed in vitro binding assays before and after SDF-1α stimulation with or without TPO in ES cell–derived megakaryocytes (Figure8). The bindings of RGS18 to Gαi or Gαq with or without AlF4− treatment are shown as controls (Figure 8A-B, lanes 9-10). RGS18 weakly and constitutively interacted with Gαi without any stimulation in megakaryocytes (Figure 8A, lane 2), and TPO alone did not enhance the binding of RGS18 to Gαi within 15 or 60 minutes after stimulation (Figure 8A, lanes 3-4). Surprisingly, however, stimulation of megakaryocytes with SDF-1α clearly enhanced the binding of RGS18 to Gαi within 15 minutes or 60 minutes after stimulation (Figure 8A, lanes 5-6), although the level of enhancement was low compared with that with AlF4− treatment (Figure 8A, lane 10). The addition of TPO did not affect the enhancement of RGS18 binding to Gαi caused by SDF-1 (Figure 8A, lanes 7-8). In contrast, little interaction of RGS18 with Gαq was detected with or without stimulation (Figure 8B, lanes 2-8). These results suggest that SDF-1 binding to its receptor CXCR4 induces modification such as depalmitoylation and/or translocation of Gαi but not of Gαq, which in turn enhances the affinity and/or capacity of Gαi binding to RGS18. Therefore, the SDF-1 receptor–mediated signaling cascade, as well as the TPO signaling pathway, may play an important role in megakaryocyte growth and differentiation.
SDF-1 affects the RGS18 binding to Gαi.
ES cell–derived megakaryocytes were stimulated with SDF-1α (lanes 5-8) for 15 minutes (lane 5) or 60 minutes (lane 6) in the presence of TPO (lanes 3, 4, 7, 8) for 15 minutes (lanes 3, 7) or 60 minutes (lanes 4, 8) or in the absence of TPO (lanes 2, 5, 6, 9, 10), and the cell lysates were prepared. In vitro bindings of Gαi (A) or Gαq (B) to GST-RGS18 were performed with the cell lysates, and the bound proteins were blotted with anti–Gαi-3 (A) or anti-Gαq/11 (B) antibody. Arrowheads indicate the bound Gα. The bindings of RGS18 to Gαi or Gαq with (lane 10) or without (lane 9) AlF4−treatment are shown as controls.
SDF-1 affects the RGS18 binding to Gαi.
ES cell–derived megakaryocytes were stimulated with SDF-1α (lanes 5-8) for 15 minutes (lane 5) or 60 minutes (lane 6) in the presence of TPO (lanes 3, 4, 7, 8) for 15 minutes (lanes 3, 7) or 60 minutes (lanes 4, 8) or in the absence of TPO (lanes 2, 5, 6, 9, 10), and the cell lysates were prepared. In vitro bindings of Gαi (A) or Gαq (B) to GST-RGS18 were performed with the cell lysates, and the bound proteins were blotted with anti–Gαi-3 (A) or anti-Gαq/11 (B) antibody. Arrowheads indicate the bound Gα. The bindings of RGS18 to Gαi or Gαq with (lane 10) or without (lane 9) AlF4−treatment are shown as controls.
Discussion
We report the isolation of an additional novel member of the RGS subfamily B. The existence of a large family of RGS proteins prompted questions about functional differences among members of this family. Our findings suggested that RGS18 expressed in megakaryocytes may act on a specific chemokine receptor–mediated signal. A variety of RGS family members may be required for the specific function of G proteins in response to various and specific stimuli in various tissues. RGS, as well as G proteins, is in general ubiquitously expressed in a variety of tissues. RGS9 is, however, specifically expressed in brain,14-16 and we found that RGS18 is specifically expressed in hematopoietic cells. Therefore, the expression of some RGS family members is restricted to specific tissues. Our findings are the first observation that the binding of endogenous RGS to specific Gα is in response to physiological stimulation, that is, to a specific chemokine receptor–mediated signal. It is crucial to identify a specific physiological stimulus on each RGS and Gα family member in specific tissues to understand this complex G-protein signaling pathway.
Immunofluorescent microscopic analysis clearly showed that RGS18 is a cytosolic protein. However, the subcellular localization of other endogenous RGS family members varies. It was recently reported that RGS2 and RGS10 accumulated in the nucleus, that RGS4 and RGS16 accumulated in the cytoplasm, and that RGSZ localized to the Golgi complex, when these RGSs were forced to be expressed in COS-7 cells.43 The study of intracellular distribution of RGS3 showed that it was diffusely localized in cytoplasm, and agonist stimulation induced its translocation from the cytosol to the plasma membrane when it was forced to be expressed in a human mesangial cell line.44 RGS1 was reported to be localized to the plasma membrane.45 Gα-interacting protein (GAIP), a member of the RGS family, reportedly was membrane bound and behaved as an integral membrane protein.46 The yeast RGS Sst2p was also found in the membrane and copurified with G proteins during cell fractionation.9 Most recently, it was also reported that RGS4 binds to membranes.47 The membrane colocalization of RGS and G proteins would be a reasonable assumption, considering the function of RGS as a Gα-binding protein. It is important to determine the subcellular localization of RGS when it is activated by physiological stimulus. Further characterizations of subcellular localization of RGS18 in response to specific chemokines in megakaryocytes have to be done.
SDF-1 is a member of the CXC family of chemokines constitutively secreted from the bone marrow stromal cells and several other cell types48 and was initially characterized as a pre-B-cell–stimulating factor and as a highly efficient chemotactic factor for T cells and monocytes.49 The biological effects of SDF-1 are mediated through its receptor CXCR4, which is expressed on leukocytes and hematopoietic stem cells.50,51 Unlike most chemokines and chemokine receptors, SDF-1 is the only known ligand for CXCR4, and CXCR4 is the only known receptor for SDF-1.52-55 Genetic elimination of SDF-1 causes perinatal lethality, owing to severe abnormalities in cardiac development, B-cell lymphopoiesis, and bone marrow myelopoiesis.56 Consistent with this last-mentioned finding, SDF-1 was reported to mediate chemotaxis of CD34+stem cells and might play an important role in migration and homing of circulating hematopoietic progenitors to the bone marrow.51,57,58 Recently, it was reported that megakaryocytes and platelets express CXCR4 and that megakaryocytes can migrate through endothelial cell monolayers in response to an SDF-1 concentration gradient.59,60 Although other chemokines, such as platelet factor 4, neutrophil activating peptide 2, macrophage inflammatory protein 1, and C10, inhibited megakaryocyte colony formation,61-64 SDF-1 was reported to augment megakaryocyte development with TPO.59 More recently, it was reported that SDF-1 displays a direct growth-promoting effect on megakaryocyte colony formation in the presence of TPO.32Little is known, however, about SDF-1 receptor–mediated signaling transduction in megakaryocytes. CXCR4 is a member of a 7-transmembrane domain, the G-protein–coupled receptor family.65 SDF-1, binding to CXCR4, mobilizes calcium and reorganizes the actin structure. The chemotactic activity of SDF-1 is blocked by pertussis toxin.49,57,58 66 These results indicate that the CXCR4 receptor transmits signals through Gαi. Consistent with these findings, we found that SDF-1 binding to its receptor CXCR4 affected the binding capacity of RGS18 to Gαi but not to Gαq in megakaryocytes. Therefore, the RGS18-mediated regulations of the Gαi signal pathway are likely to be involved in megakaryocyte differentiation, migration, and/or proliferation. To understand the development of megakaryocytes, further details of the signal transduction pathways induced by SDF-1/CXCR4 involving RGS18 must be clarified.
We thank Dr N. Suzuki for help with GAP assays and Dr T. Katada for helpful advice.
Supported by grants from the Ministry of Education, Science and Culture of Japan, the Science and Technology Agency of Japan, and American Heart Association. T.K. is an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
The isolation of the same gene was recently reported by Park et al.67
Author notes
Yuka Nagata, Tsukuba Life Science Center, The Institute of Physical and Chemical Research (RIKEN), 3-1, Koyadai, Tsukuba, Ibaraki 305-0074, Japan; e-mail: nagata@rtc.riken.go.jp.

![Fig. 2. RGS18 in RGS subfamily B. / (A) Phylogenetic tree of the RGS domains of all RGS family members. The primary amino acid sequences of RGS domains from 48 mammalian RGS proteins were aligned by means of Clustal W1.7. The phylogenetic tree was constructed by the neighbor-joining method by means of Clustal W1.7 on the basis of this alignment and visualized with the Treeview program 1.5. All known RGS proteins (except RGS10 and D-AKAP2) are grouped into 6 subfamilies A to F. Species abbreviations: h, Homo sapiens; m, Mus musculus; r, Rattus norvegicus; b, Bos taurus. The accession numbers for the sequences used in this analysis are as follows: hRGS18, AAF80227; hRGS1, NP_002913; mRGS1, NM_015811; hRGS2, NP_002914; mRGS2, O08849; rRGS2, AF279918; hRGS3,P49796; mRGS3, AF215670; hRGS4, P49798; mRGS4, O08899; rRGS4, P49799; hRGS5, NP_003608; mRGS5, O08850; rRGS5, NM_019341; hRGS6, NP_004287; mRGS6, AAC70011; hRGS7, AAD34290; mRGS7, O54829; rRGS7, BAA75635; bRGS7, O46470; rRGS8, BAA23680; hRGS9, AAC64040; mRGS9, O54828; rRGS9,AAC01959; hRGS10, NP_002916; hRGS11, AAC69175; hRGS12, O14924; rRGS12,O08774; hRGS13, AF030107; mRGS14, P97492; rRGS14, O08773; hRGS16,O15492; mRGS16, P97428; rRGS16, P56700; bRGS16, O46471; hRGS17,AAF08978; hAxin, AAC51624; mAxin, AAC53285; rAxin, AAC40066; bRET-RGS1,P79348; hRGS-GAIP, P49795; hRGSZ1, NP_003693; mRGSZ1, AF191554; mConductin, AAC26047; hp115-RhoGEF, NP_004697; mLsc, AAC52693; hPDZ-RhoGEF, BAA20834; mD-AKAP2, AAC61898. (B) Phylogenetic tree of all RGS subfamily B members constructed with the use of full-length amino acid sequences. (C) Alignment of RGS domains of all RGS subfamily B members. The RGS domain of mouse RGS18 is compared with that of other RGS subfamily B members. The regions of RGS domains are defined according to Tesmer et al.41 The conserved amino acids are shaded. The identical residues in all proteins in the alignment are displayed at the top. An asterisk indicates subfamily B–specific residue (S127 [single-letter amino acid code]). Residue numbers shown at the top correspond with mRGS18. Sources of primary sequences are the same as in panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/10/10.1182_blood.v97.10.3051/6/m_h81011045002.jpeg?Expires=1767851149&Signature=u~nObPGQmsy1bvYNnAfp7VDXjwv~4-MIoVGgBfT~jUtNDFRGfWGc8HhtiP3f2jo4H8qUeGDRgOz93PeKmxfFv31DpHQ2Aud5PP9xK5fVEylgMeZUiZCqbYUzKouNdz1vckJK81aesrZUf8fnhB-0XN3k1VciAoXpOdnfki7W2DL6xz6VDnRvjcu6XxvOEj5HI-ijD6wnPxRKk0TJE4gAuL-T1IJGVQk89h7NmjArWzQ5pRdqTbhHNMNEo56GUBio~imL3yhjIDBpJpG8egRv2sESnwc42f-FOoGSVPZ8Cb~swXuwzksMtm28kKSEO5Bwpdx4x4wOrRniUysz7YJCCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. GAP activity of RGS18 for specific Gα subunits. / Different concentrations of recombinant RGS18 or recombinant RGS4 were tested for their ability to accelerate the GTPase activity of Gαi (A), GαqR183C (B), Gαs (C), and Gα12 (D). Hydrolysis of GTP was initiated by adding the Gα subunit loaded with γ-32P]GTP to a buffer containing MgSO4 with the indicated amount of RGS protein. The reaction mixture was incubated, and aliquots were removed at the indicated times and processed, and the amount of 32Pi was counted by liquid scintillation spectrometry. HED is buffer without RGS protein. The GAP assays were performed twice with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/10/10.1182_blood.v97.10.3051/6/m_h81011045006.jpeg?Expires=1767851149&Signature=ZvdlwnBXC-Wu6w46RMwIiB~fAhUPzmuP0uJ1ENVx4Z27o4dFDCBkzlR8X3c2Wf7l~bZT6F7b1vfP1vGv3PNxTGGk~WWObePVoInw4fVnEmP2c6ovoga2F2XvVNYSu60Rq-BDCvm9VxxitsYXtWApLdb3i2so05RvxrknCY~rlf4sQiDPY4yBrpie~4Le3ptX7O04z9-sK3RJ9VbjnFPGcHD9M3A~8GjJdp~ZHXkoW84a7F009bN~g17PDqOmdKpsD9ICOm0rK6X9WBtKaQNy3P-UV~l3LakOzkuc9jLuIVtCJ4DhhRDrZFJEPVOiefzl6I2hv0SS5XbyCkZOe8QwPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal