Abstract
A higher percentage of apoptotic cells (apoptotic index or AI) is consistently found in bone marrow (BM) biopsies compared to BM aspirates of patients with myelodysplastic syndrome (MDS). Most studies have only investigated the low-density fraction (LDF) mononuclear cells from BM aspirates following density separation for AI determination. In the present study, both LDF and high-density fraction (HDF) cells for AI were examined by electron microscopy (EM) in 10 MDS patients and 4 healthy donors. Matched BM biopsies were subjected to AI detection by in situ end labeling (ISEL) of fragmented DNA. The results indicate that in LDF and HDF cells, AI is consistently higher in MDS patients (8.5% vs 1.5%, respectively; P = .039) compared to healthy donors (27% vs 4%, respectively; P = .004). The BM biopsy AI was also higher in MDS patients than in healthy donors (3+ vs 0+, respectively; P = .036). In addition, in MDS patients, more apoptotic cells were found in HDF cells than in LDF cells (27% vs 8.5%, respectively;P = .0001). All stages of maturation, ranging from blasts to terminally mature cells belonging to all 3 lineages, were represented in the dying cells in both compartments. Using EM, typical Pelger-Huett–type cells appeared to be apoptotic granulocytes. Both LDF and HDF cells should be examined for an accurate estimation of apoptotic cells because AI would be underestimated if only the LDF cells were studied. Ultrastructural studies consistently show a higher AI in BM biopsies compared to BM aspirates despite the correction factor of HDF cells provided by AI. This may represent the actual extant state, which could conceivably be due to a higher concentration of proapoptotic signals in the biopsies.
Introduction
Variable cytopenias in the face of normocellular to hypercellular bone marrow (BM) in patients with myelodysplastic syndrome (MDS) have been attributed in part to excessive intramedullary apoptosis of hematopoietic cells.1-3 Although increased rates of apoptosis have been confirmed by many independent groups using a variety of techniques,4-7 there is still an unanswered question regarding the discrepancy of the apoptotic index (AI) or the percentage of apoptotic cells between BM aspirates and biopsies. The percentage of apoptotic cells is found to be consistently higher in BM biopsies than in aspirates. In the present study, an attempt was made to address this issue. It was hypothesized that we may be underestimating AI in BM aspirate samples by only looking for apoptotic cells in the low-density fraction (LDF) of BM mononuclear cells (BMMNCs). Following separation on a density gradient, both the low- and high-density BM aspirate cells were examined by electron microscopy (EM). The BM biopsies were examined for the precise incidence of apoptosis using in situ end labeling (ISEL) of fragmented DNA. Our results indicate that large numbers of apoptotic cells can be recovered from the high-density fraction (HDF) of BM aspirate cells from MDS patients, thereby at least partly explaining the discrepancy that exists in the AI of these 2 compartments.
Materials and methods
BM biopsies from 10 MDS patients and 4 healthy donors were studied as part of this protocol. Peripheral blood, BM aspirate, and BM biopsies were obtained and transported on ice to the laboratory. The donors were asked to donate BM for research purposes, and prior to the procedures, they signed consent forms for the protocol approved by the institutional review board of Rush-Presbyterian-St Luke's Medical Center to study these samples.
Studies conducted on BM aspirates
We directly aspirated 15-20 mL BM into a syringe containing 3 cc of 2% sodium citrate. The aspirate was separated into LDFs and HDFs using Ficoll-Hypaque 1077 density gradient solution (Amersham Pharmacia Biotech, Piscataway, NJ). The LDF cells were fixed in 3% gluteraldehyde and subjected to routine transmission EM as described below. The HDF cells were further treated with sodium chloride to lyse the red blood cells. Subsequently the cells were fixed in 3% gluteraldehyde, postfixed in osmium tetroxide, dehydrated, and embedded according to the standard methods for routine EM.8
Studies conducted on BM biopsies
Long-core BM biopsies were obtained from every individual and placed in saline. Under aseptic conditions, these biopsies were cut in 2 segments. One-half was placed in Bouin's fixative and embedded in plastic using glycol methacrylate; 2- to 3-μm-thick sections were placed on Alcian blue–coated coverslips for detection of apoptosis by ISEL. The second half was used for EM as described later.
Measurement of apoptosis using ISEL of fragmented DNA
ISEL was carried out on all BM biopsies. Briefly, the cells were pretreated with sodium chloride sodium citrate (SSC) solution at 80°C and 1% Pronase (1 mg/mL in 0.15 mol/L phosphate-buffered saline [PBS]) (Calbiochem, La Jolla, CA). The sections were then incubated at 18°C with a mixture of 0.01 mol/L deoxyadenosine, deoxycytidine, and deoxyguanosine 5′-triphosphate (dATP, dCTP, and dGTP) (Promega Company, Madison, WI); 0.001 mol/L biotinylated uridine 5′-triphosphate (bio-dUTP) (Sigma Chemical Co, St Louis, MO); and 20 U/mL DNA Polymerase I (Promega Company). Incorporation of bio-dUTP was finally visualized using an avidin-biotin-peroxidase conjugate (Vectastain Elite ABC Kit; Vector, Burlingham, CA) and diamino benzindine tetrachloride. Thus, cells labeled positively for ISEL showed brown staining in their nuclei under the light microscope. The controls for these experiments were carried out as described before.9
Interpretation of slides.
All slides were observed on a televised screen by 3 different investigators. (A.R. was an investigator for each slide.) A subjective quantitative scale was formulated to determine the degree of positivity as follows: negative, low, intermediate, and high.9Negative or absent indicates that there were less than 15% ISEL-positive cells; low, 1-3+ or 15%-30%; intermediate, 4-5+ or 31%-75%; and high, 6-8+ or greater than 75%.
Statistical analysis.
The nonparametric Mann-Whitney U test was used for comparison between the 2 parameters.
Measurement of apoptosis usingEM
Modified technique.
To avoid decalcification and cell deformation during the preparation process, a modified technique, which preserves the detailed morphology of the BM biopsy, was carried out. The procedure involved perfusion of the bone with 3% gluteraldehyde, further immersion for 15 minutes, and then careful teasing of the BM by decortication. The processing for transmission EM (TEM) was carried out by using standard techniques.8 Briefly, after fixation the cells were postfixed in osmium tetroxide, treated with alcohol and propylene oxide, and embedded in Araldite (EM Sciences, Washington, PA) at 58°C for 48 hours. The biopsies were then processed for semithin and ultrathin sections. Semithin sections were stained with toluidine blue and evaluated by light microscopy. Ultrathin sections were contrasted with uranyl acetate and lead citrate and analyzed by TEM (JEOL 200, Japan).
Statistical analysis.
The Mann-Whitney U test and Spearman rank correlation were used for 2 sample comparisons of the continuous variables. Contingency tables with chi-square statistics or the Fisher exact test were used for analysis.
Results
The present study was carried out using BM samples from 10 MDS patients and 4 healthy donors. The French-American-British (FAB) classification was used to identify the various subtypes of MDS. Of the 10 MDS patients, there were 7 cases of refractory anemia (RA), and 3 of these cases had RA with ring sideroblasts (RARS). Table1 describes the details of the FAB type and apoptosis studies carried out on the different fractions of BM aspirate and biopsy using ISEL and EM.
Measurement of apoptosis using ISEL
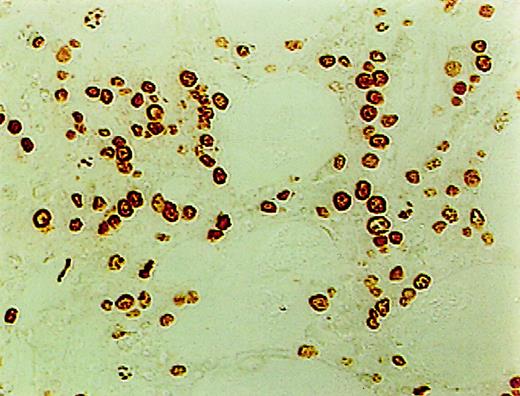
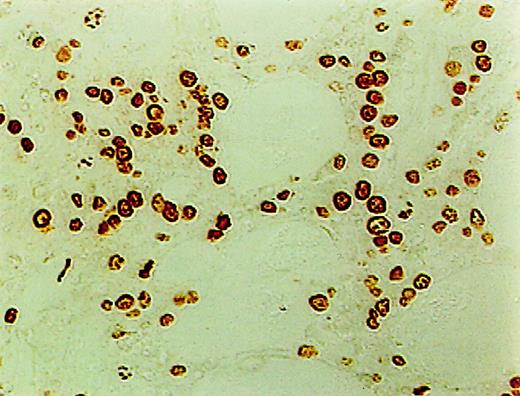
ISEL of fragmented DNA was performed on plastic embedded BM biopsies. A distinct brown staining in the nucleus identified a cell as being engaged in DNA cleavage. Among the 10 cases studied, 2 cases showed high positivity; 2, intermediate; 4, low; and 1, no ISEL positivity. When comparing MDS patients to the healthy donors, an increase in apoptosis was noted in MDS patients (3+ in MDS patients vs 0+ in donors; P = .1). All 3 lineages of hematopoietic cells, including the myeloid, erythroid, and megakaryocytic cells, were found to be undergoing apoptosis. Furthermore, in almost every case, stromal cells were also found to be apoptotic (Figure1).
High incidence of apoptosis involving hematopoietic and stromal cells in the BM of MDS patients using ISEL.
Brown staining is noted (original magnification × 400).
High incidence of apoptosis involving hematopoietic and stromal cells in the BM of MDS patients using ISEL.
Brown staining is noted (original magnification × 400).
Measurement of apoptosis using EM
Ultrastructural studies were carried out on both the LDF and HDF cells of the BM aspirate in MDS patients and the donors for the detection of apoptosis morphologically. Table2 shows the median AI in the different fractions in MDS patients and donors. The AI was calculated by counting 500-1000 cells in randomly selected fields by EM. The overall incidence of apoptosis was found to be significantly higher in both the LDF and HDF cells in MDS patients as compared to normal BM cells. The median AI in the LDF of MDS marrows was 8.5% compared to 1.5% in healthy donors (P = .0399). The HDF cells in MDS patients showed an AI of 27% compared to 4% in donor marrows (P = .0046). However, when the rate of apoptosis between the 2 fractions within individual MDS patients was compared, a significantly higher rate of apoptosis was seen in the HDF cells compared to the LDF cells (27% versus 8.5%, P = .0001).
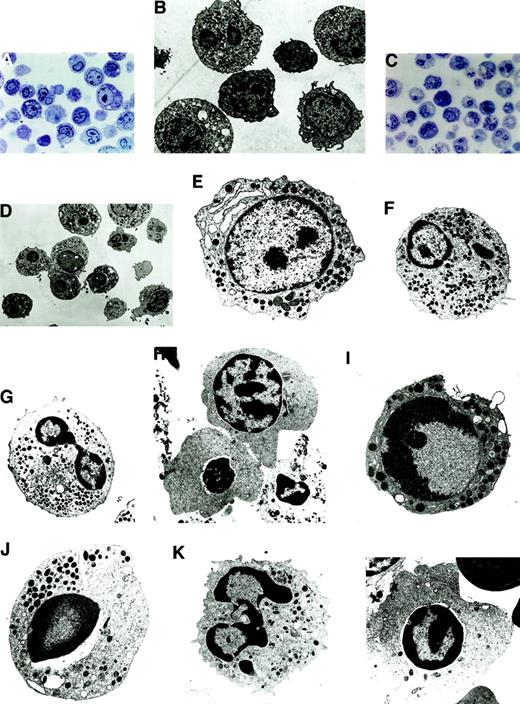
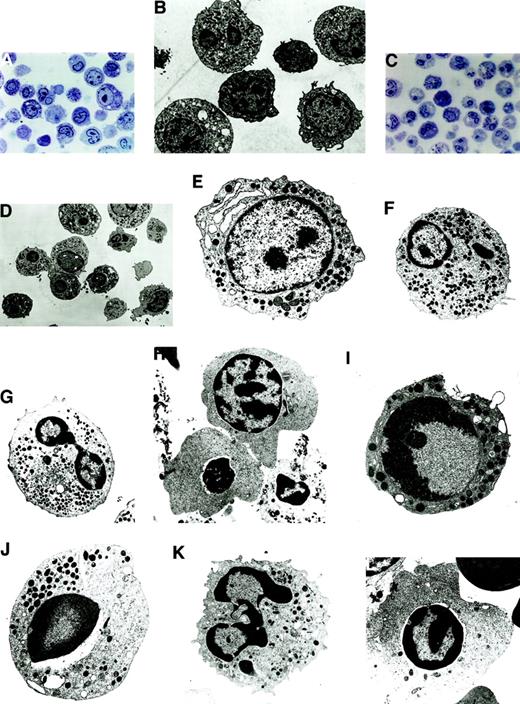
Morphologically, apoptosis was seen in all the lineages, with a predominance in the myeloid and erythroid lineages. In LDF cells, apoptosis was seen in the progenitor cells of the myeloid and erythroid lineages (Figure 2A,B). In HDF cells, the differentiated cells showed a higher degree of apoptosis (Figure 2C,D); however, both early and late stages of apoptosis were clearly recognizable under EM.
Morphologically, apoptosis was seen in all the lineages, with a predominance in the myeloid and erythroid lineages.
(A) Semithin section of LDF from MDS patient showing minimal apoptosis (toluidine blue staining, original magnification × 400). (B) Electron micrograph of semithin section A (uranyl acetate and lead citrate, original magnification × 6600). (C) Semithin section of HDF from MDS patient showing increased apoptosis in the myeloid and erythroid lineage (toluidine blue staining, original magnification × 400). (D) Electron micrograph of semithin section C. Note the characteristic appearance of apoptosis, segregation of the chromatin in sharply circumscribed masses that lie against the nuclear envelope and condensed cytoplasm (uranyl acetate and lead citrate, original magnification × 6600). (E-H) Ultrastructural features of nonapoptotic cells in MDS. (E) Note the myeloblast with euchromatin nucleus, nucleoli, and a fair number of primary and secondary granules. (F) Dysplastic promyelocyte showing a low nuclear cytoplasmic ratio and abundant granules with Auer rods. (G) As the cell matures, note the characteristic Pelger-Huet anomaly showing bilobed neutrophil. (H) Polychromatophilic megaloblast with the appearance of a few clumps among the chromatin beads (uranyl citrate, original magnification × 5000). (I-L) Ultrastructural features of apoptotic cells in MDS. (I) A myeloblast showing the early stages of apotosis with only marginal condensation of nuclear chromatin, with a prominent nucleoli and preservation of the integrity of the organelles (original magnification × 8300). (J) Apoptotic promyelocyte with a perinuclear vacuole (original magnification × 6600). (K) Mature granulocytic neutrophil undergoing apoptosis, showing nuclear budding (original magnification × 8300). (L) Apoptotic erythroid cell (uranyl acetate and lead citrate, original magnification × 6600).
Morphologically, apoptosis was seen in all the lineages, with a predominance in the myeloid and erythroid lineages.
(A) Semithin section of LDF from MDS patient showing minimal apoptosis (toluidine blue staining, original magnification × 400). (B) Electron micrograph of semithin section A (uranyl acetate and lead citrate, original magnification × 6600). (C) Semithin section of HDF from MDS patient showing increased apoptosis in the myeloid and erythroid lineage (toluidine blue staining, original magnification × 400). (D) Electron micrograph of semithin section C. Note the characteristic appearance of apoptosis, segregation of the chromatin in sharply circumscribed masses that lie against the nuclear envelope and condensed cytoplasm (uranyl acetate and lead citrate, original magnification × 6600). (E-H) Ultrastructural features of nonapoptotic cells in MDS. (E) Note the myeloblast with euchromatin nucleus, nucleoli, and a fair number of primary and secondary granules. (F) Dysplastic promyelocyte showing a low nuclear cytoplasmic ratio and abundant granules with Auer rods. (G) As the cell matures, note the characteristic Pelger-Huet anomaly showing bilobed neutrophil. (H) Polychromatophilic megaloblast with the appearance of a few clumps among the chromatin beads (uranyl citrate, original magnification × 5000). (I-L) Ultrastructural features of apoptotic cells in MDS. (I) A myeloblast showing the early stages of apotosis with only marginal condensation of nuclear chromatin, with a prominent nucleoli and preservation of the integrity of the organelles (original magnification × 8300). (J) Apoptotic promyelocyte with a perinuclear vacuole (original magnification × 6600). (K) Mature granulocytic neutrophil undergoing apoptosis, showing nuclear budding (original magnification × 8300). (L) Apoptotic erythroid cell (uranyl acetate and lead citrate, original magnification × 6600).
The normal nonapoptotic myeloblasts showed oval euchromatin nuclei, nucleoli were prominent, and a fair number of primary and secondary granules were readily found (Figure 2E). The dysplastic promyelocytes showed a low nuclear cytoplasmic ratio with the presence of Auer rods (Figure 2F). In HDF, the classical Pelger-Huet anomaly showing bilobed nucleoli was noted (Figure 2G). In the erythroid series, nonapoptotic polychromatophilic megaloblasts with the appearance of a few clumps among the chromatin beads were noted (Figure 2H).
In the apoptotic myeloblasts, initial changes in the chromatin condensation could be graphically seen (Figure 2I), thereby documenting the earliest unequivocal evidence of apoptosis. In apoptotic cells the proportion of the nuclei occupied by condensed chromatin varied with the cell type, with the early events occurring in the nucleus, followed by condensation of the cytoplasm and eventual vacuole formation. Apoptotic promyelocytes were seen with perinuclear vacuoles (Figure2J). In HDF, apoptotic neutrophils were seen in abundance (Figure 2K), and similar observations were made in the apoptotic erythroid cells (Figure 2L). In summary, all stages of maturation were unequivocally represented among the dying cells, ranging from blasts to mature cells belonging to all the 3 lineages.
Ultrastructure studies on BM biopsy
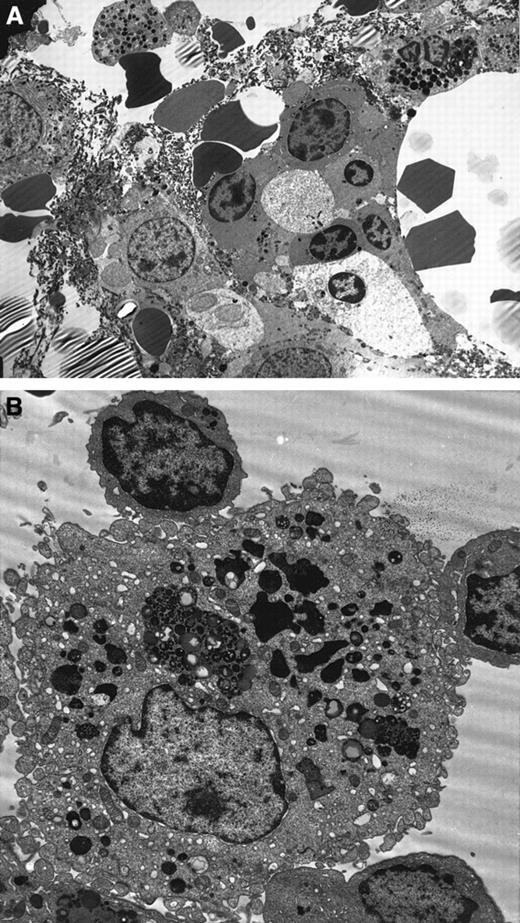
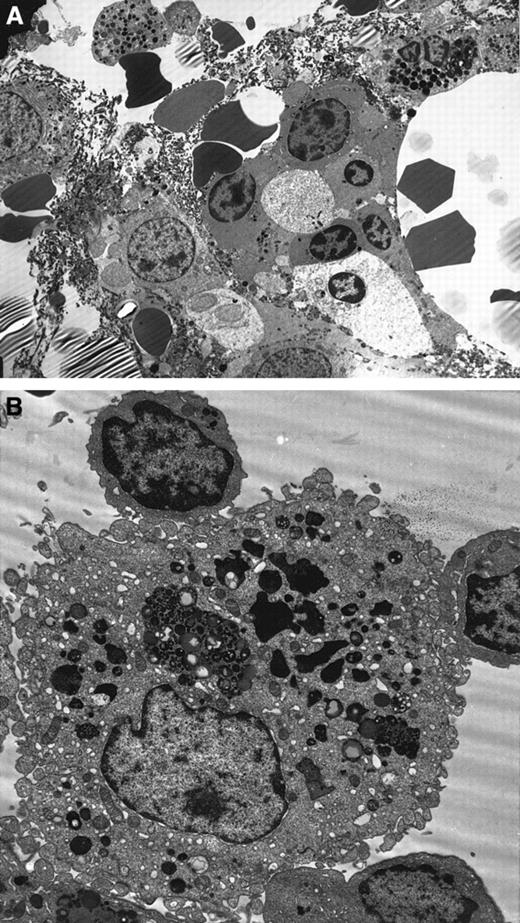
Ultrastructural details were very well preserved in the normal BM biopsies. Using the modified technique described earlier, the branching sinus network and intersinal hematopoietic tissue were seen. Remnants of fat cells were also noted. Both the stromal and parenchymal cells were clearly identified (Figure3A). In contrast, the MDS marrow showed an abundance of apoptotic cells belonging to all lineages. Again, apoptosis was predominantly noted in the myeloid and erythroid lineages. The stromal cells showed minimal apoptosis by morphology. Throughout the BM biopsy, macrophages engulfing apoptotic red cells were noted (Figure 3B).
Ultrastructure studies on BM biopsy.
(A) Electron micrograph of MDS BM biopsy showing apoptosis in the myeloid and erythroid lineages. Note the nuclear chromatin condensation and vacuoles in the cytoplasm (uranyl acetate and lead citrate, original magnification × 2600). (B) Macrophages showing partly degraded apoptotic erythroid cells in the lysosomes in the MDS BM biopsy (original magnification × 6600).
Ultrastructure studies on BM biopsy.
(A) Electron micrograph of MDS BM biopsy showing apoptosis in the myeloid and erythroid lineages. Note the nuclear chromatin condensation and vacuoles in the cytoplasm (uranyl acetate and lead citrate, original magnification × 2600). (B) Macrophages showing partly degraded apoptotic erythroid cells in the lysosomes in the MDS BM biopsy (original magnification × 6600).
Discussion
Pancytopenia, the hallmark of MDS, has long been attributed to an “ineffective hematopoiesis.” In 1990, Clark and Lampert1 published a study of 23 MDS patients whose BM biopsies demonstrated histological features of apoptosis by light microscopy in the erythroid and immature myeloid cell precursors. In 1992, Hatfill et al10 demonstrated intramedullary apoptosis in large numbers of megakaryocytes in the BM biopsies of 20 MDS patients. These observations were followed by the hypothesis of Yoshida2 in 1993 stating that apoptosis may be the mechanism for premature intramedullary cell death in MDS. The same year, we published our initial observations of excessive intramedullary apoptosis in the BM biopsies of MDS patients documented by using the ISEL technique3; this was followed by several publications confirming the findings in larger numbers of patients over the next 2 years.11-13 Using the ISEL technique, we demonstrated large numbers of apoptotic cells in BM biopsies.11
However, we could not detect either ISEL-positive cells or low-molecular DNA fragments by conventional DNA laddering on gel electrophoresis when low-density BMMNCs from BM aspirates were studied.11 Leaving BMMNCs in short-term cultures in complete medium for 4 hours and then studying these samples for the presence of low-molecular weight DNA fragments showed an increase in AI.11 Starting with our very first publication,11 we have consistently pointed out this peculiar discrepancy between the percentage of apoptotic cells in BM aspirates and matched MDS biopsies. Ultrastructural confirmation of apoptosis in BM aspirates was provided by Bogdanovic et al5in 1996.
In 1996, Lepelley et al7 published their results of 40 MDS patients in whom apoptosis was determined using the terminal deoxynucleotidyl transferase (TdT) incorporation of nucleotides on the 3′-ends of DNA (TUNEL technique [TdT–mediated dUTP nickend-labeling]) in BM aspirates. These authors found no evidence of increased apoptosis in the LDF cells of MDS patients compared to donor marrows. However, when both LDF and HDF cells of BM aspirates from 7 of 40 MDS patients were subjected to short-term cultures (18 hours), a higher incidence of apoptosis was seen by the TUNEL technique in both LDF cells (4 of 7 patients) and HDF cells (1 of 7 patients). Because this percentage of apoptotic cells ranged from 4%-7% in LDF cells and 7%-24% in HDF cells and because our results in BM biopsies had yielded more than 75% of apoptotic cells in half the MDS patients studied, Lepelley et al7 raised the question of whether apoptosis is indeed a “massive” process in MDS. This question should have only been raised if the authors had examined matched BM biopsies of their MDS patients using the same techniques that we did and had found that the biopsies yielded results similar to the aspirates.
Others who have examined BM biopsies of MDS patients have confirmed our findings precisely. For example, both Hellstrom-Lindberg et al14 and Parcharidou et al15 found more than 50% apoptotic cells in the biopsies of MDS patients. Therefore, the important question that needs to be addressed is why there is a discrepancy between the percentage of apoptotic cells found in the BM aspirates and BM biopsies; the question is not whether apoptosis in MDS is massive or not. In this paper, we asked just that question and found a possible solution.
Previously, we have been impressed by the discrepancy in the rates of cellular proliferation as well between BM aspirates and biopsies of patients with MDS and acute myeloid leukemia (AML). Following a bromododexoyuridine and/or iododexoyuridine (BrdU/IUdR) infusion, the labeling index (LI) or the percentage of S-Phase cells were 3 times higher as a rule in the biopsy compartments of MDS/AML patients than in their aspirates.16 This appeared to be a result of variable hemodilution because there are no S-Phase cells in the peripheral blood (PB) of MDS or AML patients.17 A lower AI could have a similar etiology as well because PBMNCs from MDS patients are not only nonapoptotic, but they have actually been shown to be more resistant to apoptosis than normal PBMNCs.18 Increased apoptosis and proliferation of hematopoietic cells in the biopsy compartment compared to BM aspirates could be because the levels of cytokines are much higher in biopsies than in aspirates.
Another interesting observation is that the recovery of BMMNCs is not usually high in MDS patients even though the BM is generally hypercellular. For example, it is not uncommon to recover 50-80 million BMMNCs from approximately 20 cc of citrated BM aspirate obtained from healthy donors, while the majority of BM aspirates from MDS patients yield between 20-30 million BMMNCs. Taken together, the discrepancy between the AI of BM aspirates and biopsies as well as a low recovery of BMMNCs following density separation on Ficoll-Hypaque gradient appear to indicate the possibility that an unknown but fairly substantial number of cells are unaccounted if only the LDF is examined. This study was undertaken to examine the possibility that the AI of BMMNCs was low because the apoptotic cells had migrated to the HDF cells. Indeed, that is what we found. The present study clearly demonstrates the presence of large numbers of apoptotic cells in the BM aspirates of MDS patients.
In fact, there are many more apoptotic cells in the HDF (median AI, 27%) compared to the MNCs obtained from the LDF (median AI, 8.5%;P = .0001) in MDS patients. One of the possible reasons could be that the apoptotic cells have a tendency to shrink and sink to the bottom of the HDF. So it is very important to look at both the LDF and HDF in BM aspirate to get an accurate estimation of the AI. Lepelley et al7 looked at the BMMNCs and polymorphonuclear (PMN) cells in 7 MDS patients and also found apoptotic cells in both the fractions after short-term culture. Because apoptotic cells actually lose DNA, one would expect to find more of them in the LDF cells as opposed to the HDF cells, yet a higher proportion of apoptotic cells was recovered from the latter compartment. Another obvious explanation would be that apoptosis is more common in maturing cells (HDF) as opposed to their less mature (LDF) counterparts. But this would still not explain the presence of apoptotic MNCs in HDF. We believe that the MNCs recovered from the higher density cells represent a more advanced stage in the maturational hierarchy compared to the ones found in the LDF. In the final analysis, the AI of BM aspirate cells is still not quite as high as that for the biopsy cells, and this is probably the result of hemodilution as well as the possibility that proapoptotic cytokines are more concentrated in the biopsy compartment.
Another striking feature of this study relates to the ultrastructural findings being reported here. Erythroid and/or myeloid cells belonging to all stages of maturation, ranging from immature blasts to terminally differentiated forms, were found to be apoptotic. In addition, the Pelger-Huett–type granulocytes appeared to be apoptotic neutrophils. This raises the possibility of premature apoptosis even in the granulocytes because programmed cell death appears to have preceded nuclear lobulation. In conclusion, therefore, an accurate assessment of the incidence of apoptosis can only be made in BM biopsies because BM aspirates may not be representative of the actual events.
Acknowledgments
The authors wish to thank Sandra L. Howery and Lakshmi Venugopal for excellent secretarial and administrative assistance.
Supported by grant PO1CA 75606 from the National Cancer Institute, Bethesda, MD; The Markey Charitable Trust (94-8), Miami, FL; and the Dr Roy Ringo Grant for basic research in MDS, Hindsdale, IL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vilasini Shetty, Department of Medicine, Rush Cancer Institute, 2242 West Harrison Street, Tech 2000 Building, Suite 108, Chicago, IL 60612-3515; e-mail: vravanam@rush.edu.