Abstract
Plasmodium falciparum is the most lethal form of malaria and is increasing both in incidence and in its resistance to antimalarial agents. An improved understanding of the mechanisms of malarial clearance may facilitate the development of new therapeutic interventions. We postulated that the scavenger receptor CD36, an important factor in cytoadherence of P falciparum–parasitized erythrocytes (PEs), might also play a role in monocyte- and macrophage-mediated malarial clearance. Exposure of nonopsonized PEs to Fc receptor–blocked monocytes resulted in significant PE phagocytosis, accompanied by intense clustering of CD36 around the PEs. Phagocytosis was blocked 60% to 70% by monocyte pretreatment with monoclonal anti-CD36 antibodies but not by antibodies to αvβ3, thrombospondin, intercellular adhesion molecule-1, or platelet/endothelial cell adhesion molecule-1. Antibody-induced CD36 cross-linking did result in the early increase of surface CD11b expression, but there was no increase in, or priming for, tumor necrosis factor (TNF)-α secretion following either CD36 cross-linking or PE phagocytosis. CD36 clustering does support intracellular signaling: Antibody-induced cross-linking initiated intracellular tyrosine phosphorylation as well as extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) phosphorylation. Both broad-spectrum tyrosine kinase inhibition (genistein) and selective ERK and p38 MAPK inhibition (PD98059 and SB203580, respectively) reduced PE uptake to almost the same extent as CD36 blockade. Thus, CD36-dependent binding and signaling appears to be crucial for the nonopsonic clearance of PEs and does not appear to contribute to the increase in TNF-α that is prognostic of poor outcome in clinical malaria.
Introduction
Plasmodium falciparum malaria is the world's most important parasitic disease, responsible for an estimated 300 million to 500 million cases and 1.5 million to 2.7 million deaths annually.1-3 Deaths occur primarily in young children and other nonimmune individuals who are at greatest risk for developing severe and cerebral malaria.1-3 The central pathophysiologic events in falciparum malaria are the sequestration ofP falciparum–parasitized erythrocytes (PEs) in the microvasculature of vital organs and the release of proinflammatory cytokines from cells of the monocyte/macrophage (mφ) lineage.1,4-6 A number of receptors have been implicated in the cytoadherence of PEs to endothelial cells, including intercellular adhesion molecule (ICAM)-1,7,8 vascular cell adhesion molecule (VCAM)-1,9,10 E-selectin,10platelet/endothelial cell adhesion molecule (PECAM)-1/CD31,11 chondroitin 4-sulfate,12thrombospondin (TSP),13αvβ3,14 and CD36.15-19 CD36, an 88-kd integral protein found on endothelial cells, platelets, monocytes, and mφs, has been shown to be a major sequestration receptor recognized by almost all natural isolates of P falciparum.7,20 However, its role in cerebral malaria is unclear because little CD36 is expressed on cerebral microvasculature endothelial cells.6,21 Other adhesion receptors such as ICAM-1 are expressed in cerebral endothelial cells and may be up-regulated by inflammatory cytokines such as tumor necrosis factor (TNF)-α.1 Elevated levels of proinflammatory cytokines such as TNF-α have been associated with severe malaria and a poor prognosis,22-25 and it has been reported that a genetic predisposition to overproduce TNF-α may be associated with the development of cerebral malaria.1,26-28 Taken together, these data suggest that the sequestration of PEs observed in cerebral malaria may result from up-regulated ICAM-1 and other adhesion molecules on the cerebral microvasculature because of excessive or unchecked proinflammatory responses.1,24,26 28
Phagocytic cells are an essential first line of defense against malaria. Circulating monocytes and tissue resident mφs in the liver, spleen, and elsewhere facilitate the control and resolution of infection by clearing PEs.29,30 However, the molecular mechanisms by which these cells recognize PEs is not well understood. To date most studies have concentrated on the phagocytosis of opsonized PEs31-33; however, it is unclear what role this type of clearance plays in the nonimmune individuals most at risk for severe and cerebral malaria. Monocytes can bind PEs in the absence of antibody,34 an event mediated at least in part by CD36,33 and engulf PEs even when complement and Fc receptor pathways are blocked.32 Recent studies in scavenger receptor knockout mice have suggested that this class of monocyte/mφ receptors may be involved in protection against murine malaria.35
We undertook a study of the molecular mechanisms of P falciparum phagocytosis based on the hypothesis that monocyte/mφ scavenger receptor CD36 may play a role in the nonopsonic clearance of PEs. Because a recent study demonstrated that CD36 engagement by TSP activates the p38 mitogen-activated protein kinase (MAPK),36 we further postulated that clustering of CD36 would occur during PE phagocytosis and activate the p38 and the related extracellular signal-regulated kinase (ERK) MAPKs and that this activation would contribute to the ingestion process. We found that nonopsonic phagocytosis of P falciparum PEs occurs in a CD36-dependent fashion that is associated with pronounced clustering of CD36. Both the ERK and p38 MAPKs are phosphorylated upon cross-linking of CD36, and selective pharmacologic inhibition of either MAPK decreases the nonopsonic uptake of PEs by half. Our results suggest that monocytic CD36 uses both the ERK and p38 MAPK signaling cascades to actively participate in the nonopsonic phagocytosis of PEs.
Materials and methods
Media and reagents
Endotoxin-free RPMI media were purchased from Life Technologies, Inc (Burlington, ON, Canada). Fetal calf serum (FCS) was from Hyclone (Logan, UT) and was heat-inactivated at 55°C for 30 minutes. Genistein (Calbiochem, La Jolla, CA), piceatannol (Sigma, Oakville, ON), and PD98059 (RBI, Natich, MA) were prepared in dimethyl sulfoxide. SB203580 was the kind gift of Dr J. C. Lee (SmithKline Beecham) and was suspended in dimethyl sulfoxide. The following mouse immunoglobulin (Ig) G1 monoclonal antibodies were used in phagocytosis or cross-linking studies: anti-CD36 OKM5 (Ortho Diagnostic Systems, Raritan, NJ), FA6-152 (Immunotech, Marseille, France), anti-TSP clone C6.7 (Medicorp, Montreal, QC, Canada), anti-αvβ3 clone 23C6 (Serotec, Raleigh, NC), anti–ICAM-1 clone 15.2 (Santa Cruz Biotech, CA), anti-CD49d hp2.1 (Immunotech), anti-CD45 monoclonal antibody 1214 (PDI Bioscience/Coulter, Montreal, QC, Canada), anti-FcγRIII 3G8 (Immunotech), and anti-CD18 7E4 (Immunotech). IgG2banti–HLA-DR BL2 was from Immunotech, as was goat F(ab′)2antimouse IgG. IgG2a anti-CD31 hec7 (PECAM-1) was from Endogen (Woburn, MA). Escherichia coli O111:B4 endotoxin (LPS) was purchased from Sigma and made up in sterile water. Human IgG Fc fragments were purchased from Calbiochem and trypsin-ethylenediaminetetraacetic acid (EDTA) from Sigma.
Monocyte and PE preparation
Human monocytes were isolated from the blood of healthy volunteers as previously described.37 Briefly, the buffy coat fraction from heparinized whole blood was slowly brought to an osmolarity of 360 mOsm by the addition of sterile 9% NaCl. After centrifugation over a 40/55/58 Percoll gradient at 600g for 30 minutes, the monocyte-rich layer was collected and washed 3 times in cold RPMI. This procedure consistently yields a platelet-free population of purified monocytes (> 96% neutral red granule positive; > 80% CD14+ by flow cytometry), which are not activated (minimal baseline procoagulant activity and TNF-α secretion) and have more than 98% viability by trypan blue exclusion. In some experiments, monocyte-derived mφs were prepared by in vitro incubation of purified monocytes on tissue culture wells for 5 days and maintained in RPMI–10% FCS at 37°C, 5% CO2. P falciparumcultures of the laboratory clone ITG38 were grown and synchronized by sorbitol lysis followed by 24 hours of culture as described previously.39 40 Synchronized trophozoite-stage infected erythrocytes were carefully washed 3 times in RPMI prior to phagocytosis and TNF-α assays. In experiments using trypsinised PEs, PEs were suspended in a 0.05% trypsin-EDTA solution, incubated at 37°C for 30 minutes, and washed twice in RPMI prior to phagocytosis assays.
Phagocytosis assay
About 2.5 × 105 monocytes were adhered to autoclaved glass coverslips placed in 12-well polystyrene culture plates. For studies of nonopsonic phagocytosis, Fc receptors were first blocked by incubating cells with human IgG Fc fragments (Calbiochem, San Diego, CA) at 20 μg/mL for 25 minutes at room temperature. Following incubation with Fc receptors, monocytes or culture-derived mφs were incubated with 10 μg/mL of anti-CD36, CD49d, PECAM-1, ICAM-1, TSP, or αvβ3 antibodies for an additional 25 minutes and washed with 2 changes of RPMI. PEs were suspended in 500 μL of RPMI–10% FCS–L-glutamine (L-G) and added to the monocytes/mφs at a PE:cell ratio of 20:1. In experiments to examine opsonic phagocytosis, PEs were opsonized by exposure to 50% patient serum (heat-inactivated for 30 minutes at 55°C) for 1 hour at 37°C. Control monocytes/mφs were exposed to equivalent numbers of uninfected erythrocytes (UEs). Plates were rotated gently for 4 hours at 37°C, 5% CO2. At the end of this time, nonadherent erythrocytes were washed away with 3 changes of RPMI, and adherent but nonphagocytosed erythrocytes were lysed in ice-cold distilled water for 30 seconds. Cell preparations were fixed and stained with Giemsa. Phagocytosis was assessed by light microscopy. From 500 to 1000 monocytes/mφs were counted for each coverslip and scored for the presence or absence of phagocytosed PEs. Criteria for phagocytosis required the PEs to be contained completely within the monocyte/mφ cell outline. The phagocytic index was calculated as the percentage of monocytes/mφs with clear evidence of phagocytosis.
Immunofluorescence
After lysis of nonphagocytosed PEs and UEs, monocytes were fixed and permeabilized in ice-cold 100% methanol for 60 seconds. Cells were stained for 1 hour with the monoclonal, fluorescein isothiocynate (FITC)-labeled anti-CD36 OKM5 or with murine monoclonal IC4 antibody specific to antigens expressed on the surface of P falciparum–infected erythrocytes.41 Following 5 washes in phosphate-buffered saline (PBS), Texas red–labeled goat antimouse antibody was added to the IC4 preparations and incubated for an additional hour, after which coverslips were washed in PBS and mounted. In colocalization studies, fixed cell preparations were stained first with IC4/antimouse IgG and then with FITC-labeled OKM5. Microscopy was performed with a Bio-Rad MRC 1024ES confocal microscope and analyzed with Lasersharp software.
Surface antigen cross-linking
Surface antigens were cross-linked on purified human monocytes using monoclonal antibodies as previously described.42About 5 × 105 monocytes were suspended in 100-μL RPMI–2% FCS–L-G at 4°C. Fc receptors were blocked by incubating the cells with 20-μg/mL IgG Fc fragments (Calbiochem) at 4°C for 20 minutes followed by washing in RPMI. Surface antigens were then ligated with 10 μg/mL of monoclonal antibody directed against CD36 (OKM5, FA6-152), very late antigen 4 (CD49d, hp2.1), CD18 (7E4), HLA-DR (BL2), or Fc-γRIII (3G8). After a 20-minute incubation at 4°C, cells were washed twice in RPMI, and 5-μg/mL goat antimouse F(ab′)2was added for an additional 20 minutes. After washing in cold RPMI, cells were resuspended in 500-μL RPMI–2% FCS–L-G and placed in the 37°C, 5% CO2 incubator for times ranging from 5 minutes to 4 hours.
Assessment of protein, ERK, and p38 MAPK phosphorylation
Following antigen cross-linking, monocytes were placed on ice, sedimented, and lysed in ice-cold lysis buffer containing 1% Triton X-100, 150-mmol/L NaCl, 10-mmol/L Tris-HCl (pH 7.4), 2-mmol/L sodium orthovanadate, 100-μg/mL leupeptin, 50-mmol/L NaF, 5-mmol/L EDTA, 1-mmol/L EGTA, and 1-mmol/L phenylmethylsulfonyl fluoride. Postnuclear supernatants were collected after centrifugation at 10 000gfor 5 minutes, diluted with 2 × Laemmli buffer, 0.1-mol/L dithiothreitol, and boiled for 4 minutes. Lysates prepared from 100 000 cells were separated on 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Millipore). Blots were then probed with polyclonal rabbit antiphosphotyrosine (Transduction Laboratories, Mississauga, ON, Canada) or antibody specific to the dually phosphorylated, activated forms of the ERK and p38 MAPKs (New England Biolabs, Burlington, ON, Canada). Following incubation with horseradish peroxidase–conjugated secondary antibody (Amersham, Montreal, QC, Canada), blots were developed using an ECL-based system (Amersham).
Measurement of monocyte TNF-α secretion and surface CD11b expression
Four hours after surface antigen cross-linking or exposure to PEs, cell-free supernatants were collected and assayed for soluble TNF-α using a sandwich enzyme-linked immunosorbent assay as described previously.43 Surface CD11b was measured 1 hour after cross-linking of cell surface antigens or exposure of the cells to 1-μg/mL LPS by flow cytometry. Suspended monocytes were washed in cold RPMI and resuspended in RPMI–10% FCS–L-G. Phycoerythrin-labeled anti-CD11b monoclonal antibody (Becton Dickinson) was incubated with the cells for 20 minutes at 4°C. Cells were washed in cold RPMI and evaluated for staining on a Coulter EPICS XL Cytofluorometer.
Statistical analysis
The data are represented as the mean and standard error of the indicated number of experiments. Where representative studies are shown, they are indicative of at least 3 equivalent and independent studies. Statistical comparisons were made for continuous data using one-way ANOVA with post hoc Tukey.
Results
Nonopsonic phagocytosis of P falciparum–infected erythrocytes
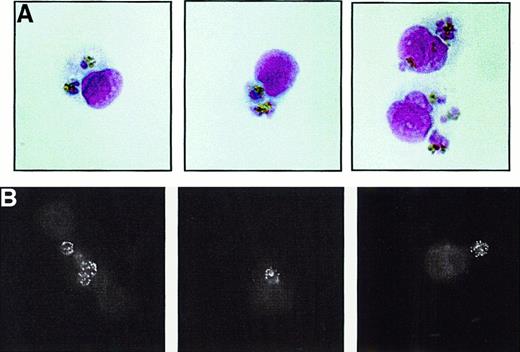
Monocytes are able to phagocytose P falciparum–infected erythrocytes in the absence of opsonization and in the relatively stringent conditions created by a low PE:monocyte ratio. As shown in Figure 1A (light microscopy) and Figure 1B (immunofluorescence with the PE-specific IC4 monoclonal antibody), the incubation of monocytes with PEs in a complement-free environment results in PE uptake despite Fc receptor blockade of the monocytes and no prior PE opsonization. To avoid quantitating PEs attached to the monocyte but not internalized, we employed both a strict lysis procedure to remove adherent erythrocytes and morphologic criteria to ensure that only phagocytosed PEs were counted. As well, PE phagocytosis was routinely confirmed by confocal microscopy. UEs are not taken up by the monocytes. Monocytes did not have to be adherent for phagocytosis to occur: leukocytes in suspension demonstrated an equivalent ability to ingest PEs (data not shown). In time course studies, we found that nonopsonic PE phagocytosis increased in a linear fashion over the 4-hour course of the assay, with 1 to 4 PEs being ingested by monocytes (data not shown).
Nonopsonic phagocytosis of
P falciparum PEs. Synchronized, nonopsonizedP falciparum trophozoite PE cultures were incubated with adherent, Fc receptor–blocked human monocytes in 10% heat-inactivated fetal calf serum for 4 hours as described in “Materials and methods.” Nonadherent erythrocytes were washed away and adherent erythrocytes removed by hypotonic lysis. After washing, monocytes were fixed and prepared for light microscopic examination (A) or for IC4 monoclonal antibody immunofluoresence (B). (A) Three panels of representative monocytes are shown under light microscopy. Each panel contains 1 or 2 monocytes, with several ingested PEs. (B) Three panels of representative monocytes analyzed by IC4 monoclonal antibody immunofluorescence following PE phagocytosis. The typical speckled pattern produced by IC4 binding to the PE surface can be readily appreciated in the ingested PEs. All results shown are indicative of results obtained on at least 3 independent occasions. Original magnification, × 1000.
Nonopsonic phagocytosis of
P falciparum PEs. Synchronized, nonopsonizedP falciparum trophozoite PE cultures were incubated with adherent, Fc receptor–blocked human monocytes in 10% heat-inactivated fetal calf serum for 4 hours as described in “Materials and methods.” Nonadherent erythrocytes were washed away and adherent erythrocytes removed by hypotonic lysis. After washing, monocytes were fixed and prepared for light microscopic examination (A) or for IC4 monoclonal antibody immunofluoresence (B). (A) Three panels of representative monocytes are shown under light microscopy. Each panel contains 1 or 2 monocytes, with several ingested PEs. (B) Three panels of representative monocytes analyzed by IC4 monoclonal antibody immunofluorescence following PE phagocytosis. The typical speckled pattern produced by IC4 binding to the PE surface can be readily appreciated in the ingested PEs. All results shown are indicative of results obtained on at least 3 independent occasions. Original magnification, × 1000.
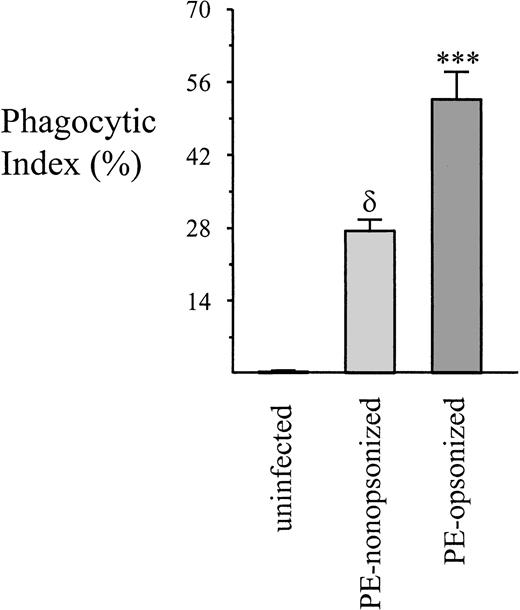
Noncomplement-mediated phagocytosis of PEs has been noted previously, albeit in previously opsonized PEs with a higher PE:monocyte ratio (200-300:1 vs 20:1).32 A direct comparison of opsonic versus nonopsonic PE phagocytosis was undertaken to determine the relative efficiencies of both processes over a 4-hour time span. As seen in Figure 2, opsonic phagocytosis leads to more PE uptake than the nonopsonic process. Moreover, opsonized PEs are consumed more avidly, with the typical monocyte ingesting 4 to 6, as compared with 1 to 4 for nonopsonized PEs. On the other hand, our results with freshly explanted monocytes likely underestimate the potential for nonopsonic PE uptake in vivo. Tissue mφs would be expected to behave more like culture-matured mφs and have been reported to express increased levels of surface CD36.44 To address this issue, we examined the ability of culture-derived mφs to phagocytose PEs. Of note, monocytes induced to differentiate into mφs by a 5-day culture on culture plates expressed significantly higher levels of CD36 and ingested significantly more nonopsonized PEs than did freshly isolated monocytes (Table1). CD36 expression in culture-derived mφs increased a mean of 83.9%, and phagocytosis increased a mean of 124.6%. Anti-CD36 antibodies significantly inhibited phagocytosis by both fresh monocytes and monocyte-derived mφs. Taken together, these results demonstrate that nonopsonic PE phagocytosis occurs and, although less efficient than opsonic uptake, nonetheless could account for considerable ingestion of PEs. In addition, the observation of increased uptake of nonopsonized PEs coincident with increased surface expression of CD36 supports a role for CD36 in this process.
A comparison of nonopsonic and opsonic PE phagocytosis.
PEs were prepared as before or were opsonized by the addition of malarial patient antiserum for 60 minutes. Phagocytosis was allowed to proceed for 4 hours, at which time nonadherent cells were washed away and adherent erythrocytes lysed. The phagocytic index for the 2 groups was calculated as described in “Materials and methods.” Data = mean ± SEM; n ≥ 8 per group. δ, P < .001 vs control UEs; ***, P < .001 vs nonopsonized PEs (ANOVA with post hoc Tukey).
A comparison of nonopsonic and opsonic PE phagocytosis.
PEs were prepared as before or were opsonized by the addition of malarial patient antiserum for 60 minutes. Phagocytosis was allowed to proceed for 4 hours, at which time nonadherent cells were washed away and adherent erythrocytes lysed. The phagocytic index for the 2 groups was calculated as described in “Materials and methods.” Data = mean ± SEM; n ≥ 8 per group. δ, P < .001 vs control UEs; ***, P < .001 vs nonopsonized PEs (ANOVA with post hoc Tukey).
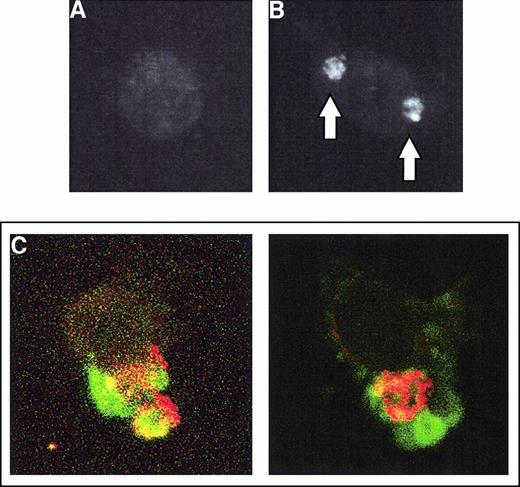
CD36 is clustered during phagocytosis of PEs
To begin to investigate the molecular mechanisms underlying nonopsonic PE phagocytosis, monocytes that had ingested PEs were stained with the OKM5 antibody specific to CD36. Cells that have not ingested PEs are outlined by antibodies specific for CD36 but do not show evidence of CD36 clustering (Figure3A). By contrast, the process of PE uptake by monocytes is accompanied by intense clustering of CD36 in the region of the phagocytosed PEs (Figure 3B). Consistent with a selective role for CD36 in the ingestion of PEs, CD36 clustering is not observed upon ingestion of latex beads coated with Fc fragments (data not shown). Confirmation of the effect is seen in Figure 3C, which demonstrates colocalization of CD36 clustering with ingested PEs as determined by confocal microscopy. As evidenced by these colocalization studies, CD36 clustering was observed in virtually all cells that had ingested PEs. This type of clustering is similar to the clustering of Fc receptors observed during Fc-dependent phagocytosis45and suggests that CD36 is actively involved in the process of PE phagocytosis.
CD36 is clustered during PE phagocytosis.
Purified human monocytes were incubated with PE preparations for 4 hours, at which time the cells were extensively washed, adherent erythrocytes lysed, and monocytes fixed in 100% methanol. (A) A typical monocyte that has not ingested any PEs is stained with FITC-labeled anti-CD36 OKM5 and analyzed by immunofluorescent microscopy. The cell outline can be readily appreciated. (B) A monocyte that has ingested 2 PEs. The position of the PEs as determined by light microscopy is indicated by arrows. The cell is stained with FITC-labeled OKM5 antibody; immunofluorescent microscopy demonstrates the dense clustering of CD36 at the site of PE ingestion. (C) After a 4-hour nonopsonic phagocytosis assay, monocytes were fixed in 100% methanol, stained first with the PE-specific IC4 monoclonal antibody, then with Texas red–labeled antimouse secondary, and finally with FITC-labeled anti-CD36 OKM5 antibody. Each panel shows single monocytes that have ingested 1 (right panel) or 2 (left panel) PEs. Note the dense clustering of CD36 (green) surrounding the PEs (red) and colocalizing with them (as evidenced by yellow staining). Results shown are representative of 4 independent experiments.
CD36 is clustered during PE phagocytosis.
Purified human monocytes were incubated with PE preparations for 4 hours, at which time the cells were extensively washed, adherent erythrocytes lysed, and monocytes fixed in 100% methanol. (A) A typical monocyte that has not ingested any PEs is stained with FITC-labeled anti-CD36 OKM5 and analyzed by immunofluorescent microscopy. The cell outline can be readily appreciated. (B) A monocyte that has ingested 2 PEs. The position of the PEs as determined by light microscopy is indicated by arrows. The cell is stained with FITC-labeled OKM5 antibody; immunofluorescent microscopy demonstrates the dense clustering of CD36 at the site of PE ingestion. (C) After a 4-hour nonopsonic phagocytosis assay, monocytes were fixed in 100% methanol, stained first with the PE-specific IC4 monoclonal antibody, then with Texas red–labeled antimouse secondary, and finally with FITC-labeled anti-CD36 OKM5 antibody. Each panel shows single monocytes that have ingested 1 (right panel) or 2 (left panel) PEs. Note the dense clustering of CD36 (green) surrounding the PEs (red) and colocalizing with them (as evidenced by yellow staining). Results shown are representative of 4 independent experiments.
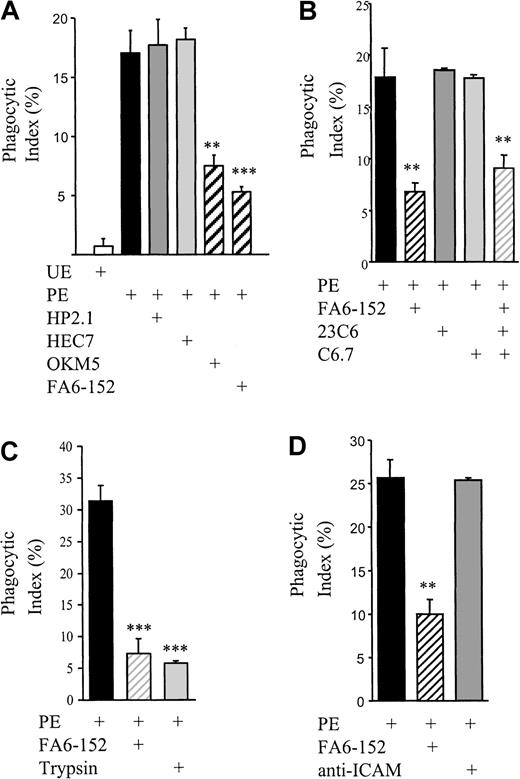
Nonopsonic phagocytosis of PEs is CD36 dependent but does not use the αvβ3-TSP-CD36 phagocytic mechanism
CD36 is involved in PE binding to monocytic cells.16 19 To assess the role of CD36 in nonopsonic PE phagocytosis, CD36 adhesive interactions were blocked by monoclonal antibodies. As seen in Figure 4, pretreatment of monocytes with OKM5 or FA6-152 results in a dramatic decrease in PE phagocytosis, whereas no inhibition was observed with the IgG isotype–matched hp2.1 (CD49d), anti–PECAM-1, or anti–ICAM-1 monoclonal antibodies. All antibodies bound to the monocytes as judged by flow cytometry (data not shown).
Nonopsonic PE phagocytosis is inhibited with anti-CD36 antibodies.
(A) Fc receptor–blocked monocytes were pretreated with 10-μg/mL monoclonal anti-CD36 (OKM5, FA6-152), anti-CD49d (HP2.1), or anti–PECAM-1 (HEC7) antibodies for 20 minutes at room temperature and then washed. Phagocytosis assays with synchronized PE cultures were performed as before. (B) Fc receptor–blocked monocytes were pretreated with 10-μg/mL monoclonal anti-TSP (C6.7), anti-αvβ3 (23C6), or anti-CD36 antibodies for 30 minutes and washed. Phagocytosis assays were performed as before. (C) Synchronized PEs were suspended in a 0.05% trypsin-EDTA solution and incubated at 37°C for 30 minutes. PEs were then washed twice, and phagocytosis assays were performed as before. Alternatively, Fc receptor–blocked monocytes were pretreated with 10-μg/mL monoclonal anti-CD36, and phagocytosis assays were performed as before. (D) Fc receptor–blocked monocytes were pretreated with 10-μg/mL monoclonal anti–ICAM-1 or anti-CD36 antibody for 30 minutes and washed. Phagocytosis assays were performed as before. In all cases, cumulative data from at least 3 independent experiments are presented as mean ± SEM; n ≥ 3 per group. **, P < .01; ***,P < .001 vs PEs (ANOVA with post hoc Tukey).
Nonopsonic PE phagocytosis is inhibited with anti-CD36 antibodies.
(A) Fc receptor–blocked monocytes were pretreated with 10-μg/mL monoclonal anti-CD36 (OKM5, FA6-152), anti-CD49d (HP2.1), or anti–PECAM-1 (HEC7) antibodies for 20 minutes at room temperature and then washed. Phagocytosis assays with synchronized PE cultures were performed as before. (B) Fc receptor–blocked monocytes were pretreated with 10-μg/mL monoclonal anti-TSP (C6.7), anti-αvβ3 (23C6), or anti-CD36 antibodies for 30 minutes and washed. Phagocytosis assays were performed as before. (C) Synchronized PEs were suspended in a 0.05% trypsin-EDTA solution and incubated at 37°C for 30 minutes. PEs were then washed twice, and phagocytosis assays were performed as before. Alternatively, Fc receptor–blocked monocytes were pretreated with 10-μg/mL monoclonal anti-CD36, and phagocytosis assays were performed as before. (D) Fc receptor–blocked monocytes were pretreated with 10-μg/mL monoclonal anti–ICAM-1 or anti-CD36 antibody for 30 minutes and washed. Phagocytosis assays were performed as before. In all cases, cumulative data from at least 3 independent experiments are presented as mean ± SEM; n ≥ 3 per group. **, P < .01; ***,P < .001 vs PEs (ANOVA with post hoc Tukey).
Previous studies examining the phagocytosis of apoptotic cells have shown that monocyte/mφ CD36 cooperates with the vitronectin receptor (αvβ3) and TSP to clear apoptotic cells.46-48 To determine whether CD36 was cooperating in a similar manner in the phagocytosis of nonopsonized PEs, CD36, αvβ3, and TSP were blocked by preincubation of monocytes with the monoclonal antibodies FA6-152, 23C6 (anti-αvβ3), and C6.7 (anti-TSP) alone and in combination. The anti-αvβ3 and anti-TSP antibodies had no effect on phagocytosis of nonopsonised PEs alone or in combination (Figure 4B, and data not shown).
PE adhesion to CD36 is mediated by the variant malarial antigen,P falciparum erythrocyte membrane protein (PfEMP)-1. PfEMP-1 is removed from the surface of the PEs following mild protease treatment.49 We investigated whether the phagocytosis of PEs is dependent upon this CD36 ligand on the infected erythrocyte surface. Proteolytic cleavage of PfEMP-1 from PEs prior to incubation with monocytes reduced their phagocytic clearance to that observed after CD36 receptor blockade with the monoclonal antibodies (Figure4C). These results suggest that CD36-mediated adhesion is central to the nonopsonic phagocytosis of PEs, although our data do not exclude the possible involvement of other receptors.
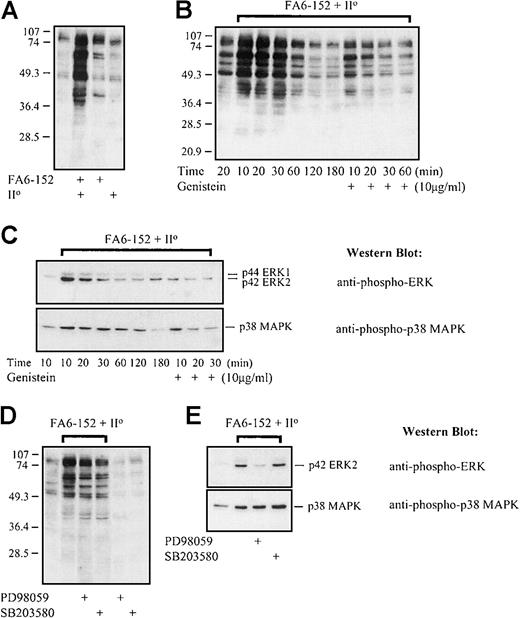
Induction of tyrosine phosphorylation, ERK, and p38 MAPK phosphorylation by CD36 cross-linking: effect of genistein, PD98059, and SB203580
A cell surface receptor actively involved in phagocytosis would be expected to generate intracellular signaling. We mimicked the intense clustering of CD36 observed during PE phagocytosis by first binding the surface antigen with specific monoclonal antibodies and then adding F(ab′)2 fragments directed against the antibody. The method allowed us to study signaling effects specific to CD36 without the confounding effect of other potential PE-monocyte interactions. Cross-linking of CD36 with primary anti-CD36 monoclonals followed by antimouse F(ab′)2 fragments results in intense clustering of CD36, similar to that observed with PE phagocytosis, and an increase in intracellular tyrosine phosphorylation (Figure5A-B), peaking 10 to 20 minutes after cross-linking and then fading (Figure 5B). Importantly, although simple antibody ligation of CD36 did generate a mild increase in tyrosine phosphorylation, cross-linking of the receptor with the addition of F(ab′)2 fragments results in considerably more signaling (Figure 5A). The F(ab′)2 fragments alone did not stimulate an intracellular signal (Figure 5A); nor did they bind to the monocytes (flow cytometric analysis, data not shown). Together these data are consistent with a signaling role for CD36 clustering.
CD36 clustering induces protein tyrosine and MAPK phosphorylation.
Monocyte surface antigen was ligated with primary murine anti-CD36 FA6-152 antibody and cross-linked with the addition of goat antimouse F(ab′)2 fragments. Ligation and cross-linking were performed at 4°C, and cellular reactions were initiated by bringing the cells to 37°C in a 5% CO2incubator. Western blot analysis was performed after lysis of the cells at the times indicated. (A) Western blot staining for tyrosine-phosphorylated proteins after simple antibody ligation of surface CD36 or clustering of CD36 by antibody cross-linking. Note the more intense pattern of tyrosine phosphorylation that follows cross-linking; the secondary F(ab′)2 alone does not induce intracellular tyrosine phosphorylation. (B) Western blot staining for tyrosine-phosphorylated proteins after cross-linking of CD36 in the presence or absence of genistein (10 μg/mL). Note the time course of phosphotyrosine accumulation, which peaks 10 to 20 minutes after cross-linking. (C) Accumulation of dually phosphorylated forms of the ERK and p38 MAPKs after cross-linking of surface CD36. In both cases, phosphorylated forms can be appreciated 60 to 120 minutes after cross-linking. Pretreatment of monocytes with genistein abolishes the induction of ERK phosphorylation and inhibits p38 MAPK phosphorylation. (D) Effect of monocyte pretreatment with PD98059 (50 μmol/L) or SB203580 (30 μmol/L) on the induction of tyrosine phosphorylation following cross-linking of CD36. Cells were lysed after 10 minutes of incubation at 37°C, 5% CO2. (E) Effect of monocyte pretreatment with PD98059 (50 μmol/L) or SB203580 (30 μmol/L) on CD36-dependent induction of ERK and p38 MAPK phosphorylation. Cells were lysed after a 20-minute incubation at 37°C, 5% CO2. All studies are representative of results obtained on at least 3 separate occasions. Although only data with the FA6-152 clone are presented, similar results were obtained with the OKM5 antibody.
CD36 clustering induces protein tyrosine and MAPK phosphorylation.
Monocyte surface antigen was ligated with primary murine anti-CD36 FA6-152 antibody and cross-linked with the addition of goat antimouse F(ab′)2 fragments. Ligation and cross-linking were performed at 4°C, and cellular reactions were initiated by bringing the cells to 37°C in a 5% CO2incubator. Western blot analysis was performed after lysis of the cells at the times indicated. (A) Western blot staining for tyrosine-phosphorylated proteins after simple antibody ligation of surface CD36 or clustering of CD36 by antibody cross-linking. Note the more intense pattern of tyrosine phosphorylation that follows cross-linking; the secondary F(ab′)2 alone does not induce intracellular tyrosine phosphorylation. (B) Western blot staining for tyrosine-phosphorylated proteins after cross-linking of CD36 in the presence or absence of genistein (10 μg/mL). Note the time course of phosphotyrosine accumulation, which peaks 10 to 20 minutes after cross-linking. (C) Accumulation of dually phosphorylated forms of the ERK and p38 MAPKs after cross-linking of surface CD36. In both cases, phosphorylated forms can be appreciated 60 to 120 minutes after cross-linking. Pretreatment of monocytes with genistein abolishes the induction of ERK phosphorylation and inhibits p38 MAPK phosphorylation. (D) Effect of monocyte pretreatment with PD98059 (50 μmol/L) or SB203580 (30 μmol/L) on the induction of tyrosine phosphorylation following cross-linking of CD36. Cells were lysed after 10 minutes of incubation at 37°C, 5% CO2. (E) Effect of monocyte pretreatment with PD98059 (50 μmol/L) or SB203580 (30 μmol/L) on CD36-dependent induction of ERK and p38 MAPK phosphorylation. Cells were lysed after a 20-minute incubation at 37°C, 5% CO2. All studies are representative of results obtained on at least 3 separate occasions. Although only data with the FA6-152 clone are presented, similar results were obtained with the OKM5 antibody.
Antibody-induced clustering of CD36 also leads to accumulation of the dually phosphorylated, active forms of the p42 ERK2, p44 ERK1, and p38 MAPK as judged by Western blot analysis (Figure 5C). Phosphorylation of both the ERK and p38 MAPK proteins peaks within 10 to 20 minutes of CD36 cross-linking but persists through 60 to 120 minutes (Figure 5C). Pretreatment of monocytes with the broad-spectrum tyrosine kinase inhibitor genistein greatly attenuates the accumulation of tyrosine phosphoproteins after CD36 cross-linking, abrogates the induction of phosphorylated ERK, and attenuates the phosphorylation of the p38 MAPK (Figure 5B-C). By contrast, CD36 cross-linking continues to induce phosphorylation of cellular proteins on tyrosine residues after monocyte pretreatment with the MEK-1 selective inhibitor, PD98059, or the p38 MAPK-selective inhibitor, SB203580 (Figure 5D). Consistent with their mechanisms of action, PD98059 abolishes CD36-dependent phosphorylation of the ERK MAPK but has no effect on p38 MAPK phosphorylation, and SB203580, which directly inhibits p38 MAPK activity, does not inhibit either ERK or p38 MAPK phosphorylation (Figure 5E). Taken together, these studies demonstrate that cross-linking of CD36 results in the prolonged phosphorylation of both the ERK and p38 MAPKs.
In PE phagocytosis studies, we also observed a similar increase in phosphorylation of the ERK and p38 MAPKs associated with the ingestion of PEs by human monocytes (data not shown). However, because it is difficult to exclude other possible PE-monocyte–induced signaling events associated with these complex cell-cell interactions, these studies are less specific than the antibody cross-linking studies described above.
PE phagocytosis is dependent upon the signaling cascades induced by CD36 clustering
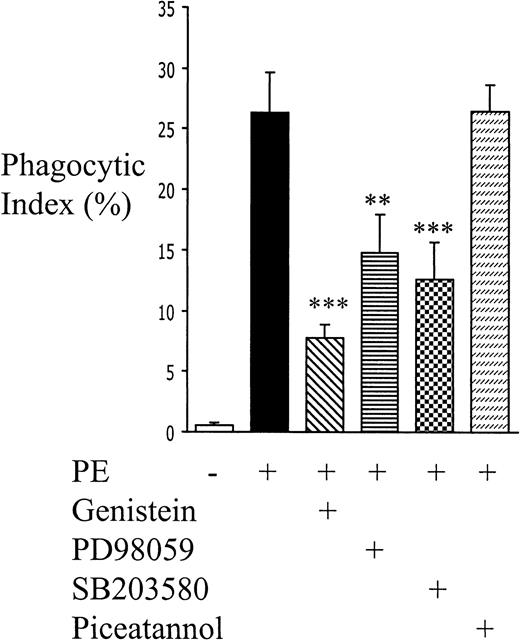
To determine whether the intracellular signals induced by CD36 cross-linking contribute to the process of PE phagocytosis, monocytes were pretreated with the broad-spectrum tyrosine kinase inhibitor genistein or with selective ERK and p38 pathway inhibitors (PD98059 and SB203580, respectively) or with the Syk kinase–specific inhibitor piceatannol. Genistein, PD98059, and SB203580 diminished phagocytosis by 60%, 40%, and 50%, respectively, and piceatannol had no inhibitory effect (Figure 6). These data suggest that the ERK and p38 MAPK signals are important to nonopsonic PE phagocytosis.
Tyrosine phosphorylation, ERK, and p38 MAPK play roles in nonopsonic PE phagocytosis.
Monocytes were pretreated with genistein (10 μg/mL), PD98059 (50 μmol/L), SB203580 (30 μmol/L), or piceatannol (30 μg/mL) for 1 hour at room temperature, and PE phagocytosis was evaluated as before. Cumulative data from at least 4 independent experiments are presented as mean ± SEM; n ≥ 6 per group. **, P < .01; ***, P < .001 vs PEs (ANOVA with post hoc Tukey).
Tyrosine phosphorylation, ERK, and p38 MAPK play roles in nonopsonic PE phagocytosis.
Monocytes were pretreated with genistein (10 μg/mL), PD98059 (50 μmol/L), SB203580 (30 μmol/L), or piceatannol (30 μg/mL) for 1 hour at room temperature, and PE phagocytosis was evaluated as before. Cumulative data from at least 4 independent experiments are presented as mean ± SEM; n ≥ 6 per group. **, P < .01; ***, P < .001 vs PEs (ANOVA with post hoc Tukey).
Several lines of evidence suggest that our results are specific and not due to nonspecific pharmacologic effects of PD98059 or SB203580. First, we found no evidence of a nonspecific toxic effect. None of the inhibitors employed exerted monocyte toxicity, as evidenced by more than 96% cell viability (trypan blue exclusion) after 6-hour incubations in their presence. Importantly, the pharmacologic agents did not affect baseline surface CD36 expression over a 6-hour incubation period (flow cytometric data, not shown) and did not affect the number of PEs adhering to the monocyte surface (data not shown). Secondly, both PD98059 and SB203580 may inhibit the cyclooxygenase enzyme.50 However, pretreatment of monocytes with a potent cyclooxygenase inhibitor, indomethacin (100 μmol/L), has no effect on the ingestion of nonopsonized PEs (data not shown). Third, in dose-response experiments we found that 1-, 10-, and 30-μmol/L doses of SB203580 were equally effective at inhibiting nonopsonic PE ingestion (data not shown). Finally, the inhibition of nonopsonized PE uptake by PD203580 and SB203580 is quantifiably different from the effects of these inhibitors on opsonized PE ingestion. In contrast to the 40% inhibition observed with nonopsonic uptake, we found that pretreatment of monocytes with PD98059 (50 μmol/L) inhibited phagocytosis of opsonized PEs by only 9.7% ± 3.5% (mean ± SEM; n = 6). Similarly, SB203580 (1 μmol/L) inhibited nonopsonic ingestion of PEs by 50%, but the same dose had little to no effect on opsonic phagocytosis (inhibition 3.0 ± 3.6%; n = 3). In addition, piceatannol,51 a known inhibitor of Fc-mediated phagocytosis,52 had no inhibitory effect on the phagocytosis of nonopsonized PEs (Figure 6). Thus, the inhibition of nonopsonized PE uptake by PD98059 and SB203580 appears to reflect selective effects of the ERK and p38 MAPK inhibitors and to differ from opsonic phagocytosis.
CD36 cross-linking induces increased CD11b expression but not increased TNF-α secretion: implications for PE phagocytosis
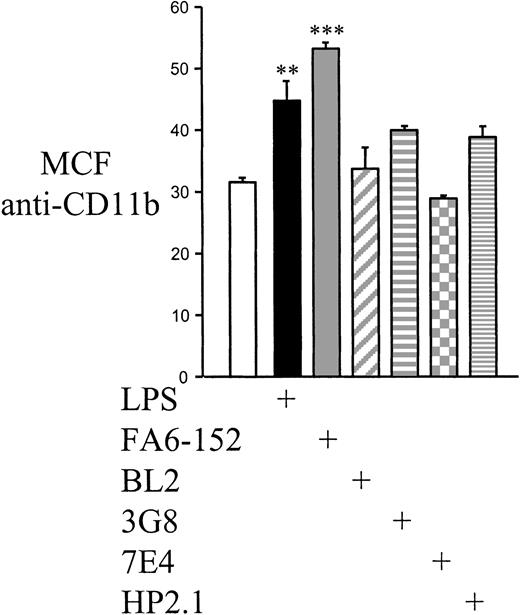
Previous work has established that CD36 engagement by monoclonal antibody or binding of PEs can result in an early increase in monocyte oxidative burst.53 Consistent with the finding that CD36 engagement triggers early monocyte responses, we found that within 1 hour of CD36 cross-linking there is increased surface expression of CD11b (Figure 7). The increase was similar to that observed with a 1-μg/mL dose of LPS; cross-linking of a variety of other monocyte surface antigens, including CD49d, CD18, HLA-DR, and FcγRIII, results in no or less marked CD11b up-regulation.
Up-regulation of surface monocyte CD11b by cross-linking of CD36.
One hour after treatment with a 1-μg/mL dose of LPS or cross-linking of surface CD36 (FA6-152), HLA-DR (BL2), FcγRIII (3G8), CD18 (7E4), or CD49d (hp2.1), monocytes were washed extensively and evaluated for surface expression of CD11b as described in “Materials and methods.” Points are taken in triplicate and are representative of results obtained in 4 separate experiments. MCF indicates median channel fluoresence; **, P < .01; ***,P < .001 vs mock–cross-linked cells (ANOVA with post hoc Tukey).
Up-regulation of surface monocyte CD11b by cross-linking of CD36.
One hour after treatment with a 1-μg/mL dose of LPS or cross-linking of surface CD36 (FA6-152), HLA-DR (BL2), FcγRIII (3G8), CD18 (7E4), or CD49d (hp2.1), monocytes were washed extensively and evaluated for surface expression of CD11b as described in “Materials and methods.” Points are taken in triplicate and are representative of results obtained in 4 separate experiments. MCF indicates median channel fluoresence; **, P < .01; ***,P < .001 vs mock–cross-linked cells (ANOVA with post hoc Tukey).
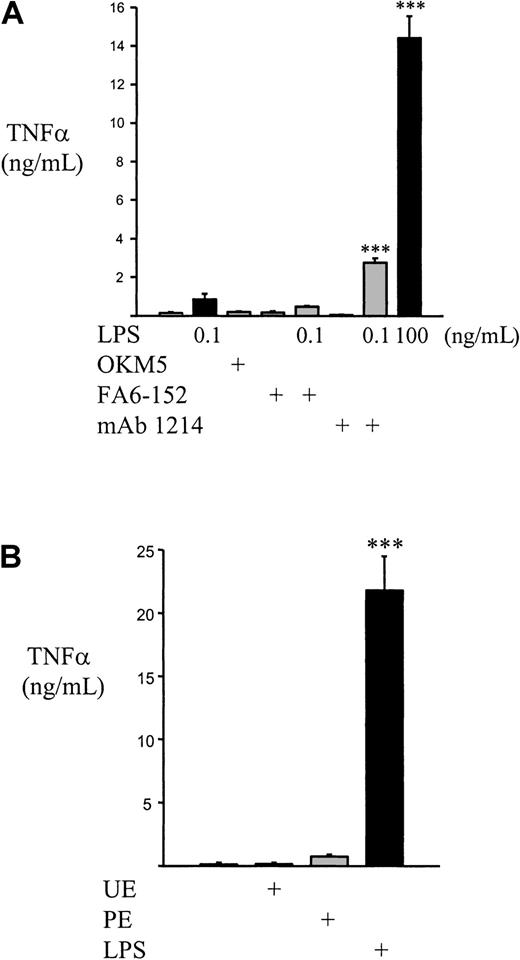
Human monocytes/mφs produce TNF-α in response to falciparum malaria, and Nagao et al5 have reported that fresh human monocytes produce significantly more TNF-α than culture-derived mφs in response to infection. To determine if CD36 binding and activation was involved in TNF-α production, we examined human monocytes for TNF-α secretion following CD36 cross-linking and CD36-mediated PE ingestion. In contrast to the observed up-regulation of CD11b, CD36 cross-linking does not result in increased secretion of TNF-α after a 4-hour incubation; a 100-ng/mL dose of LPS leads to a pronounced up-regulation of soluble TNF-α over the same time span (Figure8A). Although cross-linking of CD45 primes the monocyte for increased TNF-α secretion in response to low-dose LPS (0.1 ng/mL), CD36 cross-linking does not. Similarly, we did not find an increase in 4-hour monocyte procoagulant activity with CD36 cross-linking (data not shown). These results suggest that, despite the up-regulation of earlier monocyte responses, CD36 cross-linking does not lead to activation of monocyte cytokine secretion or to the induction of monocyte procoagulant inflammatory mediators. Similarly, the phagocytosis of intact PEs by monocytes does not lead to a significant increase in TNF-α release (Figure 8B). However, consistent with the observation that a glycosyl-phosphatidyl inositol (GPI) toxin released at schizont rupture induces TNF-α secretion,54 55 the incubation of lysed PE preparations did induce a marked increase in soluble TNF-α release (14.99 ± 1.33 ng/mL in a typical experiment; n = 3). These data suggest that CD36 clustering, either by antibody or during PE phagocytosis, leads to an increase in early markers of monocyte activation but that the secretion of TNF-α is not increased by CD36 engagement and signaling.
CD36 cross-linking and PE phagocytosis do not induce TNF-α secretion.
(A) 5 × 105 monocytes were treated with F(ab′)2 fragments alone or cross-linking with 10-μg/mL CD36 (FA6-152, OKM5) or CD45 (monoclonal antibody 1214) followed by 10-μg/mL F(ab′)2 fragments, suspended in 500-μL RPMI–10% FCS–L-G, and exposed to the presence or absence of low-dose LPS (0.1 ng/mL). High-dose (100 ng/mL) LPS stimulation was used as a positive control. Cells were incubated for 4 hours at 37°C, 5% CO2; pelleted; and soluble TNF-α determined from the supernatants by enzyme-linked immunosorbent assay. CD36 cross-linking neither increased baseline TNF-α secretion nor primed for increased secretion in response to low-dose LPS, but CD45 cross-linking did prime for increased TNF-α following low-dose LPS. (B) 2.5 × 105 monocytes were allowed to adhere to plastic culture wells and incubated with medium alone, UEs, PEs (carefully synchronized to the trophozoite stage), or 1-μg/mL LPS for 4 hours at 37°C, 5% CO2. During this time, there was minimal PE rupture. At the end of 4 hours, the medium was collected, nonadherent cells pelleted, and soluble TNF-α determined. In panels A and B, n ≥ 3 per group and data are representative of results obtained in 3 separate experiments. Data = mean ± SEM. ***,P < .001 vs mock–cross-linked or unstimulated control cells (ANOVA with post hoc Tukey).
CD36 cross-linking and PE phagocytosis do not induce TNF-α secretion.
(A) 5 × 105 monocytes were treated with F(ab′)2 fragments alone or cross-linking with 10-μg/mL CD36 (FA6-152, OKM5) or CD45 (monoclonal antibody 1214) followed by 10-μg/mL F(ab′)2 fragments, suspended in 500-μL RPMI–10% FCS–L-G, and exposed to the presence or absence of low-dose LPS (0.1 ng/mL). High-dose (100 ng/mL) LPS stimulation was used as a positive control. Cells were incubated for 4 hours at 37°C, 5% CO2; pelleted; and soluble TNF-α determined from the supernatants by enzyme-linked immunosorbent assay. CD36 cross-linking neither increased baseline TNF-α secretion nor primed for increased secretion in response to low-dose LPS, but CD45 cross-linking did prime for increased TNF-α following low-dose LPS. (B) 2.5 × 105 monocytes were allowed to adhere to plastic culture wells and incubated with medium alone, UEs, PEs (carefully synchronized to the trophozoite stage), or 1-μg/mL LPS for 4 hours at 37°C, 5% CO2. During this time, there was minimal PE rupture. At the end of 4 hours, the medium was collected, nonadherent cells pelleted, and soluble TNF-α determined. In panels A and B, n ≥ 3 per group and data are representative of results obtained in 3 separate experiments. Data = mean ± SEM. ***,P < .001 vs mock–cross-linked or unstimulated control cells (ANOVA with post hoc Tukey).
Discussion
In these studies we describe a novel role for the monocyte/mφ scavenger receptor CD36 in a P falciparum clearance mechanism that is likely to be relevant to the nonimmune patient population. Highly purified human monocytes and culture-derived mφs ingested large numbers of nonopsonized PEs over 4 hours when presented with PEs at a relatively low target:effector ratio. PE uptake was strongly inhibited by CD36 blockade or by cleavage of the CD36 ligand from the surface of PEs. PE uptake was accompanied by intense CD36 clustering around the phagocytosed PEs. Although CD36 clustering leads to increased surface CD11b within 1 hour, neither CD36 cross-linking nor PE phagocytosis increases the 4-hour secretion of TNF-α. Consistent with an active role of CD36 engagement in PE phagocytosis, cross-linking of CD36 initiates an intracellular signal characterized by protein tyrosine phosphorylation and MAPK family member recruitment. Just as the majority of nonopsonic phagocytosis is inhibited by CD36 blockade, so is PE uptake attenuated by pharmacologic inhibition of CD36-dependent signaling. These results suggest that monocytic CD36 contributes to the nonopsonic phagocytosis of P falciparum–infected erythrocytes.
In this study the majority of nonopsonic P falciparum–infected erythrocyte phagocytosis is dependent on surface CD36 and not on other described malarial receptors such as ICAM-1, TSP, αvβ3, or PECAM-1. These data are consistent with a large body of evidence demonstrating that CD36 plays an important role in PE binding to monocytes, melanoma C32 cells, and endothelial cells.15,16,18,19 Interestingly, CD36 transfection to Bowes melanoma cells has been described to directly confer the ability to phagocytose apoptotic neutrophils, lymphocytes, and fibroblasts,56 and several studies have implicated CD36 in the ingestion of apoptotic cells by primary phagocytes.46-48 These findings provide theoretical support for the participation of CD36 in phagocytic processes. It is possible that the nonopsonic phagocytosis of PEs is part of a general mechanism of clearance of senescent cells. Earlier work suggested that PE uptake was partially dependent on phosphatidyl serine,32 a phospholipid expressed in increased amounts in both senescent erythrocytes and PEs.57-60 Recent studies found that PE uptake was increased in oxidation-sensitive glucose-6-phosphate dehydrogenase–deficient erythrocytes, suggesting that the additional oxidative stress of P falciparum infection might promote red blood cell senescence and, therefore, phagocytosis of parasitized cells.31 However, increased phagocytosis was observed primarily in ring-stage PEs, not in the trophozoite-stage PEs used in our study. Furthermore, the uptake of senescent cells is clearly a function of multiple mφ surface antigens,47,48 and it has been postulated that the principal mφ receptor for senescent erythrocytes is distinct from CD36.57,61 Given that we found a large inhibition of PE uptake either by blockade of CD36 alone or by the proteolytic removal of the CD36 ligand on PEs, it seems more likely that monocyte/mφ phagocytosis of PEs is dependent on the PfEMP-1 family of variable surface proteins that are expressed in PEs and mediate adherence to CD36.49,62,63 Furthermore, the inability to block phagocytosis with anti-αvβ3 and anti-TSP antibodies suggests that the mechanism of CD36-mediated phagocytosis of PEs is distinct from the cooperative αvβ3-TSP-CD36 mechanism previously described for the clearance of apoptotic cells.46 48
It is unknown why only a small proportion of P falciparum–infected individuals develop severe or cerebral malaria. Elevated levels of TNF-α are associated with severe malaria and a poor prognosis, leading to the hypothesis that excessive secretion of proinflammatory cytokines such as TNF-α may promote cerebral malaria. The finding that monocyte CD36 engagement does not lead to increased TNF-α secretion is consistent with a protective effect in malarial pathogenesis. We found that neither CD36 cross-linking nor nonopsonic PE ingestion led to monocyte TNF-α release (Figure 8). In contrast, previous studies have noted an association between PE uptake and TNF-α secretion64; however, these were performed with less tightly synchronized P falciparum cultures than are used in the studies presented here and may reflect the effects of GPI toxins released during schizont rupture.54 Alternatively, TNF-α release may have been secondary to Fc receptor–mediated phagocytosis of opsonized PEs, because Fc receptor cross-linking has been noted to induce monocyte TNF-α secretion.65 66 These stimuli for TNF-α secretion were not present in our studies because we used only washed, tightly synchronized P falciparum cultures, and Fc receptors were blocked prior to PE phagocytosis.
Previous studies have described a role for CD36 in the induction of an early monocyte respiratory burst.53 Although we did find an early, CD36-dependent increase in surface CD11b (Figure 7), the significance of this up-regulation is unclear. CD11b functions in part to promote leukocyte adhesion, but human malaria—unlike rodent malaria—is not generally characterized by leukocyte adhesion to the vascular bed.6 Because CD11b can function in complement-mediated phagocytosis, it may be that the increased CD11b is related to an increased potential for opsonic PE phagocytosis.
It has been reported that mφ ingestion of opsonized PEs or large amounts of hemozoin results in impaired phagocytic function and decreased expression of major histocompatibility complex class II antigen.32,67,68 These observations have led to the suggestion that hemozoin loading of mφs may impair both nonspecific and specific immune responses by inhibiting phagocytosis and antigen presentation, respectively. Despite these considerations, most falciparum-infected individuals do not progress to severe or cerebral malaria and control their infections due, at least in part, to the activity of circulating and tissue-resident monocytes/mφs. Whether CD36-mediated PE phagocytosis will lead to similar mφ impairment is currently under investigation. However, it should be stressed that even if the phagocytic function of the circulating pool of monocytes is permanently decreased, these cells are replaced during the course of clinical infection by fresh phagocytes. In fact, although the early phases of falciparum infection are characterized by loss of phagocytic activity in monocyte-derived mφs, the recovery phase is accompanied by the return of highly phagocytic cells.69 In other words, the recovery phase of human malaria is coincident with an increased capacity for its clearance.
We present evidence that protein tyrosine phosphorylation and, specifically, recruitment of the ERK and p38 MAPKs follows CD36 clustering. CD36 has been associated with Src family kinases and recently in the activation of p38 MAPK,36,70,71 providing a possible link between CD36 clustering, increased tyrosine phosphorylation, and accumulation of active ERK and p38 MAPK moieties. Src family kinases have been demonstrated to be upstream of the low molecular weight GTPases involved in activation of MAPK members.72,73 Importantly, we found that clustering of surface CD36 produced far more signaling than simple divalent ligation of the receptor (Figure 5A). This mechanism of signaling is similar to that seen with integrins42 74 and argues strongly that the clustering observed during PE uptake is sufficient to induce intracellular signaling. Pharmacologic inhibition of the intracellular signals induced by CD36 clustering reduces PE phagocytosis, suggesting that CD36 is an active participant in the phagocytic process. The mechanism linking CD36 clustering to PE phagocytosis involves the ERK and p38 MAPKs (Figure 6); further studies are required to elucidate the precise link between CD36 clustering and these pathways.
Similarly, it will be of interest to define how the MAPKs contribute to monocyte PE phagocytosis. Recent studies indicate that the ERK and p38 MAPKs may be directly or indirectly associated with cytoskeletal elements such as microtubules and actin and phosphorylate regulatory proteins, providing a possible link between their activation and phagocytosis.75-77 However, the roles of the ERK and p38 MAPKs in phagocytosis are likely to be cell- and stimulus-specific.78-80
The identification of CD36 as a major sequestration receptor has led to the assumption that it contributes to the pathophysiology of severe malaria and has prompted the development of antiadherence therapies to disrupt the CD36-PE interaction.7,16,62,81 However, unlike ICAM-1, little if any CD36 is expressed on cerebral endothelial cells or renal glomeruli.6,21,82 CD36 is known to be well expressed in microvascular endothelial cells from skin, muscle, and sites rich in resident mφs, such as liver, spleen, and the reticuloendothelial system.6 Almost all natural P falciparum isolates bind CD36, but only a small proportion of infected individuals develop severe or cerebral malaria. Furthermore, recent studies have reported that significantly higher binding of PEs to CD36 occurs in cases of nonsevere malaria.83 84Collectively, these observations suggest that the CD36-PE interaction might represent a parasite-host adaptation, evolved for improved survival of the parasite with limited pathogenicity for the host (parasite sequestration in nonvital vascular beds with parasite replication balanced by host clearance). In support of this hypothesis, our data are most consistent with a protective role for monocyte/mφ CD36 by mediating clearance of P falciparum PEs and by not contributing to TNF-α release. If confirmed in vivo, these results have important therapeutic implications. First, strategies to disrupt CD36-PE interactions may be deleterious if they inhibit CD36-mediated PE phagocytosis and displace PEs from CD36 on endothelial cells in nonvital sites to receptors on the cerebral vasculature such as ICAM-1. Secondly, clinical malaria might benefit from selective up-regulation of monocyte/mφ CD36, particularly in the nonimmune host where opsonic phagocytosis would be expected to be less.
Acknowledgments
The authors thank Ziyue Lu (Toronto Hospital) for technical assistance and are indebted to Dr M. J. Phillips (Department of Pathology, University of Toronto, Toronto Hospital) for his help with light microscopic analysis, Dr I. Crandall (University of Toronto) for the kind gift of antibody IC4, and Drs. A. Gotlieb and A. Rosenthal (Department of Pathology, University of Toronto, Toronto Hospital) for their kind assistance with confocal microscopy.
Supported by the Medical Research Council of Canada operating grants MT-13721 (K.C.K.) and GR-13298 (O.R.), World Health Organization TDR Programme (TDR 920223; KC.K.), the Heart and Stroke Foundation of Canada (NA-3391; K.C.K.), and a Career Scientist Award from the Ontario Ministry of Health (K.C.K.).
I.D.M. is the recipient of a Medical Research Council of Canada fellowship. L.S. is the recipient of a Medical Research of Canada studentship.
I.D.M. and L.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kevin Kain, Tropical Disease Unit, EN G-224, Toronto General Hospital, 200 Elizabeth St, Toronto, Ontario, Canada M5G 2C4; e-mail: kevin.kain@uhn.on.ca.