Abstract
Myeloma is a unique hematologic malignancy that exclusively homes in the bone marrow and induces massive osteoclastic bone destruction presumably by producing cytokines that promote the differentiation of the hematopoietic progenitors to osteoclasts (osteoclastogenesis). It is recognized that neighboring bone marrow stromal cells influence the expression of the malignant phenotype in myeloma cells. This study examined the role of the interactions between myeloma cells and neighboring stromal cells in the production of osteoclastogenic factors to elucidate the mechanism underlying extensive osteoclastic bone destruction. A murine myeloma cell line 5TGM1, which causes severe osteolysis, expresses α4β1-integrin and tightly adheres to the mouse marrow stromal cell line ST2, which expresses the vascular cell adhesion molecule-1 (VCAM-1), a ligand for α4β1-integrin. Co-cultures of 5TGM1 with primary bone marrow cells generated tartrate-resistant acid phosphatase-positive multinucleated bone-resorbing osteoclasts. Co-cultures of 5TGM1 with ST2 showed increased production of bone-resorbing activity and neutralizing antibodies against VCAM-1 or α4β1-integrin inhibited this. The 5TGM1 cells contacting recombinant VCAM-1 produced increased osteoclastogenic and bone-resorbing activity. The activity was not blocked by the neutralizing antibody to known osteoclastogenic cytokines including interleukin (IL)-1, IL-6, tumor necrosis factor, or parathyroid hormone-related peptide. These data suggest that myeloma cells are responsible for producing osteoclastogenic activity and that establishment of direct contact with marrow stromal cells via α4β1-integrin/VCAM-1 increases the production of this activity by myeloma cells. They also suggest that the presence of stromal cells may provide a microenvironment that allows exclusive colonization of myeloma cells in the bone marrow.
Introduction
Multiple myeloma is unique among hematologic malignancies in its capacity to cause massive osteoclastic bone destruction, leading to serious skeletal complications such as intractable bone pain, pathologic bone fracture, and hypercalcemia.1,2 Histologic observations of osteolytic lesions in patients with myeloma demonstrate the occurrence of osteoclastic bone destruction adjacent to nests of myeloma cells,3 suggesting that myeloma cells influence the differentiation of hematopoietic progenitors to osteoclasts (osteoclastogenesis) and subsequent activation of these osteoclasts to destroy bone. However, the mechanism by which myeloma cells regulate osteoclastogenesis and activate osteoclasts remains unclear.
A large body of evidence suggests that the support of bone marrow stromal cells is indispensable for the differentiation, growth, and homing of cells of the B-cell lineage in the bone marrow.4-7 Moreover, recent studies also suggest that the vascular cell adhesion molecule-1 (VCAM-1) that is constitutively expressed in bone marrow stromal cells8 plays an important role in regulating the behavior of neighboring myeloma cells,9-14 which constitutively express α4β1-integrin, the receptor for VCAM-1.15 16 These observations have led us to hypothesize that cell-cell interactions between myeloma cells and marrow stromal cells via α4β1-integrin/VCAM-1 play a role in the stimulation of osteoclastogenesis and subsequent bone resorption in myeloma.
In the present study, we investigated this hypothesis using a murine myeloma cell line, 5TGM1, that causes severe osteolysis in syngeneic mice.17 5TGM1 is a subclonal cell line we established from 5T33 multiple myeloma that was originally described by Radl and colleagues18 and expresses α4β1-integrin.19 We found that cell-cell interactions between 5TGM1 myeloma cells and marrow stromal cells that are mediated through α4β1-integrin/VCAM-1 increased the production of osteoclastogenic activity by the myeloma cells. We propose that this cell-cell interaction is critical to the development and progression of myeloma-induced osteolysis.
Materials and methods
Cells
The 5TGM1 myeloma cells were initially derived from a myeloma called 5T33 that arose spontaneously in aged C57BL/KaLwRij mice.17-19 Cells were grown in Iscove modified minimal essential medium (IMDM, Life Technologies, Gaitherburg, MD) supplemented with 10% fetal bovine serum (FBS, Summit, Fort Collins, CO) and 1% penicillin-streptomycin solution (GIBCO, Grand Island, NY) at 37°C in 5% CO2 atmosphere. Inoculation of 5TGM1 cells in the inbred C57BL/KaLwRij mice induces myeloma disease exhibiting the similar features to those of human myeloma such as severe osteolysis and involvement of nonbone organs including liver and kidney.17,19 The 5TGM1 cells produce interleukin (IL)-6, but their growth is not promoted by IL-6.17 Human myeloma cell line IM-9, U266B1, and mouse B-cell lymphoma cell line RAW8.1 were purchased from American Type Culture Collection (Manassas, VA). ARH-77 human plasma cell leukemia cells, which are shown to develop myeloma bone disease,20 were obtained from Dr Roodman in our institute. These cells were grown in IMDM supplemented with 10% FBS. The ST2 bone marrow stromal cells purchased from RIKEN Cell Bank (Tsukuba, Japan) were cultured in IMDM or α-minimum essential medium (MEM) supplemented with 10% FBS. Chinese hamster ovary (CHO) cells expressing human α4-integrin21 were generously provided by Dr Takada (The Scripps Research Institute). They were cultured in Dulbecco modified Eagle minimal essential medium (DMEM) (Hazelton Biologics, Lenaxa, KS) supplemented with 10% FBS.
Antibodies
Neutralizing monoclonal antibodies against murine VCAM-1 (M/K-2.7, IgG1κ), α4β1 integrin (9428), and intracellular adhesion molecule-1 (ICAM-1, YN1/1.7), were kindly given by Dr Kensuke Miyake (Saga Medical University, Saga, Japan). Neutralizing monoclonal antibodies to murine osteoclast-activating cytokines were purchased from R & D Systems (Minneapolis, MN). Parathyroid hormone-related protein (PTH-rP) monoclonal antibody was described by Ratclife and coworkers.22 Control monoclonal antibody (MOPC-1, mouse IgG1κ) was purchased from Sigma (St Louis, MO). Isocyanate-conjugated α4-integrin antibody for flow cytometric analysis was purchased from Chemicon International (Temicula, CA).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was prepared from 5TGM1, primary culture of bone marrow stromal cells, and ST2 marrow stromal cell line by the single-step RNA isolation method using TRIzol reagent (GIBCO). RNA (3 μg) was incubated with 50 ng of random hexamer at 70°C for 10 minutes and chilled on ice, then converted to first-strand complementary DNA (cDNA) using reverse transcriptase (Perkin-Elmer, Branchburg, NJ) according to the manufacturer's instruction. The primers used for PCR were as follows: murine VCAM-1 5′-primer, 5′-OH-GCTGCGCGTCACCATTGTTCTC-3′-OH; murine VCAM-1 3′-primer, 5′-OH-ACCACCCTCTTGAAGCCTTGTG-3′-OH; murine integrin α4 5′-primer, 5′-OH-CCCCTCAACACGAACAGATAGG-3′-OH; murine integrin α4 3′-primer, 5′-OH-GCCTT- GTCCTTAGCAACACTGC-3′-OH; murine GAPDH 5′-primer, 5′-OH-TTGAAGGGTGGAGCCAAACG-3′-OH; murine GAPDH 3′-primer, 5′-OH-ACACATTGGGGGTAGGAACACG-3′-OH. PCR was performed for 30 cycles consisting of 1 minute at 94°C, 1 minute at 55°C, and 2 minutes at 72°C. The PCR reaction mixture (total 50 μL) contained 10 μL first-strand cDNA, 50 mmol/L KCI, 10 mmol/L Tris-HCI (pH 8.3), 2 mmol/L MgCl2, deoxy-NTP mix (0.2 mmol/L each), the pair of primers (0.15 μmol/L each), and 2 U Taq DNA polymerase (Perkin-Elmer). The PCR products were separated on 2.5% agarose gels containing ethidium bromide and visualized under UV light. The size of the fragments was confirmed by reference to molecular weight markers. GAPDH served as a control.
Flow cytometric analysis of VCAM-1 and α4β1-integrin expression
Cell suspensions containing either ST2 stromal cells or 5TGM1 myeloma cells (1 × 107 cells/mL) were treated with 10% normal mouse serum and subsequently incubated with either the anti–VCAM-1 antibody (M/K-2.7, 1:100 dilution), followed by the treatment with fluorescent isocyanate-conjugated goat antirat IgG or isocyanate-conjugated anti–α4-integrin antibody (1:1 dilution). Expression of the VCAM-1 or α4-integrin on cell surface was determined using a flowcytometer (FACStar plus; Becton Dickinson, San Jose, CA).
Attachment of 5TGM1 myeloma cells onto ST2 mouse bone marrow stromal cells
The ST2 cells were cultured in α-MEM supplemented with 10% FBS until confluency in 48-well culture plates (Coster, Cambridge, MA). Growing 5TGM1 cells were labeled with 10 μCi [methyl-3H] thymidine (New England Nuclear, Boston, MA) for 24 hours. 3H-labeled 5TGM1 cells (20 000 cells, 8654 ± 244 cpm) were then incubated on the ST2 cell monolayer in the absence or presence of antibodies to VCAM-1 or α4β1-integrin for 1 hour. Nonadherent cells were removed by washing with 5% trichloroacetic acid twice and phosphate-buffered saline (PBS) twice, and adherent cells were solubilized in 300 μL of 0.25 mmol/L NaOH and neutralized with the same volume of 0.25 mmol/L HCI; the radioactivity was determined in a liquid scintillation counter.
Double-staining of the co-cultures for tartrate-resistant acid phosphatase (TRAP) and VCAM-1 expression
Co-cultures were fixed with 3.7% formaldehyde and stained first for TRAP as described below. TRAP-stained cultures were next treated with 0.6% hydrogen peroxide for 15 minutes and then with 0.8% rabbit serum for 1 hour. Subsequently, the cultures were incubated with anti–VCMA-1 antibody (M/K-2.7, 1:10 dilution) at room temperature for 45 minutes, washed with PBS containing 1% rabbit serum (× 4), incubated with secondary antibody (rabbit antirat IgG, Vector Laboratories, Burlingame, CA) at room temperature for 45 minutes, washed with PBS containing 1% rabbit serum (× 4), and visualized using a commercial kit (Vectastatin Elite ABC kit, Vector Laboratories).
Formation of TRAP-positive multinucleated osteoclasts and resorption pits in co-culture of 5TGM1 myeloma cells and primary mouse bone marrow cells
Mouse bone marrow cells were obtained from 5-week-old male C57BL mice as described previously.23 24 Femurs and tibiae were dissected aseptically, cut off both ends. Bone marrow cells were flushed out, collected, and incubated in α-MEM supplemented with 10% FBS (Hyclone, Logan, UT) in 100-mm culture dishes (Becton Dickinson Labware, Bedford, MA) for 2 hours and nonadherent cells containing hemopoietic osteoclast precursors and stromal cells were harvested. Bone marrow cells (1 × 106/well) and 5TGM1 cells (1000 cells/well) in 300 μL of the culture medium were plated onto 48-well culture plates (day 1). On day 2, 300 μL of fresh culture medium was gently added to each well, and on day 4, 300 μL of spent medium was replaced with the same volume of fresh medium. On day 6, the cultures were fixed and stained for TRAP using commercial kits (Sigma). TRAP-positive multinucleated cells with more than 3 nuclei were defined as osteoclasts and manually counted under the microscope. As a positive control, a potent osteoclastogenic agent, 1α,25-dihydroxyvitamin D3 (1,25-D3, Biomol, Plymouth Meeting, PA), was added to bone marrow cultures. The co-cultures were conducted in the absence of 1,25-D3 unless indicated.
To confirm that these TRAP-positive multinucleated osteoclasts have the capacity to resorb bone, 5TGM1 cells and marrow cells were co-cultured on 5 × 5-mm whale dentine slices in the same condition, and resorption pits formed on these dentine slices were examined by scanning electron microscopy as described.25
In some experiments, co-cultures of 5TGM1 myeloma cells and marrow cells were performed using transwell inserts (24-well, Becton Dickinson Labware) to prevent the direct contact between these 2 types of cells.
Conditioned medium
The ST2 marrow cells (5 × 105/dish) and 5TGM1 myeloma cells (5 × 106/dish) were plated together onto 60-mm culture dishes (Becton Dickinson) in IMDM supplemented with 10% FBS and cultured overnight, washed with serum-free IMDM twice, and incubated in 5 mL of serum-free IMDM with 0.1% bovine serum albumin (BSA). After 48 hours, conditioned media were harvested, centrifuged to remove cell debris, and stored at −20°C until use. Conditioned medium was also harvested from the co-cultures in which 5TGM1 myeloma cells were cultured with monolayer of ST2 stromal cells that had been fixed with 2.5% paraformaldehyde.
In some experiments, 5TGM1 mouse myeloma cells and IM-9, U266B1, and ARH-77 human myeloma cells (1 × 106/mL/24-well) were cultured for 24 hours in IMDM with 5% FBS in plates coated with or without 1μg/mL recombinant soluble VCAM-1 (rsVCAM-1) that lacks transmembrane and cytoplasmic domains (kindly provided by Dr Lobb, Biogen, Cambridge, MA). Conditioned media were then harvested and assessed for bone-resorbing activity in fetal rat long bone assay as described below and for the capacity to stimulate osteoclastogenesis in mouse marrow cultures. IMDM incubated without cells in the presence and absence of rsVCAM-1 served as controls.
In other experiments, ST2 mouse bone marrow cells (2 × 105/24 well) were cultured for 24 hours on a monolayer of CHO cells that had been transfected with α4-integrin or empty vector and fixed with 2.5% paraformaldehyde. Conditioned media of these cultures were assayed for osteoclastogenic and bone-resorbing activity in mouse bone marrow assay and fetal rat long bone assay, respectively.
Organ cultures of 45Ca-labeled fetal rat long bones
Conditioned media harvested as described above were assayed for bone-resorbing activity by organ cultures of45Ca-labeled fetal rat long bones as described previously.23 Pregnant rats were injected with 250 μCi of 45Ca (New England Nuclear) on the 18th day of gestation. Radius and ulna bone shafts were excised from 19-day-old fetuses under the dissecting microscope and precultured for 24 hours in Biggers-Gwatkin-Jackson medium (Sigma) supplemented with 0.1% BSA between air and liquid phase on stainless mesh grids. Bones were then cultured in the presence of conditioned media (50% v/v) or in control medium for 120 hours. The media were changed once at 48 hours of the culture. At the end of the culture, bones were harvested and treated in ice-cold 5% trichloroacetic acid for 2 hours; 45Ca radioactivity in bones and media was determined in a liquid scintillation counter. Bone resorption was expressed as the percentage of 45Ca released into the medium from bones as calculated by 45Ca count in medium/45Ca count in medium and bone × 100.
Statisticalanalysis
All data were presented as the mean ± SEM and analyzed by analysis of variance, followed by a paired t test.
Results
Expression of VCAM-1 and α4-integrin in stromal cells and 5TGM1 myeloma cells
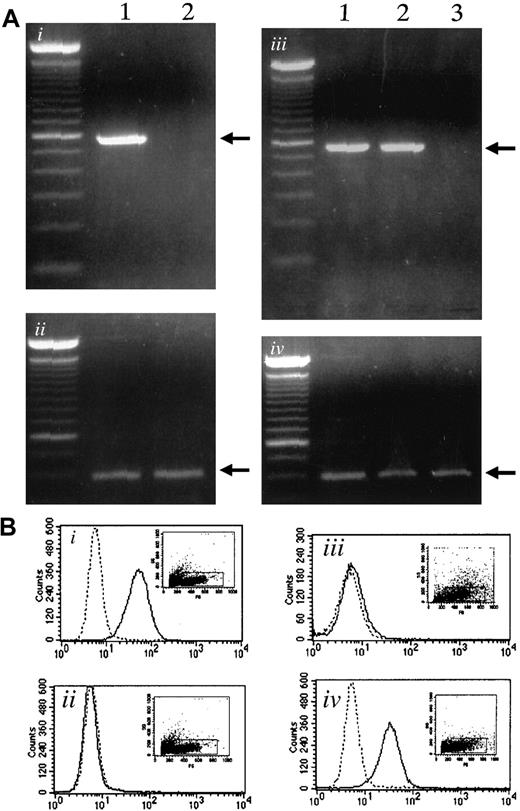
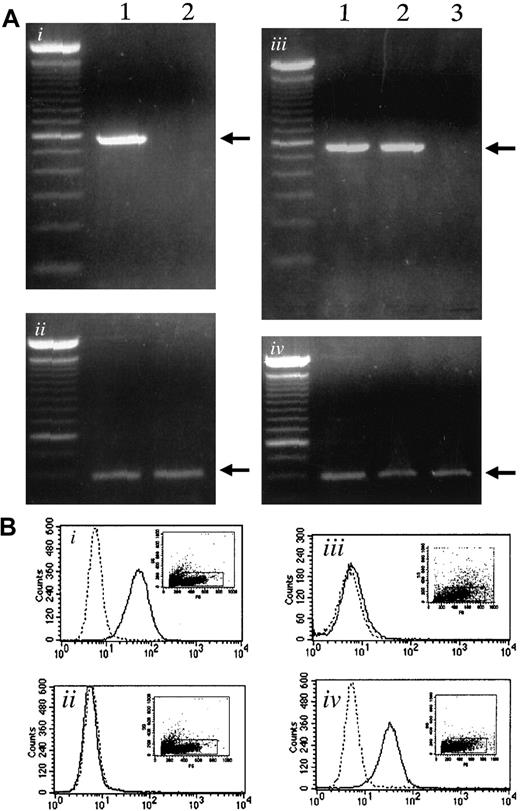
Using RT-PCR, we examined the expression of α4-integrin and VCAM-1 in myeloma cells and bone marrow stromal cells, respectively. The 5TGM1 myeloma cells expressed α4-integrin as reported previously,19whereas ST2 stromal cells did not show α4-integrin expression (Figure 1Ai). Both ST2 stromal cells and primary bone marrow stromal cells expressed VCAM-1, whereas 5TGM1 did not (Figure 1Aiii).
Expression of α4-integrin and VCAM-1 in 5TGM1 myeloma cells and in stromal cells.
(Ai) Expression of α4-integrin determined by RT-PCR. Lane 1, 5TGM1 myeloma cells; lane 2, ST2 stromal cells. (Aii), GAPDH as control. (Aiii) Expression of VCAM-1 mRNA determined by RT-PCR. Lane 1, ST2 mouse bone marrow stromal cells; lane 2, primary mouse bone marrow stromal cells; lane 3, 5TGM1 myeloma cells. (Aiv), GAPDH as control. (B) Flow cytometric analysis of VCAM-1 (Bi,Biii) and α4-integrin (Bii,Biv) expression on the cell surface of ST2 bone marrow cells (Bi,Bii) and 5TGM1 myeloma cells (Biii,Biv). Solid line indicates anti–VCAM-1 antibody (Bi,Biii), anti-α4-integrin antibody (Bii,Biv); dotted line, no primary antibody. Analysis was conducted as described in text.
Expression of α4-integrin and VCAM-1 in 5TGM1 myeloma cells and in stromal cells.
(Ai) Expression of α4-integrin determined by RT-PCR. Lane 1, 5TGM1 myeloma cells; lane 2, ST2 stromal cells. (Aii), GAPDH as control. (Aiii) Expression of VCAM-1 mRNA determined by RT-PCR. Lane 1, ST2 mouse bone marrow stromal cells; lane 2, primary mouse bone marrow stromal cells; lane 3, 5TGM1 myeloma cells. (Aiv), GAPDH as control. (B) Flow cytometric analysis of VCAM-1 (Bi,Biii) and α4-integrin (Bii,Biv) expression on the cell surface of ST2 bone marrow cells (Bi,Bii) and 5TGM1 myeloma cells (Biii,Biv). Solid line indicates anti–VCAM-1 antibody (Bi,Biii), anti-α4-integrin antibody (Bii,Biv); dotted line, no primary antibody. Analysis was conducted as described in text.
Expression of VCAM-1 on ST2 cells (Figure 1Bi) and α4-integrin expression on 5TGM1 cells (Figure 1Biv) was also demonstrated by flow cytometric analysis.
In a separate experiment, we also examined the expression of β7-integrin, because α4β7-integrin is a receptor for VCAM-1 as well. However, we did not detect β7 expression in 5TGM1 cells by RT-PCR (data not shown).
Attachment of 5TGM1 myeloma cells to ST2 cell monolayer in the absence or presence of antibodies to VCAM-1 and α4β1-integrin
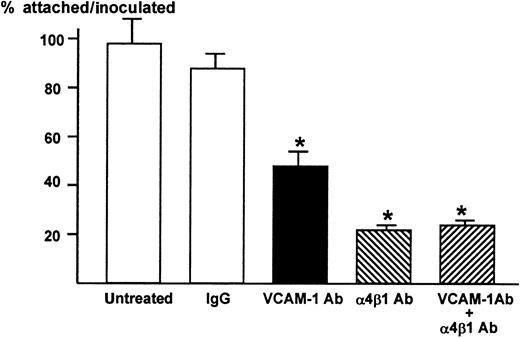
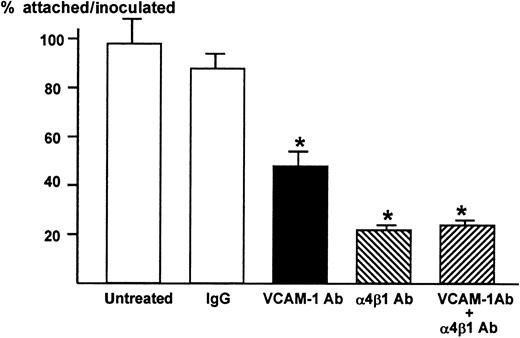
We then examined whether VCAM-1 and α4-integrin play a role in the attachment between ST2 stromal cells and 5TGM1 myeloma cells. The 5TGM1 cells grow in suspension. In contrast, almost 100% 5TGM1 cells adhered to ST2 cell monolayer (Figure2). The anti–VCAM-1 antibody (10 μg/mL) significantly and anti–α4β1-integrin antibody (10 μg/mL) more profoundly inhibited the attachment of 5TGM myeloma cells to ST2 monolayer (Figure 2), suggesting that the attachment of 5TGM1 myeloma cells to ST2 stromal cells is mediated via α4-integrin and VCAM-1. The results also demonstrate that VCAM-1 and α4β1-integrin expressed on these cells are biologically functional and that these antibodies have neutralizing activity. Increase in the concentration of these antibodies to 20 μg/mL did not show further inhibition of the attachment (data not shown). Moreover, combined treatment with anti–VCAM-1 antibody (10 μg/mL) and anti–α4β1-integrin antibody (10 μg/mL) did not further inhibit the attachment of 5TGM1 myeloma cells to ST2 monolayer (Figure 2).
Attachment of 5TGM1 myeloma cells to monolayer of mouse stromal cell line ST2 and effect of neutralizing antibodies to VCAM-1 and α4β1-integrin
. ST2 cells were cultured to confluency in 48-well culture plates.3H-Thymidine-labeled 5TGM1 cells (20 000 cells, 8654 ± 244 cpm) were seeded onto ST2 monolayer and incubated at 37°C for 1 hour in the absence or presence of 10 μg/mL of anti–VCAM-1 antibody (VCAM-1 Ab), anti-integrin α4β1-antibody (α4β1 Ab), both, or control IgG. After the incubation, nonadherent cells were removed by gentle washing with PBS, twice. Attached cells were lysed with 0.1 mol/L NaOH and radioactivity was determined by a liquid scintillation counter. Percentage of attached cells was calculated as radioactivity of adherent cells (cpm)/radioactivity of inoculated cells (cpm) × 100. Twenty μg/mL of these antibodies produced identical results (data not shown). Data are expressed as mean ± SE (n = 4). *Significantly different from IgG control (P < .01).
Attachment of 5TGM1 myeloma cells to monolayer of mouse stromal cell line ST2 and effect of neutralizing antibodies to VCAM-1 and α4β1-integrin
. ST2 cells were cultured to confluency in 48-well culture plates.3H-Thymidine-labeled 5TGM1 cells (20 000 cells, 8654 ± 244 cpm) were seeded onto ST2 monolayer and incubated at 37°C for 1 hour in the absence or presence of 10 μg/mL of anti–VCAM-1 antibody (VCAM-1 Ab), anti-integrin α4β1-antibody (α4β1 Ab), both, or control IgG. After the incubation, nonadherent cells were removed by gentle washing with PBS, twice. Attached cells were lysed with 0.1 mol/L NaOH and radioactivity was determined by a liquid scintillation counter. Percentage of attached cells was calculated as radioactivity of adherent cells (cpm)/radioactivity of inoculated cells (cpm) × 100. Twenty μg/mL of these antibodies produced identical results (data not shown). Data are expressed as mean ± SE (n = 4). *Significantly different from IgG control (P < .01).
TRAP-positive multinucleated osteoclast formation in the co-cultures of 5TGM1 myeloma cells and mouse bone marrow cells
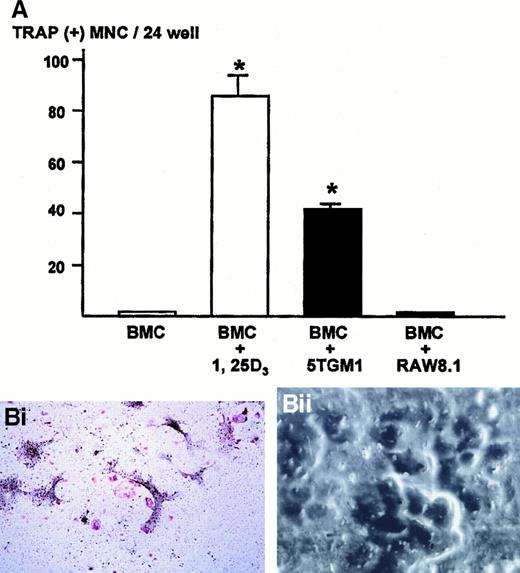
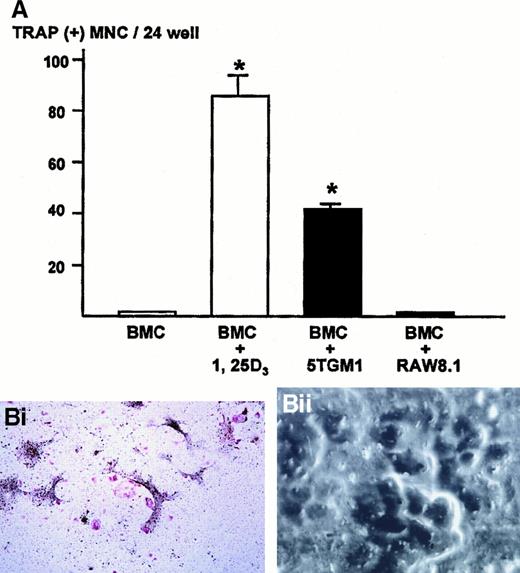
We next examined whether the cell-cell interaction between myeloma cells and stromal cells caused a generation of osteoclasts. To determine this, 5TGM1 myeloma cells were co-cultured with bone marrow cells that contain both stromal cells and hematopoietic osteoclast progenitor cells. In preliminary experiments, we found that the co-culture consisting of 1 million bone marrow cells and 1000 5TGM1 myeloma cells produced the greatest number of TRAP-positive multinucleated osteoclasts. Increase in number of 5TGM1 myeloma cells rather decreased osteoclast formation due to the overgrowth of 5TGM1 myeloma cells during 6-day culture.
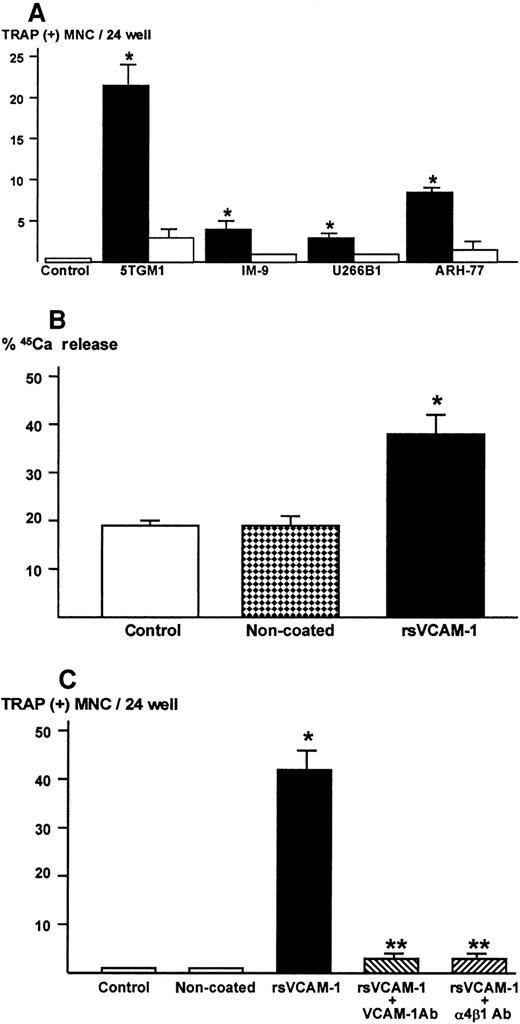
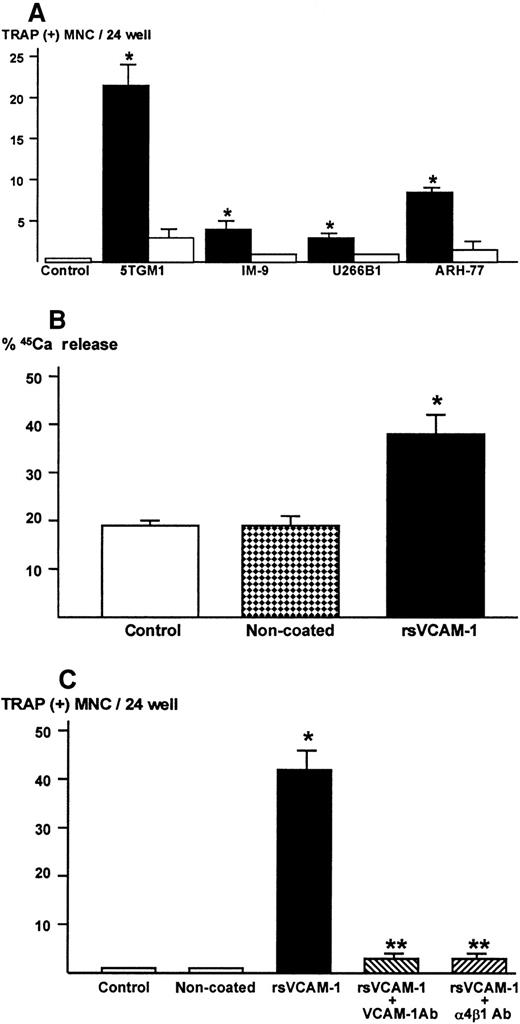
Bone marrow cells alone did not form TRAP-positive multinucleated osteoclasts (Figure 3A). In contrast, bone marrow cells cultured in the presence of 10 nmol/L 1,25-D3 formed numerous TRAP-positive multinucleated osteoclasts after 6 days of culture, as previously described.24 26 In the co-cultures of bone marrow cells and 5TGM1 myeloma cells, many TRAP-positive multinucleated osteoclasts formed (Figure 3A,Bi). These TRAP-positive multinucleated cells exhibited resorption pit formation on dentine slices (Figure 3Bii), demonstrating that these cells were capable of resorbing bone and possessed the osteoclast phenotype. When these TRAP-stained co-cultures were subsequently immunostained with anti–VCAM-1 antibody, we observed fibroblast-like cells surrounding TRAP-positive multinucleated osteoclasts were positive for VCAM-1 (Figure 3Biii), suggesting that these cells are stromal cells. Nonmyeloma RAW8.1 B-cell lymphoma cells co-cultured with bone marrow cells did not cause TRAP-positive multinucleated osteoclasts formation (Figure 3A).
Number of TRAP-positive multinucleated osteoclasts formed in the co-cultures of 5TGM1 myeloma cells and primary mouse bone marrow cells.
(A) 5TGM1 cells (1 × 103) were plated together with primary mouse bone marrow cells (BMC, 1 × 106) in 48-well plates, and cultured for 6 days. Cells were fixed and stained for TRAP activity as described in text. TRAP-positive cells with more than 3 nuclei were manually counted under a microscope as described.24,26 Data are expressed as mean ± SE (n = 6). *Significantly different from BMC alone (P < .01). (Bi) Double staining for TRAP and VCAM-1 of the co-cultures. Note that TRAP-positive large multinucleated osteoclasts (red) were surrounded by fibroblast-like VCAM-1–positive cells (black), which are most likely stromal cells. 5TGM1 myeloma cells were washed away during processing for staining. (Bii) Pit formation on dentine slices by TRAP-positive multinucleated osteoclasts formed in the co-culture. Co-culture was carried out on sperm whale dentine slices. After 6 days of culture, these dentine slices were fixed and examined by scanning electron microscopy for resorption pit formation as described.24 25
Number of TRAP-positive multinucleated osteoclasts formed in the co-cultures of 5TGM1 myeloma cells and primary mouse bone marrow cells.
(A) 5TGM1 cells (1 × 103) were plated together with primary mouse bone marrow cells (BMC, 1 × 106) in 48-well plates, and cultured for 6 days. Cells were fixed and stained for TRAP activity as described in text. TRAP-positive cells with more than 3 nuclei were manually counted under a microscope as described.24,26 Data are expressed as mean ± SE (n = 6). *Significantly different from BMC alone (P < .01). (Bi) Double staining for TRAP and VCAM-1 of the co-cultures. Note that TRAP-positive large multinucleated osteoclasts (red) were surrounded by fibroblast-like VCAM-1–positive cells (black), which are most likely stromal cells. 5TGM1 myeloma cells were washed away during processing for staining. (Bii) Pit formation on dentine slices by TRAP-positive multinucleated osteoclasts formed in the co-culture. Co-culture was carried out on sperm whale dentine slices. After 6 days of culture, these dentine slices were fixed and examined by scanning electron microscopy for resorption pit formation as described.24 25
To confirm that a direct contact between 5TGM1 myeloma cells and bone marrow stromal cells is necessary for TRAP-positive multinucleated osteoclast formation, we carried out co-culture experiments using trans-well inserts in which 5TGM1 cells were separated from bone marrow cells by a membrane. In this co-culture condition, there were very few osteoclasts formed (data not shown). The result indicates that the direct contact is essential.
Effect of antibodies to VCAM-1 and α4β1-integrin on TRAP-positive multinucleated osteoclast formation in the co-culture of 5TGM1 myeloma cells and marrow cells
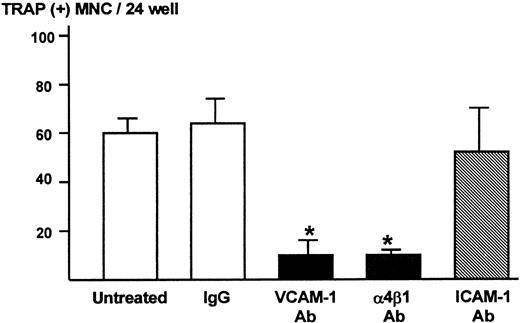
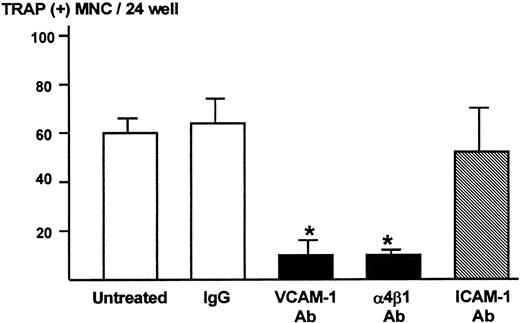
We subsequently determined the role of VCAM-1 and α4-integrin in osteoclast formation in the co-cultures using the neutralizing antibody to VCAM-1 or α4β1-integrin. Both anti–VCAM-1 antibody (VCAM-1 Ab, 10 μg/mL) and anti–α4β1-integrin antibody (α4β1 Ab, 10 μg/mL) dramatically inhibited TRAP-positive multinucleated osteoclast formation (Figure4). On the other hand, antibody against ICAM-1 (ICAM-1 Ab, 10 μg/mL) and control IgG had no effect on the osteoclast formation (Figure 4).
Effects of neutralizing antibody to VCAM-1 and α4β1-integrin on TRAP-positive multinucleated osteoclast formation in the co-cultures of 5TGM1 myeloma cells and primary mouse bone marrow cells.
A mixture of 5TGM1 cells (1 × 103) and primary mouse marrow cells (1 × 106) in suspension was inoculated in 48-well plates and cultured with or without 10 μg/mL of anti–VCAM-1 antibody (VCAM-1 Ab), anti–α4β1-integrin antibody (α4β1 Ab), anti–ICAM-1 antibody (ICAM-1 Ab), or control IgG. After 6 days of culture, cultures were fixed and the number of TRAP-positive multinucleated osteoclasts was determined. Data are expressed as mean ± SE (n = 4). *Significantly different from IgG control (P < .01).
Effects of neutralizing antibody to VCAM-1 and α4β1-integrin on TRAP-positive multinucleated osteoclast formation in the co-cultures of 5TGM1 myeloma cells and primary mouse bone marrow cells.
A mixture of 5TGM1 cells (1 × 103) and primary mouse marrow cells (1 × 106) in suspension was inoculated in 48-well plates and cultured with or without 10 μg/mL of anti–VCAM-1 antibody (VCAM-1 Ab), anti–α4β1-integrin antibody (α4β1 Ab), anti–ICAM-1 antibody (ICAM-1 Ab), or control IgG. After 6 days of culture, cultures were fixed and the number of TRAP-positive multinucleated osteoclasts was determined. Data are expressed as mean ± SE (n = 4). *Significantly different from IgG control (P < .01).
To determine whether this inhibition by VCAM-1 Ab and α4β1 Ab was specific for the 5TGM1-induced osteoclast formation, effects of these antibodies were examined on the 1,25-D3-induced osteoclast formation in the mouse bone marrow cell cultures. Neither VCAM-1 nor α4β1 Ab inhibited osteoclast formation induced by 10−8 mol/L 1,25-D3 (data not shown). The result is consistent with the notion that the interaction between VCAM-1 and α4β1-integrin is specifically critical to the osteoclast formation seen in the co-cultures of 5TGM1 myeloma cells and bone marrow cells. Feuerbach and coworkers27 have recently demonstrated that a neutralizing antibody to VCAM-1 inhibits 1,25-D3-induced osteoclast formation using a unique stromal cell line of which VCAM-1 expression is up-regulated by 1,25-D3. However, in our hands, there was no change in VCAM-1 expression in primary bone marrow stromal cells and ST2 cells used in the present study even in the presence of 1,25-D3 (data not shown). This apparent discrepancy might be due to the different biologic properties of the stromal cells used in both studies.
Effect of conditioned medium harvested from the co-cultures of 5TGM1 myeloma cells and ST2 stromal cells on bone resorption
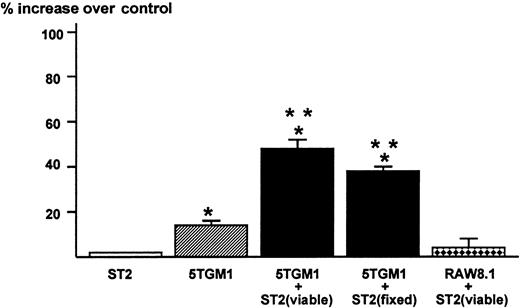
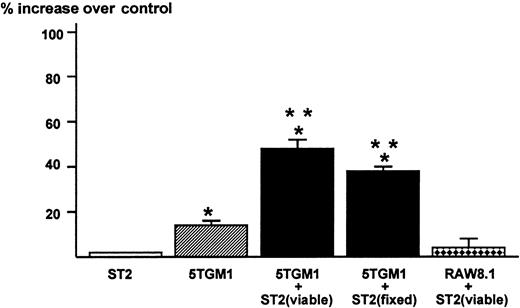
The results obtained in these co-culture experiments suggested to us that the direct contact between 5TGM1 myeloma cells and bone marrow stromal cells mediated via α4β1-integrin and VCAM-1 produces a soluble factor that stimulates osteoclast formation and function. Accordingly, we tested the effects of conditioned medium of the co-cultures of 5TGM1 myeloma cells and ST2 stromal cells on osteoclastic bone resorption. The conditioned medium at 40% (v/v) from the co-cultures showed increased bone-resorbing activity in fetal rat long bone assays (Figure5). The conditioned medium at 20% also exhibited significant bone-resorbing activity (percent increase over control = 34 ± 3) but there was no effect at 10%. The 5TGM1 myeloma cells co-cultured with ST2 stromal cells fixed with 2.5% paraformaldehyde also produced bone-resorbing activity. Conditioned medium from 5TGM1 myeloma cells alone showed marginal bone-resorbing activity. Co-culture of ST2 cells and nonmyeloma RAW8.1 B-cell lymphoma cells did not produce bone-resorbing activity. Conditioned medium from ST2 cells alone showed no bone-resorbing activity.
Production of bone resorbing activity in the co-cultures of 5TGM1 myeloma cells and ST2 mouse bone marrow stromal cells.
Conditioned media (48-hour culture) obtained from ST2 alone, 5TGM1 alone, and co-cultures of ST2 (viable or fixed) and 5TGM1 were assayed for bone resorbing activity in organ cultures of45Ca-labeled fetal rat long bones. 45Ca-labeled fetal rat long bones were cultured in the presence of conditioned media 40%, (v/v) or control medium for 120 hours. Percent 45Ca release was determined as 45Ca in the medium/45Ca in the medium plus bone × 100.45Ca release in control culture medium was 18 ± 2%. Percent increase over control was calculated as 45Ca release in test medium / 45Ca release in control medium −1 × 100. Data are expressed as mean ± SE (n = 4). *Significantly different from control (P < .05). **Significantly different from 5TGM1 alone (P < .05).
Production of bone resorbing activity in the co-cultures of 5TGM1 myeloma cells and ST2 mouse bone marrow stromal cells.
Conditioned media (48-hour culture) obtained from ST2 alone, 5TGM1 alone, and co-cultures of ST2 (viable or fixed) and 5TGM1 were assayed for bone resorbing activity in organ cultures of45Ca-labeled fetal rat long bones. 45Ca-labeled fetal rat long bones were cultured in the presence of conditioned media 40%, (v/v) or control medium for 120 hours. Percent 45Ca release was determined as 45Ca in the medium/45Ca in the medium plus bone × 100.45Ca release in control culture medium was 18 ± 2%. Percent increase over control was calculated as 45Ca release in test medium / 45Ca release in control medium −1 × 100. Data are expressed as mean ± SE (n = 4). *Significantly different from control (P < .05). **Significantly different from 5TGM1 alone (P < .05).
Production of osteoclastogenic and bone-resorbing activity in 5TGM1 myeloma cells in contact with rsVCAM-1
From the results described above, it seemed likely that 5TGM-1 cells were the producer of the bone-resorbing activity. To clarify this, 5TGM-1 myeloma cells were cultured in rsVCAM-1–coated plates in the absence of stromal cells. Of note, 5TGM1 myeloma cells became tightly attached to rsVCAM-1–coated plates. Conditioned medium was harvested from these cultures and assayed for osteoclastogenic activity using mouse bone marrow cultures. The conditioned medium showed strong osteoclastogenic activity (Figure 6A) and bone-resorbing activity in fetal rat long bone assay (Figure 6B). On the other hand, conditioned medium of 5TGM1 cells cultured on noncoated plates exhibited no activity of osteoclast formation and bone resorption. The rsVCAM-1 itself had no effects on bone resorption (data not shown).
Osteoclastogenic and bone-resorbing activity.
(A) Production of osteoclastogenic activity in 5TGM1 mouse myeloma cells and human myeloma cells cultured in rsVCAM-1–coated plates. Cells (1 × 106/mL/24-well) were cultured in plates coated with (closed bar) or without (open bar) rsVCAM-1 (1 μg/mL) in IMDM with 0.1% BSA for 24 hours. Conditioned media (40%) were assayed for osteoclastogenic activity in the mouse bone marrow cultures. Data are expressed as mean ± SE (n = 4). *Significantly different from control and conditioned media harvested from noncoated plates (P < .05). (B) Production of bone resorbing activity in 5TGM1 cells cultured in plates coated with rsVCAM-1. 5TGM1 cells (1 × 106/mL/24-well) were cultured in plates coated with or without rsVCAM-1 (1 μg/mL) in IMDM with 5% FBS for 24 hours. Conditioned media (40%) were assayed for bone-resorbing activity in organ cultures of 45Ca-labeled fetal rat long bones as described. Data are expressed as 45Ca release into the culture medium. Data are expressed as mean ± SE (n = 4). *Significantly different from conditioned medium of 5TGM1 myeloma cells cultured in noncoated plates (P < .01). (C) Effects of anti–VCAM-1 antibody (VCAM-1 Ab) or anti–α4β1-integrin antibody (α4β1 Ab) on the production of osteoclastogenic activity. 5TGM1 myeloma cells were cultured in rsVCAM-1–coated plates in the presence of 10 μg/mL of anti–VCAM-1 antibody (VCAM-1 Ab) or anti–α4β1-integrin antibody (α4β1 Ab) in IMDM with 5% FBS for 24 hours and conditioned medium (40%) was assayed for osteoclastogenic activity. Data are expressed as mean ± SE (n = 4). *Significantly different from conditioned medium of 5TGM1 cells cultured in noncoated plates (P < .01). **Significantly different from conditioned medium of 5TGM1 myeloma cells cultured in rsVCAM-1–coated plates without the antibodies (P < .01).
Osteoclastogenic and bone-resorbing activity.
(A) Production of osteoclastogenic activity in 5TGM1 mouse myeloma cells and human myeloma cells cultured in rsVCAM-1–coated plates. Cells (1 × 106/mL/24-well) were cultured in plates coated with (closed bar) or without (open bar) rsVCAM-1 (1 μg/mL) in IMDM with 0.1% BSA for 24 hours. Conditioned media (40%) were assayed for osteoclastogenic activity in the mouse bone marrow cultures. Data are expressed as mean ± SE (n = 4). *Significantly different from control and conditioned media harvested from noncoated plates (P < .05). (B) Production of bone resorbing activity in 5TGM1 cells cultured in plates coated with rsVCAM-1. 5TGM1 cells (1 × 106/mL/24-well) were cultured in plates coated with or without rsVCAM-1 (1 μg/mL) in IMDM with 5% FBS for 24 hours. Conditioned media (40%) were assayed for bone-resorbing activity in organ cultures of 45Ca-labeled fetal rat long bones as described. Data are expressed as 45Ca release into the culture medium. Data are expressed as mean ± SE (n = 4). *Significantly different from conditioned medium of 5TGM1 myeloma cells cultured in noncoated plates (P < .01). (C) Effects of anti–VCAM-1 antibody (VCAM-1 Ab) or anti–α4β1-integrin antibody (α4β1 Ab) on the production of osteoclastogenic activity. 5TGM1 myeloma cells were cultured in rsVCAM-1–coated plates in the presence of 10 μg/mL of anti–VCAM-1 antibody (VCAM-1 Ab) or anti–α4β1-integrin antibody (α4β1 Ab) in IMDM with 5% FBS for 24 hours and conditioned medium (40%) was assayed for osteoclastogenic activity. Data are expressed as mean ± SE (n = 4). *Significantly different from conditioned medium of 5TGM1 cells cultured in noncoated plates (P < .01). **Significantly different from conditioned medium of 5TGM1 myeloma cells cultured in rsVCAM-1–coated plates without the antibodies (P < .01).
The 5TGM1 myeloma cells did not attach to rsVCAM-1–coated plates in the presence of anti–VCAM-1 Ab (10μg/mL) or anti–α4β1-integrin Ab (10 μg/mL) during the entire culture period. Conditioned medium of 5TGM1 cells cultured in rsVCAM-1–coated plates in the presence of these antibodies showed marked decrease in the osteoclastogenic activity (Figure 6C).
Production of osteoclastogenic activity in ST2 stromal cells in contact with α4-integrin
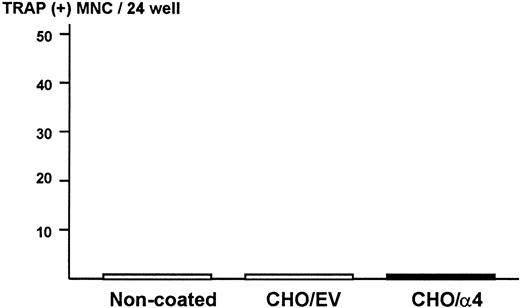
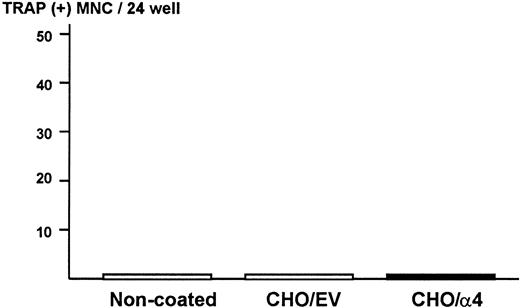
To exclude the possibility that ST2 bone marrow cells produce osteoclastogenic activity when they contact α4β1-integrin expressed on 5TGM1 myeloma cells in the co-cultures, ST2 cells were cultured on monolayer of fixed CHO cells expressing α4-integrin. We observed that attachment of ST2 cells to fixed CHO cells expressing α4-integrin was increased compared with fixed CHO cells with empty vector or noncoated plates (data not shown). However, none of conditioned media harvested from these cultures showed osteoclastogenic activity in the bone marrow culture assay (Figure7). These data strongly suggest that 5TGM1 myeloma cells are responsible for the production of osteoclastogenic and bone-resorbing activity in the co-cultures.
Production of osteoclastogenic activity in ST2 mouse bone marrow cells in contact with α4-integrin.
Confluent CHO cells expressing α4-integrin or empty vector were fixed with 2.5% paraformaldehyde and ST2 cells (2 × 105/24-well) were cultured on the monolayer of the fixed CHO cells for 24 hours. Conditioned media of these cultures were assayed for osteoclastogenic activity in mouse bone marrow cultures. EV indicates empty vector.
Production of osteoclastogenic activity in ST2 mouse bone marrow cells in contact with α4-integrin.
Confluent CHO cells expressing α4-integrin or empty vector were fixed with 2.5% paraformaldehyde and ST2 cells (2 × 105/24-well) were cultured on the monolayer of the fixed CHO cells for 24 hours. Conditioned media of these cultures were assayed for osteoclastogenic activity in mouse bone marrow cultures. EV indicates empty vector.
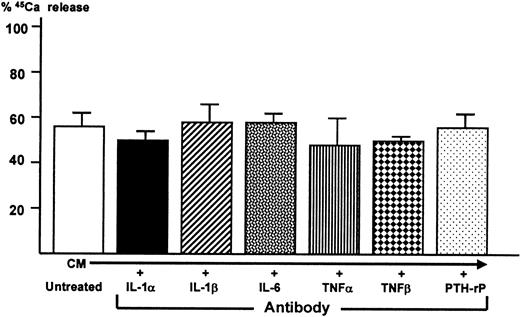
Effect of neutralizing antibodies to known osteoclastogenic cytokines on bone-resorbing activity producer by 5TGM1 cells
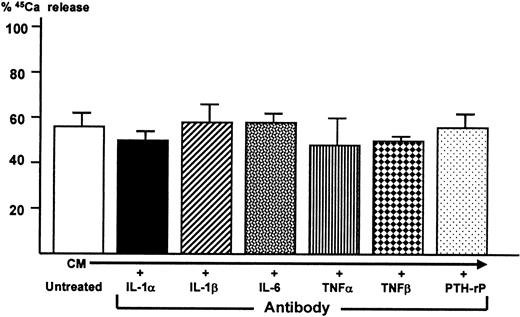
In an attempt to identify 5TGM1-derived cytokine responsible for osteoclastogenic activity and bone resorption, conditioned media from the co-cultures were treated with a saturating concentration of neutralizing antibodies to several known bone resorption-stimulating cytokines including IL-1α, IL-1β, IL-6, tumor necrosis factor-α (TNFα), tumor necrosis factor-β (TNFβ), and PTH-rP. Bone-resorbing activity of the antibody-treated conditioned media were then determined in the fetal rat long bone assay. In a preliminary experiment using the fetal rat long bone assay, 10−7 mol/L IL-1α and IL-1β, 500 ng/mL IL-6 together with 500 ng/mL soluble IL-6 receptor, 10−7 mol/L TNFα and TNFβ, and 25 ng/mL PTH-rP markedly stimulated bone resorption. We confirmed that each of the antibodies at a concentration used here blocked the bone-resorbing activity of each corresponding cytokine in this assay (data not shown). However, none of these antibodies blocked bone-resorbing activity present in the conditioned media (Figure8).
Effect of neutralizing antibodies to known osteoclast-activating cytokines on bone-resorbing activity of conditioned medium harvested from co-cultures of 5TGM1 myeloma cells and ST2 mouse bone marrow stromal cells.
Conditioned medium (CM) was treated with 10 μg/mL neutralizing antibodies to IL-1α and IL-1β, IL-6, TNFα, TNFβ, and PTH-rP (20 μg/mL)22 for 1 hour at room temperature. Subsequently, antibody-treated conditioned medium was assessed for its bone-resorbing activity in the fetal rat long bone assay as described in Figure 5.45Ca release in control culture medium was 17 ± 1% (not shown). Data are expressed as mean ± SE (n = 4).
Effect of neutralizing antibodies to known osteoclast-activating cytokines on bone-resorbing activity of conditioned medium harvested from co-cultures of 5TGM1 myeloma cells and ST2 mouse bone marrow stromal cells.
Conditioned medium (CM) was treated with 10 μg/mL neutralizing antibodies to IL-1α and IL-1β, IL-6, TNFα, TNFβ, and PTH-rP (20 μg/mL)22 for 1 hour at room temperature. Subsequently, antibody-treated conditioned medium was assessed for its bone-resorbing activity in the fetal rat long bone assay as described in Figure 5.45Ca release in control culture medium was 17 ± 1% (not shown). Data are expressed as mean ± SE (n = 4).
Discussion
Progressive osteoclastic bone destruction is one of the most detrimental complications of multiple myeloma. Earlier studies have suggested that production of osteoclast-activating cytokines by myeloma cells is responsible for the aberrant increase in osteoclastic bone destruction in patients.2,3 Here, we studied the murine myeloma cell line, 5TGM1, which causes typical myeloma bone diseases with extensive osteoclastic osteolysis in syngeneic mice,17 for its capacity to produce bone-resorbing cytokines to understand the mechanism responsible for myeloma-induced bone disease. Like many other human and murine myeloma cells,2 conditioned medium from 5TGM1 cultures showed marginal bone-resorbing activity. In contrast, our data showed that 5TGM1 cells in direct contact with the ST2 bone marrow stromal cells or primary mouse bone marrow stromal cells in the co-cultures produced increased osteoclastogenic activity. Prevention of contact between 5TGM1 and stromal cells in trans-well cultures resulted in a marked decrease in the production of this activity. More importantly, the results that 5TGM1 cells cultured in rsVCAM-1–coated plates produced osteoclast-stimulating activity in the absence of bone marrow stromal cells indicate that 5TGM1 myeloma cells are responsible for producing this activity in the co-cultures. These data demonstrate that the cell-cell contact with bone marrow stromal cells is essential for 5TGM1 myeloma cells to cause osteoclast stimulation. It is, therefore, suggested that the absence of stromal cells, a critical cellular component of bone marrow, may explain the failure of myeloma cells that are freshly isolated from patients with extensive osteolytic skeletal lesions or currently available human and murine myeloma cell lines to consistently produce discernible osteoclast-activating cytokines in culture. Figure 6A in which several human myeloma cell lines show the production of osteoclastogenic activity only in the presence of rsVCAM-1 further supports this notion.
We next examined which CAMs were involved in the direct cell-cell interactions between 5TGM1 cells and marrow stromal cells that are necessary for the production of osteoclastogenic cytokines. Our experiments using neutralizing antibodies to VCAM-1 and α4β1-integrin indicate that VCAM-1 and α4β1-integrin are involved in this cell-cell interaction. It has been previously reported that VCAM-1 is constitutively expressed in bone marrow stromal cells8 and that myeloma cells express α4β1-integrin, which serves as the receptor for VCAM-1.12,16VCAM-1/α4β1-integrin–mediated cross-talk with marrow stromal cells has been shown to be critical to the growth,9,12 metabolism,12 cellular signaling,13 specific localization,15 and homing7 of myeloma cells in the marrow cavity. Other studies have suggested the importance of the marrow microenvironment on the expression of the malignant phenotype of myeloma cells.7,9 Our results are consistent with these earlier studies. In addition, recent reports have demonstrated that CHO cells overexpressing α4β1-integrin increase osteoclast formation in vitro28 and preferentially develop bone metastases.21 They have also shown that administration of neutralizing antibodies to VCAM-1 or α4β1-integrin selectively decreases bone metastases but not pulmonary metastases.21 These results suggest that VCAM-1/α4β1-integrin interactions also play a critical role in the preferential colonization of solid tumors to bone. Our findings indicate that VCAM-1/α4β1-integrin interactions are, at least in part, responsible for the cell-cell communication between marrow stromal cells and 5TGM1 myeloma cells, which consequently leads to increased production of osteoclast-activating cytokines. In further support of this notion, our data demonstrate that several human myeloma cell lines produce osteoclastogenic activity when they contact rsVCAM-1 in culture.
However, it should be noted that solid tumors may behave differently from myeloma in this regard. For example, others have shown that stable transfection of α4β1-integrin cDNA into B10 melanoma cells decreases the capacity of these cells to invade and metastasize.29 In addition, several other studies also reported that α4β1-integrin inhibited metastasis.30 The reasons for these differences are unknown. It is speculated that solid tumor cells, which do not necessarily have predilection for homing to the bone marrow, might elicit different cytoplasmic or nuclear events as a consequence of these cell-cell interactions with host cells compared with myeloma cells that exclusively colonize in the bone marrow.
Myeloma cells express not only α4β1-integrin but also a variety of other integrins.12,16 To determine the involvement of other integrins in the interactions between 5TGM1 cells and stromal cells, we examined α4β7-integrin, which has been shown to be expressed in myeloma cells12,16 and is also a receptor for VCAM-1. However, the β7-integrin was not detected in 5TGM1 cells by RT-PCR. Fibronectin, which is also a ligand for α4β1-integrin and expressed in marrow stromal cells,31 may also be involved in mediating the interactions between 5TGM1 cells and bone marrow stromal cells. In a preliminary experiment, we observed that 5TGM1 cells attached onto fibronectin-coated plates (data not shown), suggesting that fibronectin may also play a role in cell-cell contact between 5TGM1 cells and bone marrow stromal cells. Further studies are required to clarify this point. Nevertheless, our results strongly suggest that α4β1-integrin constitutively expressed in myeloma cells and VCAM-1 constitutively expressed in the marrow stromal cells are critical to establish the partnership between these 2 types of cells in the bone marrow cavity during the progression of massive bone destruction in myeloma.
Different osteoclast-activating cytokines such as IL-1,32IL-6,33,34 TNF-β,35 PTH-rP36and hepatocyte growth factor37 have been implicated in the pathogenesis of myeloma-induced osteolysis. We have not as yet identified the osteoclast-stimulating activity produced in the co-cultures, and this is likely to be an extensive undertaking. Neutralizing antibodies to several known osteoclast-activating cytokines including IL-1α and β, IL-6, TNF-α and -β, and PTH-rP failed to block the osteoclast-stimulating activity in the conditioned medium of the co-cultures. These preliminary observations raise the possibility that a novel cytokine may be responsible. Recent studies reported independently from 2 groups suggest that the transmembranous receptor activated nuclear factor (NF)-κB ligand (RANKL)/osteoclast differentiation factor (ODF)/osteoprotegerin ligand (OPGL) expressed in stromal cell/osteoblasts is the final common mediator of osteoclast-activating factors such as 1,25-D3, parathyroid hormone (PTH), prostaglandin E2, and IL-11.38,39 Of interest, our preliminary experiments have shown that both 5TGM1 and ST2 cells express RANKL/ODF/OPGL and that the expression is up-regulated in the co-cultures.40 These results suggest that not only myeloma cells but also bone marrow stromal cells directly enhance osteoclastogenesis and bone resorption. It should be noted, however, that RANKL/ODF/OPGL is a membrane-bound protein and thus not soluble. Whether RANKL/ODF/OPGL also plays a role in the pathogenesis of bone destruction in myeloma is currently unknown but is obviously an important issue to be explored to further understand the molecular mechanism of this devastating complication of myeloma.
In conclusion, our data suggest that the direct cell-cell contact between myeloma cells and marrow stromal cells via α4β1-integrin/VCAM-1 is critical to the production of a yet-unidentified soluble factor(s) that stimulates osteoclastogenesis and bone resorption. We believe this is one of the mechanisms by which myeloma cells exclusively home in the bone marrow and subsequently cause profound bone destruction. If this is proved to be the case universally in myeloma, then disruption of this interaction is a potential new therapeutic intervention for the treatment of myeloma-induced bone disease.
Acknowledgments
The authors are grateful to Dr David Roodman for valuable comments and discussion, Dr Roy Lobb for helpful discussion and providing the recombinant soluble VCAM-1, and Dr Kensaku Miyake for supplying the antibodies to VCAM-1, ICAM-1, and α4β1-integrin. The authors also thank Miss Mie Masuda, Ms Fran Ramirez, and Ms Christa Martinez for their excellent secretarial assistance.
Supported by NIH grants PO1-CA40035, R01-AR28149, and RO1-DK45229.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toshiyuki Yoneda, Division of Endocrinology and Metabolism, Department of Medicine, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr, San Antonio, TX 78284-7877; e-mail: yoneda@uthscsa.edu.