Abstract
Interferon (IFN)-α has proven useful for treating several clinical conditions, including chronic viral hepatitis and chronic myeloproliferative and lymphoproliferative disorders. In addition to its well-known antiviral effects, the cytokine exerts antiproliferative effects on many cell types, helping to explain its therapeutic usefulness in these latter conditions. However, this same property accounts for several undesirable effects, including thrombocytopenia, which can interfere with the successful clinical application of IFN-α. Unfortunately, the mechanisms responsible for the myelosuppressive effects of the cytokine are incompletely understood. The effects of IFN-α on megakaryocyte (MK) development were studied. Using several marrow cell purification techniques and quantitative culture methods, it was found that IFN-α directly inhibits thrombopoietin (TPO)-induced MK growth. Previous studies indicated that Janus kinase (JAK) and its substrates mediate the effects of TPO on cellular proliferation and survival. It was found that IFN-α directly suppresses TPO-induced phosphorylation of the JAK2 substrates c-Mpl and STAT 5 in a TPO-dependent hematopoietic cell line and of Mpl and STAT3 in primary murine MK. Moreover, IFN-α induces SOCS-1 production in these cells, which has been shown to inhibit TPO-induced cell growth. Because SOCS protein expression is induced by many cytokines and has been reported to extinguish signaling from several hematopoietic cytokine receptors, these results identify a molecular mechanism responsible for cytokine receptor cross-talk.
Introduction
Interferon (IFN)-α has proven a useful therapeutic agent in a number of clinical settings, including chronic viral infections (eg, hepatitis B, C, and G)1-3 and several neoplastic disorders (eg, chronic myelogenous leukemia [CML],4 essential thrombocythemia,5 Hodgkin disease,6 and non-Hodgkin lymphoma7). The efficacy of the cytokine is in large measure mediated by its antiproliferative effect on a variety of cells, at least in the latter settings. However, in addition to its desired effect to reduce viral or pathologic cell growth, the antiproliferative effects of the cytokine are associated with detrimental actions. For example, in several series of patients treated with IFN-α for viral hepatitis, thrombocytopenia developed8-12 and interfered with continued full-dose therapy in the more severely affected patients.8-10
Although some previous studies suggest that immune mechanisms mediate the thrombocytopenic effects of IFN-α,13,14 other investigations have focused on the myelosuppressive effects of the cytokine. Several studies using murine or human marrow cells have shown that IFN-α can suppress megakaryocyte (MK) formation. Concentrations of 10 to 1000 U/mL IFN-α inhibit MK colony formation by 30% to 60% and MK growth in suspension cultures by a similar degree.15-18 However, all the reported studies have used whole marrow cells or minimally purified fractions of whole marrow (eg, low-density, adherence-depleted cells). Because IFN-α has also been shown to inhibit the growth of marrow-derived fibroblasts,16,19 cells that are present in whole marrow cultures and in minimally fractionated cell populations and that form a large part of the hematopoietic microenvironment, it is possible that IFN-α exerts only an indirect effect on megakaryopoiesis. Work with other hematopoietic cells also suggests that the effects of IFN-α may be indirect, mediated by the marrow microenvironment. For example, neoplastic cells derived from patients with CML adhere poorly to marrow stromal cells; IFN-α has been shown to enhance CML cell adhesion to marrow stromal cells, re-establishing the capacity of the marrow microenvironment to regulate hematopoietic cell growth.20Moreover, IFN-α–induced erythroid cell suppression is reportedly caused by a soluble factor released from T lymphocytes.21Unfortunately, whether these IFN-α effects on the marrow stroma, cellular adhesion, or T cells underlie its role in the growth of MK and the molecular mechanisms by which IFN-α leads to myelosuppression is not yet fully understood.
Recently, work from other laboratories and from our own has led to the cloning and characterization of thrombopoietin (TPO), the primary regulator of MK and platelet production.22 Like other members of the type 1 family of hematopoietic cytokine receptors, the TPO receptor (c-Mpl) induces its biologic effects on ligand-induced multimerization by the activation of Janus kinase (JAK). Although a member of the type 2 family of cell surface receptors, the IFN receptors also transduce cellular effects by the induction of JAK, which, in turn, triggers many of the same signaling pathways initiated by TPO and other hematopoietic cytokines. These findings suggest that some form of cross-talk between TPO and IFN receptors might mediate the effects of IFN-α on MK development. The recent cloning and characterization of suppressors of cytokine signaling (SOCS; alternatively termed cytokine-inducible SH-2 protein [CIS], signal transduction and activators of transcription [STAT]-induced STAT inhibitor [SSI], and JAK binding [JAB] protein), a recently discovered family of STAT-induced proteins that serves to extinguish cytokine signaling (reviewed in Hilton23), provide an attractive molecular mechanism to link these 2 events.
To address these issues we investigated the mechanism(s) of IFN-α inhibition of TPO-induced MK growth in suspension and semisolid clonal assays, using whole marrow cells and purified populations of marrow progenitors. Additionally, to investigate the molecular basis of the effects of IFN-α on hematopoiesis, we tested whether the cytokine inhibits TPO-induced signaling, specifically through the induction of SOCS proteins. Our results indicate that IFN-α acts directly on MK progenitors to inhibit their proliferation and to reduce TPO-induced Mpl receptor signaling and that the induction of SOCS-1 is likely, at least in part, responsible for these findings.
Materials and methods
Reagents and cell lines
Murine IFN-α was purchased from Calbiochem (San Diego, CA). Dr Donald Foster (ZymoGenetics, Seattle, WA) kindly provided purified recombinant (r), murine (m), and human (h) TPO. Dr Douglas Hilton (Walter and Eliza Hall Institute, Melbourne, Australia) provided SOCS-1 cDNA, and Dr Akihito Yoshimura (Kurume University, Kurume, Japan) provided SOCS-3 cDNA.
BaF3 cell culture and analysis
BaF3/mMpl cells and derived cell lines were cultured in IL-3, as previously described,24 until use. Aliquots of 1 × 104 cells were incubated in triplicate 0.1-mL cultures in the presence of various concentrations of mTPO, with or without increasing concentrations of mIFN-α, at 37°C in a fully humidified environment for 48 hours. Cell growth was assessed by the capacity of cultures to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma, St Louis, MO) as previously described.25
Marrow cell purification
Marrow cells were flushed from the femurs and tibias of 8- to 10-week-old B6D2F1 mice (Jackson Laboratories, Bar Harbor, ME) that had been injected for 3 to 5 days with 2 μg rhTPO. To produce a low-density marrow cell fraction, whole marrow cells were applied to an Optiprep discontinuous gradient (density, 1.080 g/mL; Nycomed, Oslo, Norway) and subjected to 400g centrifugation for 20 minutes, and interface cells were collected, washed twice, and resuspended in phosphate-buffered saline containing 5% fetal calf serum. A lineage depleted (Lin−) marrow fraction was obtained using a titrated mixture of rat antimouse monoclonal antibodies (7/4; Serotec, Raleigh, NC), B220, CD5, TER119, Mac-1 (Pharmingen, San Diego, CA), and Dynabeads M-450 (Dynal, Great Neck, NY) coated with sheep antirat IgG as previously described.26 Finally, a purified MK progenitor cell population was obtained by fluorescence-activated cell sorter (FACS) purification of Lin− cells. Phycoerythrin (PE)–conjugated anti-CD41 (an IgG1) and fluorescein isothiocyanate (FITC)–conjugated Gr-1 (IgG2b; both from Pharmingen) were added for 15 minutes on ice, and the CD41+/Gr-1− cells were obtained by cell sorting on FACStar. Both PE-conjugated IgG1 and FITC-conjugated rat IgG2b were used as isotype controls. Finally, to obtain purified mature MK, whole marrow cells were placed in culture for 3 days in the presence of 10 ng/mL murine TPO, and the cells were applied to a discontinuous albumin gradient, as previously described.27 The resultant cell preparation was 90% or more pure, as assessed by acetylcholinesterase (AChE) staining.
Megakaryocyte cultures and analysis
Whole bone marrow mononuclear cells were cultured at 5 or 10 × 104/0.1 mL and purified CD41+/Lin− MK progenitors at from 1 to 1.5 × 103/0.1 mL in triplicate. Results from all cell concentrations were virtually identical, allowing us to pool them from all experiments for analysis. Cultures contained Iscove modified Dulbecco medium supplemented with 10% fetal calf serum and varying concentrations of rmTPO, with or without murine IFN-α, and were incubated for 4 days at 37°C in a fully humidified environment. The cultures were then assessed for MK mass by AChE activity, for DNA ploidy by culturing sorted CD41+/Gr-1− cells with TPO for 3 days and staining with propidium iodide, and for cell size by microscopic evaluation as previously described.28,29 Additional agar-containing cultures to enumerate colony-forming unit–MK-derived colonies were performed in parallel using previously described methods,28 except that whole agar plates were stained with AChE before enumeration to enhance the accuracy of counting.
Western blotting for intracellular signaling mediators
Polyclonal Mpl antiserum raised against the extracytoplasmic domain of the human Mpl protein was provided by Don Foster (ZymoGenetics), and an anti-STAT5b antibody was provided by James Ihle. Anti-phosphotyrosine mouse monoclonal antibody 4G10 and JAK2 rabbit antisera were purchased from Upstate Biotechnology (Lake Placid, NY). STAT3 polyclonal IgG and SOCS-3 antipeptide antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Dr Akihito Yoshimura (Osaka, Japan) kindly provided a polyvalent rabbit antiserum to SOCS-3. BaF3/mpl and MK lysates were prepared as described.25Typically, 1 μg antibody was added to 500 μg protein extract for 2 hours at 20°C or overnight at 4°C, followed by protein A agarose beads (Santa Cruz Biotechnology), and the immunoprecipitate was size-fractionated by 7.5% polyacrylamide gel electrophoresis alongside prestained molecular weight markers. Proteins were transferred to nitrocellulose membranes and probed by standard techniques, typically using 1 μg/mL primary antibody 4G10, horseradish peroxidase-conjugated antimurine immunoglobulin antibody as a secondary antibody, and chemiluminescence reagents (Amersham Pharmacia, Buckinghamshire, UK).
Reverse transcription–polymerase chain reaction analysis for SOCS gene expression
Whole-cell RNA was obtained from BaF3/mMpl and from Lin−/CD41+ MK grown for 3 days in mTPO using the RNA preparation kit from Qiagen (Santa Clarita, CA). First-strand cDNA was synthesized using oligo-dT primers and AMV reverse transcriptase (Gibco) and was subjected to 24 to 30 cycles of polymerase chain reaction (PCR) using murine SOCS-1–specific primers (sense, 5′ CACTCCGATTACCGGCGCATCAC 3′; antisense, 5′ GCTCCTGCAGCGGCCGCACG 3′), murine SOCS-3-specific primers (sense, 5′ AAAAGCGACTACCAGCTGGTGGT 3′; antisense, 5′ TCTCGCCCCCAGAATAGATGTAG 3′), or murine CIS-specific primers (sense, 5′ CTGGAGCTGCCCGGGCCAGCC 3′; antisense, 5′ TTTCAGGTGCACTGCAGTAGCCAC 3′), and the PCR reagents from Promega (Madison, WI). The PCR products were visualized by ethidium bromide staining of agarose gels, and their identities were verified by DNA sequencing. Amplification of murine glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA served as a loading control. Only experiments in which GAPDH band densities were in the linear range were assessed.
Statistical analysis
All statistical comparisons were performed using a Student 2-tailed t test for paired values.
Results
IFN-α directly inhibits thrombopoietin-induced megakaryopoiesis
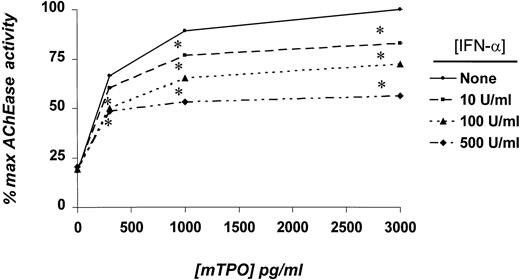
Numerous cellular assays were performed to identify the effects of IFN-α on TPO-induced MK development. Using whole marrow cell cultures and AChE assays, we found that IFN-α significantly inhibited TPO-induced MK growth in a dose-dependent manner by up to 45% at 500 U/mL (Figure 1). Megakaryopoiesis was significantly inhibited at essentially all doses of TPO tested if at least 100 U/mL IFN-α was present, a level readily attainable in patient plasma with commonly used doses of the drug. We also performed marrow cell cultures in semisolid medium to determine whether the effects of IFN-α extended to MK colony-forming cells. As shown in Table 1, 500 U/mL IFN-α inhibited colony formation by a statistically significant 34% to 36% at the 2 concentrations of TPO assessed.
Effects of IFN-α on AChE activity in whole marrow cell cultures.
Whole marrow cells were obtained from mice previously injected for 3 days with TPO. Five to 10 × 105 cells/mL were placed in suspension culture for 4 days with the indicated concentrations of mTPO and IFN-α. Results represent the mean AChE values of triplicate cultures of 5 independent experiments. *P < .05 in comparison with cultures devoid of IFN-α.
Effects of IFN-α on AChE activity in whole marrow cell cultures.
Whole marrow cells were obtained from mice previously injected for 3 days with TPO. Five to 10 × 105 cells/mL were placed in suspension culture for 4 days with the indicated concentrations of mTPO and IFN-α. Results represent the mean AChE values of triplicate cultures of 5 independent experiments. *P < .05 in comparison with cultures devoid of IFN-α.
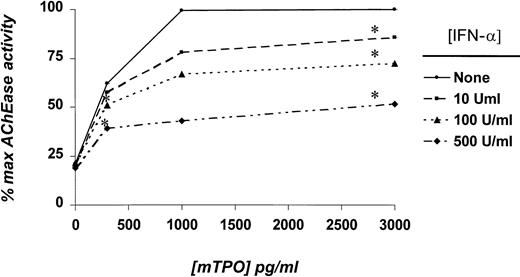
Because both semisolid and suspension cultures of whole marrow cells contained numerous accessory cells that could have mediated the IFN-α effect, we subsequently used purified MK progenitor cell populations in TPO and IFN-α dose-response assays. As did results using whole marrow cultures, IFN-α exerted a dose-dependent inhibition of TPO-induced AChE activity in cultures of low-density, lineage-depleted CD41+ cells (Figure 2). Megakaryocyte number, size, and level of polyploidy were also affected by IFN-α (Table 2). These results indicated that the cytokine directly affected the MK and its progenitors and did not depend on the presence of accessory cells in the culture.
Effects of IFN-α on AChE activity in cultures of CD41+/Lin− marrow cells.
Purified MK progenitor cells were obtained as described in “Materials and methods,” and 1 to 1.5 × 104/mL was placed in suspension culture with the indicated concentrations of mTPO and IFN-α. Results represent the mean AChE values of triplicate cultures of 4 independent experiments, except for the 1000 pg/mL mTPO data, for which only a single experiment was performed (hence, the absence of statistical significance). *P < .05 in comparison with cultures devoid of IFN-α.
Effects of IFN-α on AChE activity in cultures of CD41+/Lin− marrow cells.
Purified MK progenitor cells were obtained as described in “Materials and methods,” and 1 to 1.5 × 104/mL was placed in suspension culture with the indicated concentrations of mTPO and IFN-α. Results represent the mean AChE values of triplicate cultures of 4 independent experiments, except for the 1000 pg/mL mTPO data, for which only a single experiment was performed (hence, the absence of statistical significance). *P < .05 in comparison with cultures devoid of IFN-α.
IFN-α blunts thrombopoietin-induced signaling
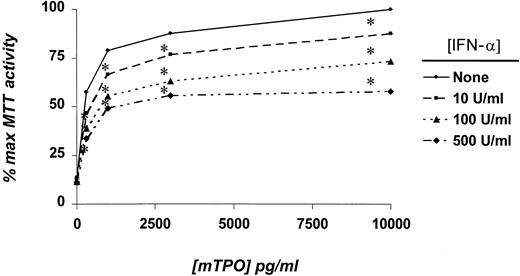
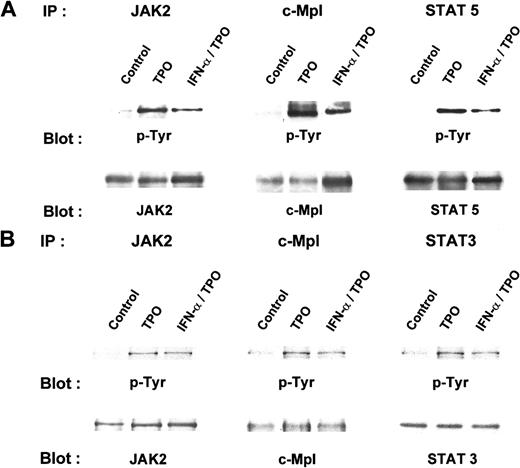
Numerous studies have indicated that the addition of TPO to Mpl receptor-bearing cells leads to the rapid phosphorylation and activation of JAK2, TYK2, STAT3, STAT5, the mitogen-activated protein kinases (MAPKs) ERK1 and ERK2, and several other signaling intermediates.30-34 Our results with purified MK progenitors suggested that TPO-induced signaling might be altered by the presence of IFN-α. To develop a model system to study the interaction between TPO and IFN-α signaling, we tested whether the latter cytokine affected TPO-induced cell growth in BaF3/mMpl cells. Because BaF3 is a prolymphocytic cell line, we anticipated the expression of IFN receptors. We found that 10 to 500 U/mL IFN-α blunted TPO-induced BaF3/mMpl cell proliferation to a degree similar to that found for MK progenitor cells (Figure3). To test whether IFN-α affected TPO signaling, we pretreated BaF3/mMpl cells and mature MK with IFN-α for 4 hours before stimulation with TPO and assessed the phosphorylation of c-Mpl, JAK2, STAT3, and STAT5. In BaF3/mMpl cells, IFN-α inhibited the phosphorylation of TPO-induced Mpl, JAK2, and STAT5 phosphorylation, commensurate with the level of inhibition seen in the AChE assays (Figure 4). When the intensity of the bands shown in Figure 4 was measured by densitometry and adjusted for the amount of each immunoprecipitated protein detected by Western blot analysis, phosphorylation of JAK2, Mpl, and STAT5 was reduced in the presence of TPO plus IFN-α to 45%, 40%, and 25%, respectively, that seen in cells cultured in TPO alone. Curiously, STAT3 phosphorylation did not change in 3 separate experiments (data not shown). In primary murine MK cultured in TPO plus IFN-α, Mpl, JAK2, and STAT3, phosphorylation was reduced to 40%, 75%, and 67%, respectively, compared to cultures containing TPO alone (Figure 4B). Because we failed to identify significant phosphorylation of mature MK STAT5 in response to TPO in a previous study,27 the phosphorylation of STAT5 was not investigated in the current experiments.
Effects of IFN-α on the proliferation of BaF3/mMpl cells.
BaF3/mMpl cells were washed free of IL-3 and placed in culture in the indicated concentrations of mTPO and IFN-α. After 2 days, MTT assays were performed; the mean value of 7 experiments is shown. *P < .05 in comparison with cultures devoid of IFN-α.
Effects of IFN-α on the proliferation of BaF3/mMpl cells.
BaF3/mMpl cells were washed free of IL-3 and placed in culture in the indicated concentrations of mTPO and IFN-α. After 2 days, MTT assays were performed; the mean value of 7 experiments is shown. *P < .05 in comparison with cultures devoid of IFN-α.
IFN-α blunts Mpl, JAK2, and STAT-5 phosphorylation in BaF3/mMpl cells and primary murine megakaryocytes.
(A) BaF3/mMpl cells were grown in IL-3 and were washed and starved for 16 hours. Four hours before the addition of 10 ng/mL mTPO, 500 U/mL IFN-α or sham was added to the culture; 10 minutes after TPO was added, the cells were lysed and immunoprecipitated with antibodies to mMpl, JAK2, or STAT5. Immunoprecipitates were size fractionated by denaturing PAGE, and the proteins were transferred and blotted for phosphotyrosine. After probing, the blots were stripped and reprobed for c-Mpl, JAK2, and STAT5. Similar results were obtained from 3 additional experiments. (B) A similar protocol to that in A was followed, except that MK was obtained by elution from an albumin gradient and the cells were starved for 4 hours before the addition of TPO after a 4-hour pretreatment with IFN-α or control. This experiment was repeated twice with similar results. Densitometry of bands in the JAK2 lanes reveal a 20% reduction in JAK2 phosphorylation in MK pretreated with IFN-α and TPO compared with TPO alone.
IFN-α blunts Mpl, JAK2, and STAT-5 phosphorylation in BaF3/mMpl cells and primary murine megakaryocytes.
(A) BaF3/mMpl cells were grown in IL-3 and were washed and starved for 16 hours. Four hours before the addition of 10 ng/mL mTPO, 500 U/mL IFN-α or sham was added to the culture; 10 minutes after TPO was added, the cells were lysed and immunoprecipitated with antibodies to mMpl, JAK2, or STAT5. Immunoprecipitates were size fractionated by denaturing PAGE, and the proteins were transferred and blotted for phosphotyrosine. After probing, the blots were stripped and reprobed for c-Mpl, JAK2, and STAT5. Similar results were obtained from 3 additional experiments. (B) A similar protocol to that in A was followed, except that MK was obtained by elution from an albumin gradient and the cells were starved for 4 hours before the addition of TPO after a 4-hour pretreatment with IFN-α or control. This experiment was repeated twice with similar results. Densitometry of bands in the JAK2 lanes reveal a 20% reduction in JAK2 phosphorylation in MK pretreated with IFN-α and TPO compared with TPO alone.
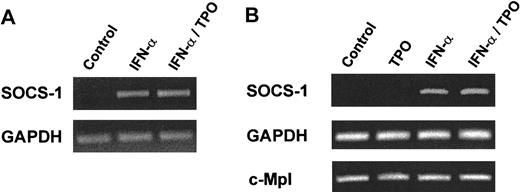
IFN-α, but not thrombopoietin, induces SOCS-1 expression
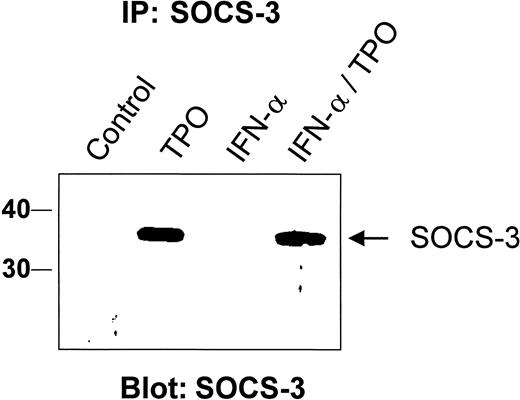
In addition to turning on a growth factor or a cytokine-induced signal, cells must retain the capacity to turn that signal off. Several pathways that extinguish cytokine signaling have been identified. For example, tyrosine phosphatases such as SHP-1 have been shown to reduce the level of erythropoietin (EPO) signaling,35 and nuclear phosphatases that act on STAT proteins have been identified.36 Moreover, ligand-induced receptor internalization can down-regulate the effects of cytokines on hematopoietic cells. Recently, another mechanism of cytokine signal suppression has been identified, mediated by members of the SOCS family of proteins, which act to inhibit JAK-induced signaling events.37 Thus, we tested whether IFN-α or TPO induced SOCS gene expression in BaF3/mMpl cells or purified MK. Using a specific reverse transcription (RT)-PCR assay, we found that TPO was a poor stimulus of mRNA specific for SOCS-1 in RT-PCR assays of BaF3/mMpl cells or purified MK (Figure 5; data not shown). In contrast, IFN-α was a potent stimulus in both cell types. Induction of SOCS-1 mRNA was maximal at 1 hour and began to wane by 4 hours after stimulation with IFN-α. In contrast, the other SOCS protein known to inhibit hematopoietic cytokine receptor and STAT activation, SOCS-3, was markedly induced by TPO but only poorly induced by IFN-α in BaF3/mMpl cells (Figure 6). The addition of IFN-α to TPO failed to augment the SOCS-3 response to TPO, either at low or high concentrations of the latter. We also used RT-PCR to evaluate CIS induction but found little difference in levels of CIS mRNA in the presence or absence of IFN-α, TPO, or both (data not shown).
RT-PCR analysis of BaF3/mMpl and MK RNA after stimulation with TPO or IFN-α.
BaF3/mMpl (A) or purified MK (B) were starved for 14 or 7 hours, respectively, and either untreated or stimulated with 10 ng/mL mTPO for 10 minutes, 500 U/mL IFN-α for 4 hours, or both. After RNA harvest, RT-PCR was conducted with SOCS-1–specific oligo-deoxynucleotide primers for 30 (A) or 24 (B) cycles, and the products were analyzed by ethidium bromide staining of agarose gels. Amplification of both c-Mpl and GAPDH served as controls. Similar experiments were performed twice with BaF3/mMpl cells and 3 times with MK.
RT-PCR analysis of BaF3/mMpl and MK RNA after stimulation with TPO or IFN-α.
BaF3/mMpl (A) or purified MK (B) were starved for 14 or 7 hours, respectively, and either untreated or stimulated with 10 ng/mL mTPO for 10 minutes, 500 U/mL IFN-α for 4 hours, or both. After RNA harvest, RT-PCR was conducted with SOCS-1–specific oligo-deoxynucleotide primers for 30 (A) or 24 (B) cycles, and the products were analyzed by ethidium bromide staining of agarose gels. Amplification of both c-Mpl and GAPDH served as controls. Similar experiments were performed twice with BaF3/mMpl cells and 3 times with MK.
Thrombopoietin, but not IFN-α, induces SOCS-3 protein expression.
Cell lysates from BaF3/mMpl were prepared from unstimulated cells or those grown for 4 hours with 10 ng/mL TPO, 500 U/mL IFN-α, or both. Thirty minutes before lysate preparation, a proteosome inhibitor, MG132, was added to the cells. A polyvalent antiserum raised against the carboxyl terminus of SOCS-3 was used to immunoprecipitate specific protein, which was then size fractionated by SDS-PAGE, transferred to nitrocellulose, and probed for SOCS-3 with a monoclonal antibody to the protein. This experiment was repeated 3 times with identical results. Relative molecular weight markers are shown on the left in kilodaltons.
Thrombopoietin, but not IFN-α, induces SOCS-3 protein expression.
Cell lysates from BaF3/mMpl were prepared from unstimulated cells or those grown for 4 hours with 10 ng/mL TPO, 500 U/mL IFN-α, or both. Thirty minutes before lysate preparation, a proteosome inhibitor, MG132, was added to the cells. A polyvalent antiserum raised against the carboxyl terminus of SOCS-3 was used to immunoprecipitate specific protein, which was then size fractionated by SDS-PAGE, transferred to nitrocellulose, and probed for SOCS-3 with a monoclonal antibody to the protein. This experiment was repeated 3 times with identical results. Relative molecular weight markers are shown on the left in kilodaltons.
Discussion
The most important findings of this report are that IFN-α acts directly on megakaryocytic progenitor cells to inhibit their TPO-induced development, IFN-α blunts the JAK/STAT signaling response induced by TPO, and IFN-α induction of SOCS-1 appears responsible for impaired TPO signaling. These conclusions derive from cell growth and signaling studies carried out in an IFN-α and TPO-responsive cell line and, most important, in primary murine megakaryocytes, lending physiologic relevance to the conclusions. The findings also offer a mechanism to help explain the common finding of cytokine receptor cross-talk.
In the current study we found that IFN-α inhibits the growth of murine MK in the presence of purified rmTPO. Essentially identical results were obtained using either whole marrow cells or highly purified MK progenitors. Although previous studies using whole marrow cells and plasma as a source of MK stimulating activity have recognized the inhibitory effect of IFN-α on megakaryopoiesis, the experiments reported here establish that the cytokine adversely affects MK directly, even in the presence of large doses of purified rmTPO. Hence, these observations extend our understanding of the thrombocytopenia that can occur in patients treated with IFN-α for chronic viral hepatitis or neoplastic disorders, and they suggest that treatment of such patients with recombinant TPO may not alleviate the adverse effects of IFN-α.10
Since the purification of IFNs and the cloning of their receptors in the mid-1980s, numerous investigators have examined the basis by which these cytokines exert their physiologic effects. Several studies suggest a direct inhibitory effect of IFN on growth factor–induced proliferation pathways. For example, IFN-α has been shown to augment double-stranded RNA-activated protein kinase activity, an enzyme that inhibits translation initiation factor-2, implicating a reduction of the growth factor–induced protein synthesis necessary for cytokine response.38 We have not investigated this possibility in primary cells, but we believe it could contribute to the inhibitory effect of IFN-α on TPO-induced MK growth because cell size was reduced slightly in our studies with purified progenitor cells. Other studies have suggested that IFN induces expression of the cell cycle inhibitor p27kip1, an event that arrests cells in G0/G1.39 We have not investigated cell cycle inhibitors in the current study, but it is known that several lymphokines reduce p27kip1 expression in lymphocytes,40 making it possible that the blockade of TPO signaling by IFN-α might also be associated with increased MK p27kip1.
The effects of IFN-γ on megakaryopoiesis have been more controversial, with reports suggesting both suppression and stimulation of MK progenitor cell growth.17,41-43 Substantial insights into the antiproliferative hematopoietic response to IFN have come from investigations of the effects of IFN-γ on erythropoiesis, studies showing that the cytokine promotes programmed cell death in cultures of purified erythroid progenitor cells.44 However, in contrast to this effect of IFN-γ, we failed to find evidence for enhanced apoptosis in purified MK progenitor cells treated with 500 U/mL IFN-α (data not shown). Given the profound differences in the biologic activities of IFN-α and IFN-γ and in how these 2 cytokines signal, it is perhaps not surprising that the results of our studies of IFN-α and those of IFN-γ differ.
Thrombopoietin is the primary regulator of MK and platelet production.22 The hormone acts by binding to its high-affinity cell surface receptor, c-Mpl, first recognized in altered form as the transforming oncogene of the murine myeloproliferative leukemia virus.45 The TPO receptor is a member of the type 1 family of hematopoietic cytokine receptors.46 Much is known of the mechanisms by which hematopoietic growth factors affect the survival, proliferation, and differentiation of cells that give rise to all the blood lineages.47 Signal transduction in this system is initiated by ligand-induced receptor oligomerization or conformational changes, events that induce JAK cross-phosphorylation and activation, resulting in tyrosine phosphorylation of a number of critical intracellular substrates. Included among the downstream mediators of hematopoietic growth factor receptor activation are JAK, STAT, MAPK, and Phosphoinositol 3 kinase (PI3K), signaling molecules that directly impact cellular development by modifying gene expression or the activity of molecules vital to cell proliferation and survival. Other investigators and we27,30-34 have determined that each of these signal transduction pathways is activated in TPO-stimulated Mpl-bearing cell lines and primary marrow-derived cells. In contrast, IFN acts through members of the type II cytokine receptor family, which recruits a plethora of cytoplasmic mediators to initiate signaling cascades.48-51 Of note, many of the secondary signaling pathways common to the interleukins, hematopoietic growth factors, and protein hormones, including JAK, STAT, MAPK, and PI3K, have been described as mediators of IFN-α activity. In fact, the STAT proteins were first recognized as ligand-induced transcription factors in IFN-treated cells.52 These findings immediately suggested that IFN-α affects TPO-induced signaling. Thus, our results that IFN-α reduced TPO-induced phosphorylation (activation) of the Mpl receptor, JAK2, STAT3, and STAT5 (Figure 4), the timing and degree of which were commensurate with the IFN-α–induced blockade of TPO-induced cell growth, lend support to the hypothesis.
Recently, Jaster et al38 reported that IFN-α reduced IL-3–induced growth of Ba/F3 cells, a prolymphocytic cell line also used in the current studies. In contrast to our results, these investigators concluded that IFN-α had no effect on the IL-3–induced activation of STAT5. Although it is possible that the opposing conclusions can be attributed to true differences between IL-3 and TPO, this is not likely. Alternatively, we believe the difference lies in the assays used to detect receptor activation. We studied Mpl, JAK2, and STAT phosphorylation using Western blot analysis. Jaster et al38 studied the capacity of Ba/F3 protein extracts to shift a STAT5 DNA binding probe in electrophoretic mobility shift assays, without providing a quantitative assessment of STAT5 activation. Thus, though their suggestion that reduced protein translation is responsible for the IFN-α effect on IL-3–induced cell growth may also be correct, their experimental approach cannot exclude the molecular mechanism we propose—reduced growth factor–induced signaling events.
In addition to responding to extracellular signals for growth and metabolic activation, cells must have mechanisms to extinguish growth factor–induced processes. Previous studies53,54 have identified a number of such mechanisms, including the down-modulation of receptor expression and the activation of phosphatases that quench growth factor receptor–mediated signals. The importance of these counter-regulatory pathways is illustrated by the pathologic expansion of hematopoiesis that can occur when either process fails.55 More recently, another mechanism of growth factor signal termination has been identified, mediated by the SOCS proteins. The cloning of a STAT-inducible gene, CIS,56 and several additional genes that bear substantial sequence homology37,57 has yielded a family of proteins that can directly suppress growth factor receptor–induced signals. Because IFNs are potent inducers of STAT1 activation, they also lead to the production of several SOCS proteins. In turn, SOCS proteins act to eliminate the signal that initially led to their production. On the basis of these findings, we tested whether SOCS proteins might be candidates to mediate IFN-α–induced blunting of TPO signaling. Because only 3 of the 8 CIS and SOCS proteins thus far identified have been shown to play important roles in cytokine signaling (CIS, SOCS-1, and SOCS-3),23 we concentrated our efforts on these genes.
Hence, we tested whether CIS, SOCS-1, or SOCS-3 was inducible in BaF3/mMpl or murine MK treated with IFN-α. We found that CIS was not inducible at the RNA level in either cell system (data not shown) and that SOCS-3 was not induced in BaF3 cells by IFN-α at the protein level, though TPO was a potent stimulus (Figure 6). It was also possible that IFN-α might augment TPO-induced SOCS-3 expression to help explain its inhibition of TPO signaling. We failed to obtain evidence to support this hypothesis; the addition of IFN-α to TPO failed to increase the SOCS-3 expression induced by the latter, either at high-dose (Figure 6) or low-dose (data not shown) TPO. In contrast, our results pointed to a role for SOCS-1 in the inhibition of TPO signaling. In BaF3/mMpl cells, we found that TPO could induce only low-level expression of SOCS-1 mRNA, a level that was consistently lower than the levels found after stimulation with IFN-α. In murine MK, IFN-α, but not TPO, induced the expression of SOCS-1 mRNA. Unfortunately, we could not confirm these conclusions at the protein level because the several antibodies to SOCS-1 we tested failed in the Western blotting experiments. Nevertheless, the induction of SOCS-1 by IFN-α could explain the ability of the cytokine to interfere with TPO-induced MK development.
Several lines of evidence support the hypothesis that SOCS-1 is responsible for IFN-α–induced diminution of the TPO response. First, the timing of IFN-α effects on TPO-induced cell signaling is consistent with an SOCS protein-mediated process. Pretreatment with IFN-α for 4 hours was required to exert an effect on TPO-induced JAK, Mpl, and STAT phosphorylation, consistent with the need for IFN-α–induced STAT activation, SOCS mRNA transcription, and protein synthesis. Second, IFN-β, which acts through the same receptor as IFN-α, was reported to abrogate IL-6–induced growth of a myeloma cell line by interfering with the formation of essential signaling complexes leading to the activation of p21 Ras,58 findings consistent with the interruption of IL-6–induced JAK-mediated signals. Moreover, the approximately 50% degree of IFN-β–mediated inhibition of IL-6–induced Ras activation in that study is remarkably similar to the level of IFN-α–induced inhibition of TPO-induced proliferation we found in the experiments presented here. Third, SOCS-1 has been shown to block signaling from IL-3 and TPO.23,59 Fourth, SOCS-1, but not SOCS-3, confers resistance to IFN-β signaling in myeloid leukemia cells.60 Fifth, the pattern of inhibition of signaling molecules exerted by IFN-α in our studies—reduction of JAK2, Mpl, and STAT3/5 phosphorylation—is most consistent with SOCS-1 action for several reasons. In contrast to CIS, which acts to inhibit growth factor signaling by competing for JAK substrate binding to tyrosine-phosphorylated cytokine receptor scaffolds (and, hence, reducing phosphorylation of STAT but not JAK or the receptors), SOCS-1 and SOCS-3 bind to the activation loop of JAK, inhibiting JAK activation and all downstream signaling events.61,62 Our finding that JAK2, Mpl, and STAT phosphorylation were blunted by IFN-α is most consistent with the action of an SOCS protein that binds to and directly inhibits JAK. The differential capacity of IFN-α to induce SOCS-1, but not SOCS-3, expression in the experiments reported herein also argues for a role for SOCS-1 in IFN-α–induced suppression of TPO-induced MK development. Sixth, mice nullizygous for SOCS-1, in which much of the pathologic condition is caused by the enhanced biologic activity of IFN,63 are thrombocytopenic.64 Because the administration of IFN-γ does not suppress thrombopoiesis in vivo,65 66 and though some other MK-suppressive cytokine may be overactive in SOCS-1−/− mice, these observations are again supportive of a role for SOCS-1 in IFN-α–mediated blunting of TPO-induced MK development.
We found that SOCS-3 is induced by TPO in primary murine MKs. To our knowledge, this is the first report of the responsiveness of this signaling inhibitor to TPO. Numerous cytokines have been shown to induce SOCS-3 production, including growth hormone, prolactin, leptin, IL-2, IL-10, and EPO. In fact, the most obvious physiologic effect in mice genetically engineered to eliminate SOCS-3 expression is fetal erythrocytosis, likely caused by unregulated EPO signaling.67 Thus, given the similarity of signaling pathways used by EPO and TPO, our finding that TPO also induces SOCS-3 was not unexpected.
Finally, the results reported here may have broader implications. As noted, most of the signaling pathways used by TPO are shared with a large number of interleukins, hematopoietic growth factors, and other cytokines, mediators exerting stimulatory and inhibitory effects on cell development. Several of these molecules have also been demonstrated to induce SOCS protein production, and SOCS proteins block signaling from many stimulatory cytokines.23 Of interest, it was recently reported that IL-10 down-modulates IFN-α and IFN-γ signaling in monocytes and acts to induce the expression of SOCS-3, suggesting a causal link between the 2 observations.68 By demonstrating that IFN-α is associated with the induction of SOCS-1 and can blunt TPO-induced proliferation, we extend those studies and provide additional support for an SOCS-based cross-talk hypothesis. It thus appears that SOCS proteins can not only extinguish the signals generated by the inducing cytokine but also attenuate cellular responses to other cytokines simultaneously present. These results provide a molecular mechanism to help explain a common finding in cytokine-responsive cells: receptor cross-talk.
Acknowledgments
We thank Dr Don Foster at ZymoGenetics for the kind gift of recombinant murine and human TPO and anti-Mpl antiserum, Dr James Ihle for the anti-STAT5b antibody, Dr Douglas Hilton for the murine SOCS-1 cDNA and antiserum to the protein, and Dr Akihito Yoshimura for the SOCS-3 cDNA and the antiserum to SOCS-1 and SOCS-3 proteins.
Supported by National Institutes of Health grants R01 CA31615 and R01 DK 49855 (K.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth Kaushansky, Division of Hematology, University of Washington School of Medicine, Box 357710, 1959 NE Pacific St, Seattle, WA 98195; e-mail:kkaushan@u.washington.edu.