Abstract
The binding of von Willebrand factor (vWF) to glycoprotein (GP) Ib-IX-V stimulates transmembrane signaling events that lead to platelet adhesion and aggregation. Recent studies have revealed that the signaling protein 14-3-3ζ binds directly to the cytoplasmic domain of GP Ib. In this study, the dynamic association of 14-3-3ζ with GP Ib-IX, the phosphoinositide 3-kinase (PI 3-kinase), or both, was investigated in resting, thrombin, or vWF and botrocetin-stimulated platelets by analysis of discrete subcellular fractions. Results of this study demonstrate maximal coimmunoprecipitation of 14-3-3ζ with GP Ib-IX in the nonstimulated cytosolic fraction and in the actin cytoskeletal fraction of thrombin- or vWF-stimulated human platelets. Immunoprecipitated 14-3-3ζ or GP Ib from cytosolic fractions contained PI 3-kinase enzyme activity and an 85-kd polypeptide recognized by antibodies to the p85 subunit of PI 3-kinase. After platelet activation, the level of association between these species decreased in the cytosolic fraction. However, increased complex formation between 14-3-3ζ and GP Ib-IX and between PI 3-kinase and GP Ib-IX was detected in actin cytoskeletal fractions derived from thrombin- or vWF-stimulated platelets. Recombinant glutathione S-transferase-14-3-3ζ fusion protein (14-3-3ζ–GST) inhibited affinity-captured PI 3-kinase enzyme activity up to 70% at 2 μmol/L 14-3-3ζ–GST. However, increasing concentrations up to 5 μmol/L 14-3-3ζ–GST resulted in the 3-fold enhancement of PI 3-kinase enzyme activity. We propose that the association between PI 3-kinase and 14-3-3ζ with GP Ib-IX serves to promote the rapid translocation of these signaling proteins to the activated cytoskeleton, thereby regulating the formation of 3-position phosphoinositide-signaling molecules in this subcellular compartment.
Platelet adhesion to the vascular subendothelium provides a mechanism for the arrest of bleeding and also contributes to the occlusion of diseased vessels in pathologic states. The platelet receptor for von Willebrand factor (vWF), the glycoprotein (GP) Ib-IX-V complex (GP Ib-IX-V), mediates the initial adhesion of platelets to vWF in the exposed subendothelium.1-4 In addition, platelet activation and aggregation are induced by the interaction between GP Ib-IX-V and vWF at sites of turbulence in occluded vessels.
The GP Ib-IX-V complex is composed of 4 polypeptide subunits5 comprising the disulfide-linked GP-Ibα and GP-Ibβ subunits, which form a 1:1 complex with GP IX. On the platelet plasma membrane, the GP Ib-IX complex forms a 2:1 complex with GPV, which is dissociated by detergents such as Triton X-100. Binding of vWF to the GP Ib-IX-V complex initiates a number of intracellular signaling events that lead to platelet activation. These include tyrosine phosphorylation of platelet proteins,6,7 activation of protein kinase C, activation of phosphoinositide 3-kinase (PI 3-kinase),8 elevation of intracellular calcium,9 and synthesis of thromboxane A2.10Although these intracellular signaling events are not precisely delineated, they result in platelet activation, reorganization of the platelet cytoskeletal actin filaments leading to shape change and spreading, and activation and exposure of the other platelet adhesion receptors including integrin GP IIbIIIa (αIIbβ3) and P-selectin. These receptors mediate, respectively, the platelet–platelet and platelet–leukocyte interactions essential for normal and pathologic thrombosis.
A recently described mechanism for coordinating GP Ib-IX-V transmembrane signaling comes from the identification that the scaffolding protein 14-3-3ζ forms a complex with GP Ib-IX-V.11 A member of the 14-3-3 highly conserved family of proteins that bind and regulate a diverse number of intracellular signaling proteins,12 14-3-3ζ copurifies with GP Ib-IX from detergent extracts of human platelets. Amino acid sequences have been identified from the cytoplasmic domains of GP Ibα, GP Ibβ, and GPV that may participate in the binding of 14-3-3ζ to the receptor complex.13 The C-terminal 5 amino acids of GP Ibα bind 14-3-3ζ,13,14 whereas sequences within GP Ibβ (Arg-160 to Arg-175) and GPV (Lys-529 to Gly-544) also form complexes with 14-3-3ζ.13 In vitro peptide-binding studies suggest that the phosphorylation of GP Ibβ at the protein kinase A phosphorylation site (Ser 166)15,16 may result in the enhanced association of 14-3-3ζ.13 In addition, studies using the yeast 2-hybrid assay have shown that the interaction of 14-3-3ζ with GP Ibβ is significantly reduced after the mutation of serine 166.17
PI 3-kinase phosphorylates various forms of phosphoinositide in the D3 position of the inositol ring.18 Thrombin stimulation of platelets results in a rapid transient formation of phosphatidylinositol 3,4,5-trisphosphate (PtdIns[3,4,5]P3) and phosphatidylinositol 3,4-bisphosphate (PtdIns[3,4]P2).19-21 After platelet aggregation, a sustained, delayed accumulation of PtdIns(3,4)P2 occurs that is associated with irreversible platelet aggregation.22,23 The production of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 correlates with the translocation of PI 3-kinase to the platelet actin cytoskeleton and an increase in PI 3-kinase enzyme activity.20,24 A role for PI 3-kinase in platelet activation may be to maintain the receptor αIIbβ3 in its active conformation to ensure irreversible platelet aggregation.25
In this study, we have shown that GP Ib-IX forms a complex with 14-3-3ζ and PI 3-kinase in platelet subcellular fractions. After thrombin or vWF stimulation, the association of 14-3-3ζ and PI 3-kinase with GP Ib decreases in the platelet cytosol. GP Ib in complex with 14-3-3ζ or PI 3-kinase increases in the actin cytoskeleton platelet activation. We propose that this may represent a mechanism whereby the rapid translocation of PI 3-kinase to the activated actin cytoskeleton is mediated by association with 14-3-3ζ and interaction with GP Ib.
Materials and methods
Materials
[γ32P]Adenosine triphosphate (ATP) was purchased from Dupont– New England Nuclear (Boston, MA). PtdIns, PtdSer, bovine serum albumin, and N-ethylmaleimide were purchased from Sigma (St. Louis, MO). Actigel ALD Superflow resin was obtained from Sterogene (Arcadia, CA). Synthetic peptides Y751 (based on PDGF receptor β), A7-7 and A7-10(based on the cytoplasmic domain of GP Ibα), B2 (based on the cytoplasmic domain of GP Ibβ), and pS-Raf 259 (based on the 14-3-3ζ binding site on Raf-1) were purchased from Chiron (Clayton, Australia).
Preparation of washed platelets and platelet subcellular fractions
Platelets were obtained from healthy volunteers and were washed using a previously described method.24 Washed platelets were left unstimulated or were stimulated with 1 U/mL thrombin with or without stirring or with 10 μg/mL vWF and 3 μg/mL botrocetin. In the indicated experiments, platelets were treated with 2 mmol/L RGDS before stimulation with thrombin. After activation with thrombin or with vWF and botrocetin, the platelets were lysed with 1 vol Triton X-100 lysis buffer (200 mmol/L Tris-HCl, pH 7.4, 10% Triton X-100, 50 mmol/L EGTA, 4 mmol/L leupeptin, 4 mmol/L aprotinin, 2.5 mg/mL phenylmethylsulfonyl fluoride (PMSF)) to 9 volumes of platelets and rocked at 4°C for 1 hour. Lysates were centrifuged at 15 400g for 5 minutes to separate the Triton X-100 soluble and insoluble (actin cytoskeletal) extracts and platelet subcellular fractions isolated as previously described.8 The membrane cytoskeletal fraction was obtained by centrifugation of the Triton-soluble cytosol at 100 000g for 1 hour. The resultant supernatant represented the Triton-soluble cytosol, and the pellet represented the membrane cytoskeleton. This pellet was solubilized by incubation with 2 × RIPA buffer (20 mmol/L NaH2PO4, pH 7.0, 300 mmol/L NaCl, 4 mmol/L EDTA, 250 μg/mL PMSF, 400 μmol/L leupeptin, 400 μmol/L aprotinin, 2% Nonidet P-40, 2% sodium deoxycholate, 0.2% sodium dodecyl sulfate [SDS]) for 1 hour at 4°C and by centrifugation at 15 400g for 10 minutes.
Affinity capture of PI 3-kinase
Platelet cytosol was prepared and isolated as described.26 The peptide (Y751) DMSKDESVDYpVPMLDMK (where Yp represents phosphotyrosine), which corresponds to the binding site on the platelet-derived growth factor (PDGF) β receptor for the p85 subunit of PI 3-kinase, was coupled to Actigel Superflow resin (Sterogene) as described.27 Sixty microliters of the Y751-coupled resin was mixed with 600 μL platelet cytosol overnight at 4°C. The resin was pelleted and washed 3 times with 1 mL of 20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl followed by 3 × 1 mL washes with 20 mmol/L HEPES, pH 7.4, 1 mmol/L EGTA, and 5 mmol/L MgCl2.
Antibodies
Immunoprecipitation of 14-3-3ζ, GP Ib, or PI 3-kinase
Immunoprecipitation of 14-3-3ζ was performed as follows: 5 μL polyclonal antisera directed against 14-3-3ζ was incubated overnight at 4°C with 600 μL human platelet cytosol (5 mg/mL) or the Triton-soluble cytosolic, actin cytoskeletal, and membrane cytoskeletal platelet fractions and 60 μL of a 50% slurry of protein-A–Sepharose preequilibrated with 20 mmol/L Tris, pH 7.4, and 150 mmol/L NaCl. The pelleted protein-A–Sepharose was washed 6 times with 1 mL 20 mmol/L Tris, pH 7.4, and 150 mmol/L NaCl. The GP Ib-IX complex and the p85 subunit of PI 3-kinase were immunoprecipitated using 5 μL polyclonal antibody to glycocalicin or polyclonal antibody to the p85 subunit of PI 3-kinase (Upstate Biotechnology), respectively. The conditions used for immunoprecipitation were as for 14-3-3ζ. In indicated experiments, 1 mmol/L N-ethylmaleimide was added to the platelet fractions before immunoprecipitation.
PI 3-kinase assay
The PI 3-kinase captured on protein-A–Sepharose pellets with associated anti-p85, anti–14-3-3ζ, or antiglycocalicin immunoprecipitates was washed 3 times with ice-cold 20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 1 mmol/L sodium orthovanadate, and 250 μg/mL PMSF. This was followed by 3 washes with ice-cold kinase assay buffer (20 mmol/L HEPES, pH 7.4, 1 mmol/L EGTA, 5 mmol/L MgCl2). Immunoprecipitates were resuspended in kinase buffer and mixed with sonicated PtdIns (200 μmol/L) and PtdSer (300 μmol/L) and with [γ32P]ATP (50 μmol/L, 1 μCi/nmol) to a final reaction volume of 100 μL. Reactions were stopped after 20 minutes at room temperature, lipid products were analyzed by thin-layer chromatography (TLC),28 and products were excised from the TLC and counted using liquid-scintillation counting.
Effect of 14-3-3ζ–GST on PI 3-kinase activity
Recombinant 14-3-3ζ–glutathione-S-transferase (GST) fusion protein (14-3-3ζ–GST) or GST was induced and purified.29PI 3-kinase affinity captured on Actigel Superflow resin (Sterogene) was washed 3 times with ice-cold 20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 1 mmol/L sodium orthovanadate, and 250 μg/mL PMSF and 3 times with 20 mmol/L HEPES, pH 7.4, 1 mmol/L EGTA, and 5 mmol/L MgCl2. The resin with associated PI 3-kinase was resuspended in kinase buffer and mixed with sonicated PtdIns (200 μmol/L) and PtdSer (300 μmol/L), [γ32P]ATP (50 μmol/L, 1 μCi/nmol), and 0.5 to 5 μmol/L 14-3-3ζ–GST fusion protein, or with GST alone, to a final reaction volume of 100 μL. The reactions were stopped after 20 minutes at room temperature, lipid products were analyzed using TLC,28 and products were excised from the TLC and counted using liquid-scintillation counting.
Results
Expression of 14-3-3 and GP Ib-IX in platelet subcellular fractions
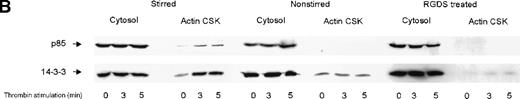
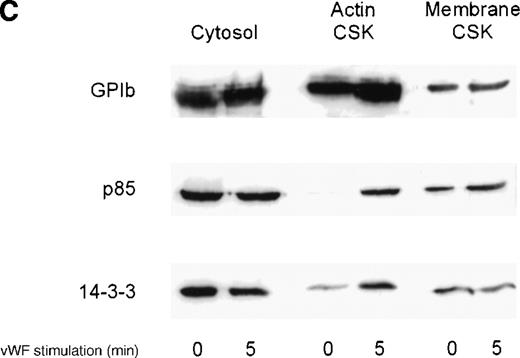
We investigated the subcellular compartmentalization of 14-3-3ζ and GP Ib-IX in resting, thrombin-stimulated, or vWF-stimulated platelets compared to the p85 subunit of the PI 3-kinase. In unstimulated platelets, 14-3-3ζ was detected in the cytosolic and membrane cytoskeletal fraction, with only low levels in the cytoplasmic actin cytoskeleton. However, both 14-3-3ζ and GP Ib were always present in the unstimulated platelet actin cytoskeletal fraction. In contrast, as shown by many studies, the p85 subunit of PI 3-kinase is not detectable in the actin cytoskeletal fraction of unstimulated platelets.30 However, after platelet activation, the enzyme translocates to the cytoplasmic actin cytoskeleton. We observed, after stimulation with thrombin or vWF and botrocetin, a time-dependent translocation of 14-3-3ζ to the actin cytoskeleton (Figure1A-C). Densitometric analysis of the intensity of the immunoreactive 14-3-3ζ in resting platelet actin cytoskeleton, compared with that of the thrombin-activated actin cytoskeleton, demonstrated a 1.8 ± 0.4 (n = 4)-fold increase in the cytoskeleton after platelet activation. Translocation of 14-3-3ζ to the actin cytoskeleton was dependent on platelet aggregation and integrin engagement because translocation was not observed when platelets were not stirred or when they were preincubated with RGDS peptide (Figure 1B).
Activation-dependent translocation of PI 3-kinase, GP Ib, and 14-3-3 to the cytoskeleton.
Washed platelets were stimulated with thrombin (1 U/mL) (A) or vWF and botrocetin (C) for the indicated times. Triton-soluble cytosol (Cytosol), actin cytoskeletal (actin CSK), and membrane cytoskeletal (membrane CSK) fractions were isolated. Thirty microliters of each fraction was analyzed by SDS-PAGE and immunoblot analysis using antibodies to glycocalicin (GP Ib), the p85 subunit of PI 3-kinase (p85), or 14-3-3ζ. (B) Platelets were stimulated with thrombin (1 U/mL), with or without stirring, or were preincubated with 2 mmol/L RGDS for 10 minutes before thrombin stimulation for 0, 3, or 5 minutes. Thirty microliters of the cytosolic or actin cytoskeletal fractions was analyzed by SDS-PAGE and by immunoblot analysis using antibodies to p85 or 14-3-3ζ. This figure is representative of 3 similar experiments.
Activation-dependent translocation of PI 3-kinase, GP Ib, and 14-3-3 to the cytoskeleton.
Washed platelets were stimulated with thrombin (1 U/mL) (A) or vWF and botrocetin (C) for the indicated times. Triton-soluble cytosol (Cytosol), actin cytoskeletal (actin CSK), and membrane cytoskeletal (membrane CSK) fractions were isolated. Thirty microliters of each fraction was analyzed by SDS-PAGE and immunoblot analysis using antibodies to glycocalicin (GP Ib), the p85 subunit of PI 3-kinase (p85), or 14-3-3ζ. (B) Platelets were stimulated with thrombin (1 U/mL), with or without stirring, or were preincubated with 2 mmol/L RGDS for 10 minutes before thrombin stimulation for 0, 3, or 5 minutes. Thirty microliters of the cytosolic or actin cytoskeletal fractions was analyzed by SDS-PAGE and by immunoblot analysis using antibodies to p85 or 14-3-3ζ. This figure is representative of 3 similar experiments.
Analysis of the association between 14-3-3ζ and GP-Ib in stimulated platelets
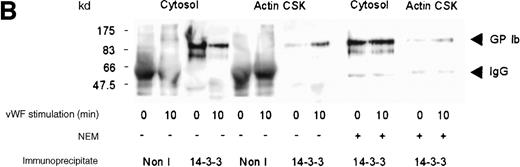
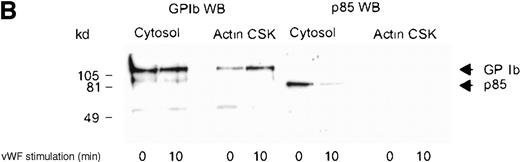
Although several reports have documented the association of the GP Ib-IX complex with 14-3-3ζ,13,14,17 this association has not been examined systematically on thrombin- or on vWF- and botrocetin-induced platelet stimulation. Platelet subcellular fractions were isolated from resting or stimulated platelets, and the proteins were extracted from washed actin cytoskeletal fractions using RIPA buffer (10 mmol/L NaH2PO4, pH 7.0, 150 mmol/L NaCl, 2 mmol/L EDTA, 250 μg/mL PMSF, 400 μmol/L leupeptin, 400 μmol/L aprotinin, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% SDS). Platelet subcellular fractions were immunoprecipitated using polyclonal antibodies to 14-3-3ζ. The immunoprecipitates were captured on protein-A–Sepharose, washed, and immunoblotted using antibodies to glycocalicin (the extracellular domain of GP Ib). 14-3-3ζ formed a complex with GP Ib in platelet cytosol, and this association decreased by 37% after thrombin or vWF and botrocetin stimulation, as determined by densitometric analysis of immunoblots (Figure 2A,B; Table 1). Concomitantly, a 3-fold (n = 5) increased association between 14-3-3ζ and GP Ib was observed in the actin cytoskeletal fraction of thrombin or vWF-activated platelets, as determined by densitometric analysis of the intensity of immunoreactive GP Ib detected in 14-3-3ζ immunoprecipitates. In some studies, platelet lysates were preincubated with N-ethylmaleimide (1 mmol/L); this has been shown to dissociate actin-binding protein from the receptor complex.26 However, this did not affect the level of GP Ib present in 14-3-3ζ immunoprecipitates (Figure 2A). Collectively, these studies demonstrate that 14-3-3ζ forms a complex with the GP Ib-IX receptor in resting platelets, and the association between the 2 species is not static. After platelet activation with either thrombin or vWF, 14-3-3ζ bound to GP Ib-IX decreased in the cytosolic fraction and increased in the actin cytoskeletal fraction.
Association of 14-3-3 with GP Ib-IX complex.
One milliliter cytosol or the RIPA-extracted actin cytoskeletal (actin CSK) fraction from resting, thrombin-activated (1 U/mL) (A), or vWF- and botrocetin-activated platelets (B) was immunoprecipitated using 5 μL 14-3-3ζ antibody or nonimmune sera (Non I) (5 μL) and was immunoblotted using antibodies to the extracellular domain of GP Ib (anti-glycocalicin). As indicated, in some experiments the platelet subcellular fractions were preincubated with 1 mmol/LN-ethylmaleimide (NEM) to displace actin-binding protein. This figure is representative of 5 similar experiments.
Association of 14-3-3 with GP Ib-IX complex.
One milliliter cytosol or the RIPA-extracted actin cytoskeletal (actin CSK) fraction from resting, thrombin-activated (1 U/mL) (A), or vWF- and botrocetin-activated platelets (B) was immunoprecipitated using 5 μL 14-3-3ζ antibody or nonimmune sera (Non I) (5 μL) and was immunoblotted using antibodies to the extracellular domain of GP Ib (anti-glycocalicin). As indicated, in some experiments the platelet subcellular fractions were preincubated with 1 mmol/LN-ethylmaleimide (NEM) to displace actin-binding protein. This figure is representative of 5 similar experiments.
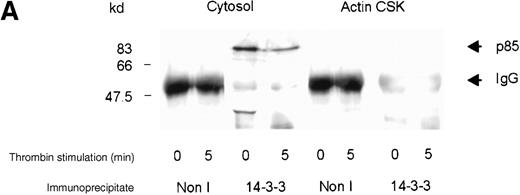
Complex formation between 14-3-3ζ and PI 3-kinase
The binding of vWF to the platelet GP Ib-IX complex stimulates the activation and cytoskeletal localization of PI 3-kinase and pp60c-src.8 We investigated whether PI 3-kinase forms a complex with 14-3-3ζ in stimulated or resting platelets. 14-3-3ζ was immunoprecipitated from platelet subcellular fractions of resting, thrombin-, or vWF- and botrocetin-stimulated platelets, and the washed immunoprecipitates were immunoblotted using p85 or GP Ib antiserum. PI 3-kinase and 14-3-3ζ formed a complex in the cytosolic fraction of human platelets (Figure3A,B). After thrombin or vWF and botrocetin stimulation, the level of p85 detected in complexes with 14-3-3ζ decreased by 50%, as assessed by densitometric analysis of immunoreactive p85 (Table 1). We were unable to detect complex formation between PI 3-kinase and 14-3-3ζ in the RIPA-extracted actin cytoskeletal fraction, despite the demonstration of complex formation between 14-3-3ζ and GP Ib-IX in the same fraction (Figure 3B). We cannot exclude that the extraction of the actin cytoskeleton with RIPA buffer prohibits immunoprecipitation of a 14-3-3ζ–PI 3-kinase complex. In addition, the detection of PI 3-kinase versus GP Ib in 14-3-3ζ immunoprecipitates is dependent on both the amount of enzyme or receptor in complex with 14-3-3ζ and on the affinity of PI 3-kinase compared with GP Ib antibodies. These results however, demonstrate that 14-3-3ζ associates with GP Ib and the p85 subunit of PI 3-kinase in platelet cytosol, and the level of association decreases after platelet activation.
Association of p85 with 14-3-3.
(A) One milliliter cytosol or the RIPA-extracted actin-cytoskeletal (actin CSK) fraction derived from resting or thrombin-stimulated platelets (2 × 109/mL) was immunoprecipitated using the antibody to 14-3-3ζ (5 μL) or nonimmune sera (Non I) (5 μL) and immunoblotted using antibodies to the p85 subunit of PI 3-kinase. This figure is representative of 3 similar experiments. (B) One milliliter cytosol or the RIPA-extracted actin cytoskeletal (actin CSK) fraction derived from resting or vWF-stimulated platelets (2 × 109/mL) was immunoprecipitated using antibody to 14-3-3ζ (5 μL) and immunoblotted using antibody to either GP Ib or the p85 subunit of PI 3-kinase. This figure is representative of 3 similar experiments.
Association of p85 with 14-3-3.
(A) One milliliter cytosol or the RIPA-extracted actin-cytoskeletal (actin CSK) fraction derived from resting or thrombin-stimulated platelets (2 × 109/mL) was immunoprecipitated using the antibody to 14-3-3ζ (5 μL) or nonimmune sera (Non I) (5 μL) and immunoblotted using antibodies to the p85 subunit of PI 3-kinase. This figure is representative of 3 similar experiments. (B) One milliliter cytosol or the RIPA-extracted actin cytoskeletal (actin CSK) fraction derived from resting or vWF-stimulated platelets (2 × 109/mL) was immunoprecipitated using antibody to 14-3-3ζ (5 μL) and immunoblotted using antibody to either GP Ib or the p85 subunit of PI 3-kinase. This figure is representative of 3 similar experiments.
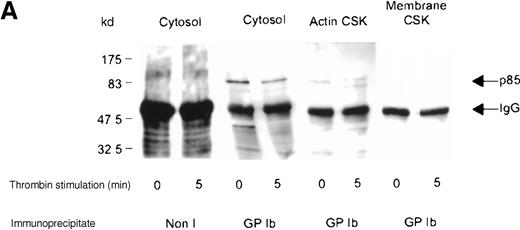
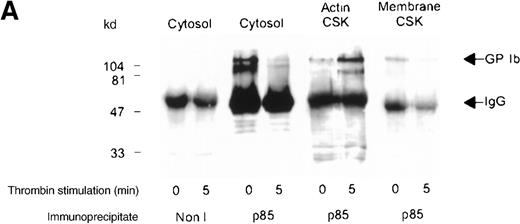
Association of the PI 3-kinase with the GP Ib receptor complex
We sought evidence of complex formation between p85 and GP Ib. Receptor immunoprecipitates were immunoblotted using anti-p85 serum. An association between p85 and the receptor complex was detected in the Triton-soluble cytosolic fraction of resting and thrombin- or vWF-stimulated platelets (Figure 4A,B). The level of association between the GP Ib receptor and PI 3-kinase decreased by 45% in the cytosolic fraction after thrombin or vWF stimulation, as assessed by densitometric analysis of immunoblots (Table 1). In addition, complex formation between GP Ib and p85 was also observed in the activated RIPA-extracted cytoskeleton. However, this was routinely less than that detected in the cytosolic fraction (Figure 4B).
Association of p85 with GP Ib.
(A) One milliliter cytosol, actin cytoskeletal (actin CSK), or membrane cytoskeletal (membrane CSK) platelet subcellular fractions from thrombin-stimulated platelets (2 × 109/mL platelets) or (B) vWF- and botrocetin-stimulated platelets were immunoprecipitated using 5 μL anti-glycocalicin antibody or nonimmune sera (Non I) (5 μL) and immunoblotted using antibody to the p85 subunit of PI 3-kinase. This figure is representative of 4 similar experiments.
Association of p85 with GP Ib.
(A) One milliliter cytosol, actin cytoskeletal (actin CSK), or membrane cytoskeletal (membrane CSK) platelet subcellular fractions from thrombin-stimulated platelets (2 × 109/mL platelets) or (B) vWF- and botrocetin-stimulated platelets were immunoprecipitated using 5 μL anti-glycocalicin antibody or nonimmune sera (Non I) (5 μL) and immunoblotted using antibody to the p85 subunit of PI 3-kinase. This figure is representative of 4 similar experiments.
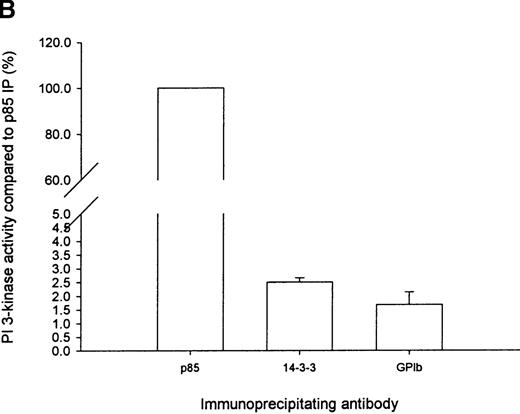
To substantiate the observation that PI 3-kinase forms a complex with 14-3-3ζ, GP Ib-IX, or both, in the cytosolic fraction of human platelets, we assayed 14-3-3ζ and GP Ib-IX immunoprecipitates for PI 3-kinase enzyme activity using PtdIns as a substrate (Figure5A). PI 3-kinase enzyme activity was observed in 14-3-3ζ and GP Ib immunoprecipitates but not in nonimmune precipitates. Enzyme activity was slightly greater in 14-3-3ζ (2.5% ± 0.35%; n = 5) versus GP Ib-IX (1.7% ± 0.9%; n = 4) immunoprecipitates as a percentage of that observed in p85 immunoprecipitates. However, this was not statistically significant and might have reflected the differing affinities of the 2 antibodies (Figure 5B). In addition, we could not exclude the possibility that either of these antibodies sterically inhibited PI 3-kinase enzyme activity under the assay conditions used or that the association with 14-3-3ζ, GP Ib-IX, or another unidentified protein in the complex reduced lipid kinase enzyme activity.
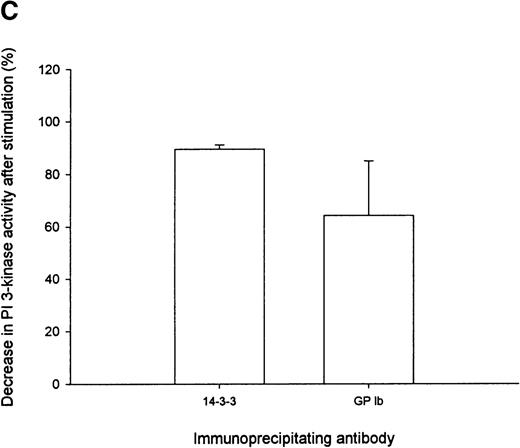
Immunoprecipitation of PI 3-kinase activity with 14-3-3 or GP Ib from platelet cytosol.
(A) Six hundred microliters of human platelet cytosol (5 mg/mL) was immunoprecipitated using 5 μL nonimmune, anti-p85, anti–14-3-3ζ, or antiglycocalicin antiserum, captured on protein A–Sepharose, and washed, and PI 3-kinase activity was determined using PtdIns as a substrate as described in “Materials and methods.” (B) The PtdIns 3-P formed in immunoprecipitates of nonstimulated platelet Triton-soluble cytosolic fractions using the indicated antibodies was expressed as a percentage of that observed in p85 immunoprecipitates (IP). Values represent the mean ± SD of 4 experiments. (C) Platelets were stimulated for 5 minutes with thrombin (1 U/mL), and PI 3-kinase assays were performed on washed immunoprecipitates using the indicated antibodies. The decrease in enzyme activity is expressed as a percentage relative to that observed in nonstimulated immunoprecipitates using the same antibody. Values represent mean ± SD of 4 experiments.
Immunoprecipitation of PI 3-kinase activity with 14-3-3 or GP Ib from platelet cytosol.
(A) Six hundred microliters of human platelet cytosol (5 mg/mL) was immunoprecipitated using 5 μL nonimmune, anti-p85, anti–14-3-3ζ, or antiglycocalicin antiserum, captured on protein A–Sepharose, and washed, and PI 3-kinase activity was determined using PtdIns as a substrate as described in “Materials and methods.” (B) The PtdIns 3-P formed in immunoprecipitates of nonstimulated platelet Triton-soluble cytosolic fractions using the indicated antibodies was expressed as a percentage of that observed in p85 immunoprecipitates (IP). Values represent the mean ± SD of 4 experiments. (C) Platelets were stimulated for 5 minutes with thrombin (1 U/mL), and PI 3-kinase assays were performed on washed immunoprecipitates using the indicated antibodies. The decrease in enzyme activity is expressed as a percentage relative to that observed in nonstimulated immunoprecipitates using the same antibody. Values represent mean ± SD of 4 experiments.
The association between PI 3-kinase and 14-3-3ζ or GP Ib, as assessed by PI 3-kinase enzyme activity, decreased by 90% and 64%, respectively, in the cytosol after thrombin stimulation (Figure 5C). These results were consistent with p85 immunoblot analysis, which revealed that the intensity of the p85 subunit of PI 3-kinase detected in GP Ib immunoprecipitates decreased by 45% after thrombin stimulation (Table 1). We were unable to confirm the presence of PI 3-kinase enzyme activity in 14-3-3ζ or GP Ib immunoprecipitates derived from the thrombin-activated actin cytoskeleton because the presence of SDS (0.1%) in the RIPA-extraction buffer completely inhibited enzyme activity. These studies, nevertheless, demonstrate that 14-3-3ζ, the GP Ib-IX receptor, and PI 3-kinase form a complex in resting platelet cytosol. PI 3-kinase association with 14-3-3ζ or GP Ib-IX decreases in the cytosolic fraction after thrombin or vWF and botrocetin activation. Whether PI 3-kinase dissociates from 14-3-3ζ bound to GP Ib or from 14-3-3ζ not associated with the receptor and translocates in complex with either 14-3-3ζ or GP Ib to the actin cytoskeleton remains to be determined.
Association between the GP Ib receptor and PI 3-kinase in the actin cytoskeleton
To investigate whether PI 3-kinase formed a complex with the GP Ib-IX receptor in the thrombin-activated cytoskeleton, PI 3-kinase was immunoprecipitated from this fraction using antibodies to the p85 subunit of PI 3-kinase and immunoblotted using anti-glycocalicin antibodies. The GP Ib-IX receptor was detected in p85 immunoprecipitates of platelet cytosolic fractions. The association between the 2 species decreased by approximately 35% in the cytosol after thrombin stimulation (Table 1). Little GP Ib-IX receptor was associated with PI 3-kinase in the nonstimulated platelet actin cytoskeleton. However, after thrombin or vWF and botrocetin activation, a significantly increased association between GP Ib-IX and p85 was detected in the actin cytoskeleton (Figure6A,B). In control studies, no GP Ib-IX receptor was immunoprecipitated using nonimmune sera.
Association of GP Ib with p85 in the actin cytoskeleton.
(A) One milliliter cytosol, actin cytoskeletal (actin CSK), or membrane cytoskeletal (membrane CSK) platelet subcellular fractions from thrombin-stimulated platelets or (B) botrocetin- and vWF-stimulated platelets was immunoprecipitated using 5 μL p85 antiserum or 5 μL nonimmune sera (Non I) and was immunoblotted using antiglycocalicin antibody. These figures are representative of 4 similar experiments.
Association of GP Ib with p85 in the actin cytoskeleton.
(A) One milliliter cytosol, actin cytoskeletal (actin CSK), or membrane cytoskeletal (membrane CSK) platelet subcellular fractions from thrombin-stimulated platelets or (B) botrocetin- and vWF-stimulated platelets was immunoprecipitated using 5 μL p85 antiserum or 5 μL nonimmune sera (Non I) and was immunoblotted using antiglycocalicin antibody. These figures are representative of 4 similar experiments.
There are 2 possible mechanisms that could mediate the association between PI 3-kinase and the GP Ib-IX receptor. The enzyme could directly associate with the receptor through yet unrecognized motifs in the cytoplasmic domain of the receptor complex, or PI 3-kinase could associate with the receptor by binding to 14-3-3ζ. Two experimental approaches were undertaken to resolve this. First, displacement of 14-3-3ζ from the receptor using specific peptides encompassing the proposed binding sites on GP-Ib-IX was attempted.13However, no displacement of 14-3-3ζ, or of PI 3-kinase, was observed in GP Ib-IX immunoprecipitates using a range of peptides (results not shown). Second, we reasoned that if PI 3-kinase was complexed to the receptor by 14-3-3ζ, then immunoprecipitation of 14-3-3ζ to completion from platelet subcellular fractions should also immunodeplete PI 3-kinase in complex with the GP Ib-IX. Unfortunately, because of the relatively low affinity of the 14-3-3ζ polyclonal antibodies, complete immunoprecipitation of 14-3-3ζ from platelet lysates was not achieved using maximal concentrations of antiserum or repeat immunoprecipitations (results not shown).
Recombinant 14-3-3ζ has a biphasic effect on PI 3-kinase enzyme activity
It has been shown that 14-3-3τ protein binds directly to the p110 catalytic subunit of PI 3-kinase.31Overexpression of 14-3-3τ inhibits PI 3-kinase enzyme activity, suggesting that the association of 14-3-3 with PI 3-kinase regulates receptor-mediated activation of the enzyme. We determined the effect of recombinant 14-3-3ζ on PI 3-kinase enzyme activity in vitro using platelet cytosol as a source of PI 3-kinase enzyme. The phosphorylated peptide (CDESVDYPVPML), which represents the p85 binding domain of the PDGF β receptor,27 was coupled to Actigel Superflow resin (Sterogene) and PI 3-kinase affinity captured from human platelet cytosol, as previously described.32 PI 3-kinase assays were performed using PtdIns as a substrate, and the formation of PtdIns 3-P was determined in the presence of increasing concentrations of recombinant GST or 14-3-3ζ–GST. Recombinant 14-3-3ζ–GST had a biphasic effect on PI 3-kinase activity. Progressive inhibition in the formation of PtdIns 3-P was observed in the presence of up to 2 mmol/L recombinant 14-3-3ζ–GST. At these concentrations of 14-3-3ζ–GST, more than 50% inhibition of PI 3-kinase activity was observed. However, with increasing concentrations of 14-3-3ζ–GST, enhanced PI 3-kinase activity was detected that was maximal at the highest concentration of 14-3-3ζ–GST tested, 5 mmol/L (Figure 7). In contrast, the recombinant GST protein had no effect on affinity-purified PI 3-kinase enzyme activity in concentrations up to 5 mmol/L in the assay.
Effect of 14-3-3 on PI 3-kinase enzyme activity.
A peptide (Y751) corresponding to the p85 binding site on the PDGF receptor was coupled to Actigel Superflow resin. Six hundred microliters of platelet cytosol (12 mg/mL) was incubated with 60 μL of a 50% slurry of the peptide-coupled resin. The resin was pelleted and incubated with the indicated concentrations of recombinant GST (gray bars) or 14-3-3ζ–GST (black bars), and PI 3-kinase assays were performed on washed pellets using PtdIns as the substrate. Enzyme activity is expressed as a percentage of that observed in control reactions performed in the absence of recombinant GST or 14-3-3ζ–GST. Results represent the mean ± SD of 3 experiments.
Effect of 14-3-3 on PI 3-kinase enzyme activity.
A peptide (Y751) corresponding to the p85 binding site on the PDGF receptor was coupled to Actigel Superflow resin. Six hundred microliters of platelet cytosol (12 mg/mL) was incubated with 60 μL of a 50% slurry of the peptide-coupled resin. The resin was pelleted and incubated with the indicated concentrations of recombinant GST (gray bars) or 14-3-3ζ–GST (black bars), and PI 3-kinase assays were performed on washed pellets using PtdIns as the substrate. Enzyme activity is expressed as a percentage of that observed in control reactions performed in the absence of recombinant GST or 14-3-3ζ–GST. Results represent the mean ± SD of 3 experiments.
Discussion
GP Ib-IX binding to vWF induces signal transduction that activates the ligand binding function of integrin αIIbβ3 and integrin-dependent cell functions.33-35 The first major evidence for a mechanism by which the receptor may mediate intracellular signaling was derived from the observation that the scaffolding protein 14-3-3ζ binds to the cytoplasmic domain of GP Ib.13,14 17 It is an attractive hypothesis that GP Ib-IX-V may signal through proteins complexed to 14-3-3ζ, and the current study presents evidence that this may indeed be the case, with the identification that 14-3-3ζ, GP Ib-IX, and PI 3-kinase form a dynamic complex in the cytosol and the thrombin- or vWF-activated actin cytoskeleton. The dynamic association between these species could be mediated by 2 potential mechanisms. The PI 3-kinase may bind 14-3-3ζ and thereby complex with GP Ib, and this complex may dissociate after platelet activation and reassociate in the activated cytoskeleton on platelet activation. Alternatively, it is possible that a proportion of the GP Ib-IX receptor complex and the associated 14-3-3ζ or PI 3-kinase becomes directly incorporated into the actin cytoskeleton, thus mediating the observed translocation of these signaling molecules.
We originally reported that PI 3-kinase enzyme activity was absent in GP Ib-IX immunoprecipitates, using monoclonal antibodies against the receptor complex.8 However, in the current study using polyclonal, rather than monoclonal, antibodies to the receptor, we demonstrated in GP Ib immunoprecipitates the association of PI 3-kinase enzyme activity, albeit low, and the presence of an 85-kd polypeptide recognized by specific antibodies to the p85 adapter subunit. These results are consistent with complex formation between PI 3-kinase and the GP Ib-IX receptor. Several lines of evidence suggested that PI 3-kinase binding to the receptor complex may be mediated by its association with 14-3-3ζ. First, in the cytosolic fraction, we never detect PI 3-kinase associated with the receptor in the absence of 14-3-3ζ. Second, the dissociation of PI 3-kinase with the receptor correlates with 14-3-3ζ dissociation. The PI 3-kinase–GP Ib complex decreases in the Triton-soluble and membrane cytoskeletal fractions of thrombin-activated platelets, which correlates with decreased 14-3-3ζ and p85 detected in GP Ib immunoprecipitates. This, coupled with the observation that the association of both 14-3-3ζ and PI 3-kinase with GP Ib increases in the activated actin cytoskeleton, suggests that the mechanism of PI 3-kinase association with GP Ib is through 14-3-3ζ. However, we were unable to demonstrate an association between 14-3-3ζ and PI 3-kinase in the actin cytoskeleton of stimulated platelets. The complex may dissociate when treated with SDS in the RIPA extraction buffer. More likely, because of limitations with the available antibodies used (ie, the combination of 14-3-3ζ immunoprecipitation and PI 3-kinase immunoblot detection), the complex exists below detectable levels. In addition, we cannot exclude the possibility that PI 3-kinase directly associates with the GP Ib-IX receptor complex or with another protein that then binds to the receptor. However, this mechanism of association appears unlikely given the absence of potential binding sites within the cytoplasmic domain of GP Ib-IX. Because 14-3-3ζ appears constitutively associated with the GP Ib-IX receptor, it is highly likely that PI 3-kinase also binds 14-3-3ζ in platelet cytosol and to the receptor through 14-3-3ζ.
It has been proposed that the p110 subunit of PI 3-kinase can directly bind 14-3-3τ.31 However, the p85 subunit of human, but not of mouse, PI 3-kinase contains the RSXSpXP motif found in many proteins known to associate with 14-3-3.36 The phosphorylation status of the serine residue (Ser 231) of the p85 subunit of PI 3-kinase is unknown. Increasing evidence suggests that 14-3-3 can bind proteins in a phosphoserine-independent manner, which involves the same binding site as does interaction with phosphoserine-containing proteins.37-39 In support of this contention, we have demonstrated that recombinant GST-p85 can affinity capture 14-3-3ζ from platelet cytosol and that peptides encompassing this proposed 14-3-3ζ binding site on the p85 subunit of PI 3-kinase can bind to recombinant 14-3-3ζ with high affinity (results not shown).
Results of 2 previous studies are consistent with our observation that the interaction between 14-3-3 and PI 3-kinase negatively regulates lipid kinase enzyme activity. First, 14-3-3 binding of PI 3-kinase in human T cells inhibits enzyme activity after T-cell receptor activation.31 Second, 14-3-3 binds to IRS-1 and thereby modulates insulin-dependent PI 3-kinase signaling in 3T3L1 adipocytes.40 The negative regulation of PI 3-kinase enzyme activity by 14-3-3ζ attached to the GP Ib receptor complex would in part explain the difficulties we experienced detecting enzyme activity in receptor immunoprecipitates, though the p85 subunit of PI 3-kinase was readily detected by Western blotting. Using in vitro assays, we showed that recombinant 14-3-3ζ inhibited platelet cytosolic PI 3-kinase activity, but the maximum inhibition of activity observed at any concentration of 14-3-3ζ was never more than 70%. The purpose of localization of the lipid kinase to the receptor in the resting cell is unclear if only a small proportion of the enzyme is associated and enzyme activity is inhibited. Based on our studies, less than 5% of total platelet PI 3-kinase is associated with GP Ib-IX. However, we also demonstrated at higher concentrations that recombinant 14-3-3ζ increased PI 3-kinase enzyme activity. Because increased PI 3-kinase activity has consistently been observed in the thrombin- or vWF-activated cytoskeleton, complex formation with 14-3-3ζ may be a potential mechanism for the observed translocation and activation of the kinase. This may, in turn, mediate the localized synthesis of PtdIns(3,4,5)P3 and PtdIns(3,4)P2and thereby regulate irreversible platelet aggregation.
Supported by a grant from the National Health and Medical Research Council of Australia (no. 9936645), and supported in part by the National Heart Foundation of Australia.
Reprints:Christina A. Mitchell, Department of Biochemistry and Molecular Biology, Monash University, Clayton, Victoria, 3168 Australia; e-mail: christina.mitchell@med.monash.edu.au.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.