Abstract
Recent experiments point to the great value of lentiviral vectors for the transduction of human hematopoietic stem cells (hHSCs). Vectors used so far, however, have been poorly satisfying in terms of either biosafety or efficiency of transgene expression. Herein is described the results obtained with human immunodeficiency virus–based vectors optimized in both of these aspects. It is thus shown that vectors containing the EF1α and, to a lesser extent, the phosphoglycerate kinase (PGK) promoter, govern high-level gene expression in human hematopoietic progenitors as well as derived hematopoietic lineages of therapeutic relevance, such as erythrocytes, granulocytes, monocytes, dendritic cells, and megakaryocytes. EF1α promoter-containing lentiviral vectors can also induce strong transgene expression in primary T lymphocytes isolated from peripheral blood. A self-inactivating design did not affect the performance of EF1α promoter-based vectors but significantly reduced expression from the PGK promoter. This negative effect could nevertheless be largely rescued by inserting the post-transcriptional regulatory element of woodchuck hepatitis virus upstream of the vector 3′ long terminal repeat. These results have important practical implications for the genetic treatment of lymphohematologic disorders as well as for the study of hematopoiesis via the lentivector-mediated modification of hHSCs.
Introduction
Gene therapy via the transduction of human hematopoietic stem cells (hHSCs) represents a very promising approach for the treatment of a number of inherited and acquired lymphohematologic disorders. The stable genetic manipulation of long-term repopulating hHSCs with existing gene delivery systems, however, was until recently impossible to achieve at an efficiency compatible with therapeutic realities. Oncoretroviral vectors derived from Moloney murine leukemia virus (MLV), for instance, although highly appealing because they integrate their cargo into the chromosomes of target cells, cannot transduce hHSCs that have not been first treated with inducers of proliferation.1,2 Indeed, the nuclear transport of the MLV preintegration complex requires the breakdown of the nuclear envelope that occurs at mitosis.3,4Unfortunately hHSCs, whether harvested from the bone marrow, the umbilical cord, or mobilized in the peripheral circulation, are mostly quiescent and lose their pluripotentiality after stimulation and proliferation.5-7 Recent reports, however, have shown that a significant fraction of pluripotent cells as well as cells capable of long-term engraftment in nonobese diabetic/severe combined immunodeficient (NOD/SCID) cells, also called SCID-repopulating cells (SRCs), can be maintained, transduced, and even expanded using specific stimulation conditions.7-10
Lentiviruses are a subgroup of retroviruses that can infect nondividing cells owing to the karyophilic properties of their preintegration complex, which allow for its active import through the nucleopore. Correspondingly, lentiviral vectors derived from human immunodeficiency virus type 1 (HIV-1) can mediate the efficient delivery, integration, and long-term expression of transgenes into nonmitotic cells both in vitro and in vivo.11-13 In particular, HIV-based vectors can efficiently transduce human CD34+ hematopoietic cells in the absence of cytokine stimulation,14-18 and these cells are capable of long-term engraftment in NOD/SCID mice.17 Furthermore, bone marrow from these primary recipients can repopulate secondary mice with transduced cells, confirming the lentivector-mediated genetic modification of very primitive hematopoietic precursors, most probably bona fide stem cells.36 Because none of the other currently available gene delivery systems have such an ability, lentiviral vectors provide a previously unexplored basis for the study of hematopoiesis and for the gene therapy of inherited and acquired lymphohematopoietic disorders via the genetic modification of HSCs.
The demonstration of this important point, however, was provided with an early generation of lentiviral vectors unsuitable for therapeutic applications, either because they failed to meet biosafety requirements14-16 or because they induced levels of transgene expression that were dismissingly low.17,18 20Our recent efforts have resulted in the development of lentiviral vectors that are improved in both of these aspects. Accordingly, the present article describes gene transfer vehicles that appear particularly well suited for the transduction of human hematopoietic precursor cells (HPCs) and for the expression of transgenes in differentiated blood lineages. Our results should facilitate the further use of lentiviral vectors for the genetic manipulation of lymphohematopoietic cells, be it for research or therapeutic purposes.
Materials and methods
Vectors
Production of MLV- and HIV-derived vectors pseudotyped with the vesicular stomatitis virus (VSV) G envelope protein was achieved by transient cotransfection of 3 plasmids into 293T epithelial cell line as previously described.11 MLV vector particles were produced using the cytomegalovirus (CMV)-GagPol plasmid as packaging construct21 and a vector derived from pSLX, which expressed green fluorescent protein (GFP) from the CMV promoter. The HIV-derived packaging construct used was pCMVR8.91, which encodes the HIV-1 Gag and Pol precursors as well as the regulatory proteins Tat and Rev.22 VSV G was expressed from pMD.G. The HIV vector plasmids were derivatives of the original pHR′ backbone,11 with the following modifications. Self-inactivating (SIN) vectors were produced from the previously described SIN-18 vector, which contains a deletion in the U3 region of the 3′ long terminal repeat (LTR) from nucleotide −418 to nucleotide −18, removing all the transcriptionally active sequences.23 Insertion of the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) between the reporter gene and the 3′ LTR was done as previously described.24The phosphoglycerate kinase (PGK) promoter corresponded to nucleotides 424 to 930 of the murine PGK gene (GenBank accession number M18735). The EF1α promoter was derived from the pEF-BOS plasmid25and comprised nucleotides 373 to 1561 of the human elongation factor EF1α gene (GenBank accession number J04617). After transient transfection of the 3 plasmids by calcium phosphate in 293T cells, the supernatant was harvested, concentrated by 2 rounds of ultracentrifugation, and resuspended in serum-free medium (Cellgro SCGM, Cellgenix, Freiburg, Germany). Viral stocks were stored at −70°C and titers determined by transduction and flow cytometry analysis of GFP expression in HeLa cells as previously described.22 Titers were composed of between 5 × 107 and 108 HeLa-transducing units (TU) per milliliter.
Purification and transduction of CD34+cells
Cord blood samples were obtained according to institutional guidelines, and CD34+ cells were purified as described.26 In brief, cord blood mononuclear cells recovered after Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient centrifugation were incubated on ice with anti-CD34 M450 Dynabeads (Dynal, Oslo, Norway) as described by the manufacturer. After several washes to eliminate unbound cells, CD34+ cells were recovered from the beads by incubation for 15 minutes at 37°C with the “Detach-a-bead” included in the kit. Cells were immediately washed and analyzed by flow cytometry. The percentage of purified CD34+ cells was 89% ± 7.0%. For transduction, 105 cells were seeded in 96-well plates in 100 μL of Cellgro medium supplemented with antibiotics (Gibco BRL, Life Technologies Ltd, Paisley, Scotland) with 10−4-mol/L dithiothreitol (Fluka Biochemika, Buchs, Switzerland) and thrombopoietin (TPO). After overnight incubation, 106(typically) or 105 to 5 × 106 (for dose-response analysis) HeLa-TU of vector was added per well, and the volume was adjusted to 200 μL with Cellgro SCGM medium containing TPO. After 24 hours, cells were washed, diluted to 400 μL in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum (both from Gibco BRL), antibiotics, and FLT3-L, TPO, and stem cell factor (SCF) for 3 days. Cells were either directly analyzed for GFP and CD34 expression or further cultured with the 3 growth factors. For experiments shown in Figure 1, concentrated viral stocks were treated with DNAse I (Roche Diagnostics, Rotkreuz, Switzerland) prior to incubation with cells to eliminate DNA contaminants that may interfere with further polymerase chain reaction (PCR) analysis. Briefly, 106 TU of vector was incubated for 30 minutes at 37°C in a final volume of 20 μL containing 20-μg/mL DNAse I and 10-mmol/L MgCl2. Then, 80 μL of Cellgro medium was added, and the resulting 100 μL was added to 100 μL of Cellgro medium containing 105 CD34+ cells. Cells were then cultured as described above.
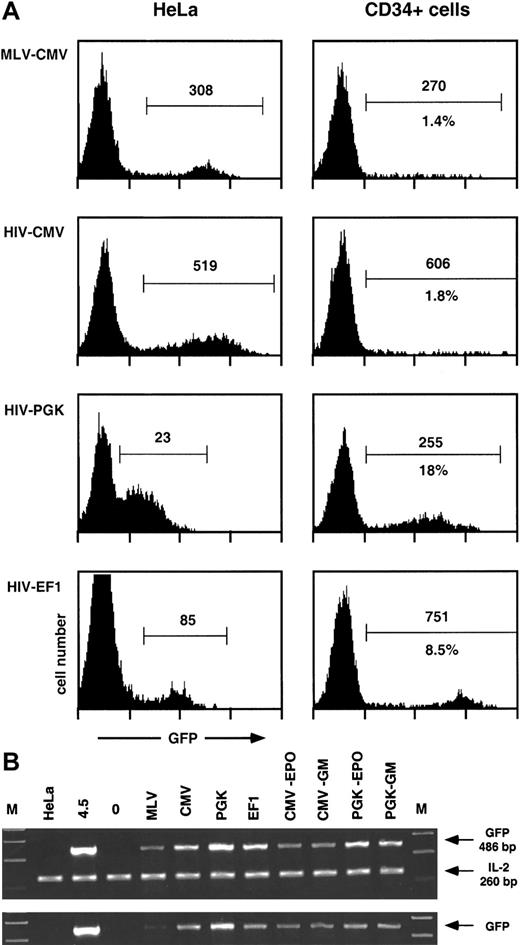
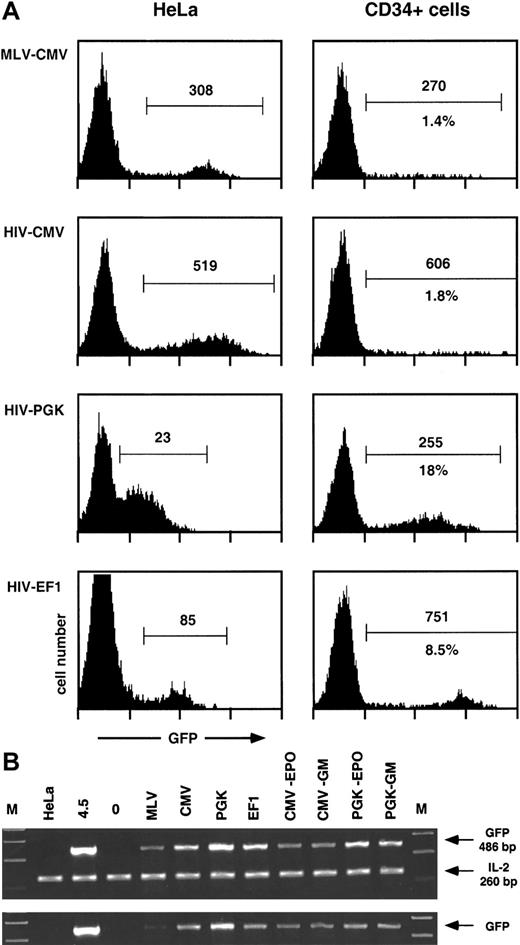
Transgene transfer and expression after transduction of human CD34+ cells with MLV and HIV vectors.
Human CD34+ cells from umbilical cord blood (105 cells) were transduced with 106 HeLa-TU of MLV- or the indicated HIV-derived GFP encoding vectors, previously treated with DNAse I, as described in “Materials and methods.” After 4 days, cells were analyzed by flow cytometry for GFP expression (A) and lysed for PCR analysis of the presence of GFP transgene (B). Results from 1 experiment representative of 2 independent evaluations are shown. (A) Expression of the GFP transgene after transduction of human CD34+ cells or HeLa cells. Results are represented as histograms of GFP fluorescence intensity (x-axis, 4-log scale) versus cell number (y-axis, linear). GFP+ cells (within marker) were analyzed for percentage (lower number) and median of fluorescence intensity (upper number). Percentages were omitted in HeLa cells because the histograms were obtained from titration experiments of the corresponding vectors. (B) Presence of the GFP transgene after transduction and expansion of human CD34+ cells. Cellular extracts equivalent to 5000 cells (upper panel) were amplified as described in “Materials and methods” with GFP-specific primers, together with IL-2–specific primers as internal controls. Sizes of the corresponding PCR products are indicated. Lanes are as follows: M, molecular weight marker; HeLa (negative control for GFP, positive control for IL-2); 4.5, a clone of HeLa containing 1 copy of HIV-CMV-GFP vector; 0, untransduced CD34+ cells; MLV-CMV-PGK-EF1, CD34+ cells transduced with the corresponding vectors (see A); CMV-EPO, CD34+ cells transduced with HIV-CMV vector (lane CMV) and analyzed after expansion and differentiation into erythroid cells (see text); CMV-GM, same as CMV-EPO but analyzed after expansion and differentiation into monocytic cells (see text); PGK-EPO and PGK-GM, same as CMV-EPO and CMV-GM but from CD34+ cells transduced with HIV-CMV vector (lane PGK). To ensure proportionality, cellular extracts equivalent to 1700 cells (lower panel) were amplified separately.
Transgene transfer and expression after transduction of human CD34+ cells with MLV and HIV vectors.
Human CD34+ cells from umbilical cord blood (105 cells) were transduced with 106 HeLa-TU of MLV- or the indicated HIV-derived GFP encoding vectors, previously treated with DNAse I, as described in “Materials and methods.” After 4 days, cells were analyzed by flow cytometry for GFP expression (A) and lysed for PCR analysis of the presence of GFP transgene (B). Results from 1 experiment representative of 2 independent evaluations are shown. (A) Expression of the GFP transgene after transduction of human CD34+ cells or HeLa cells. Results are represented as histograms of GFP fluorescence intensity (x-axis, 4-log scale) versus cell number (y-axis, linear). GFP+ cells (within marker) were analyzed for percentage (lower number) and median of fluorescence intensity (upper number). Percentages were omitted in HeLa cells because the histograms were obtained from titration experiments of the corresponding vectors. (B) Presence of the GFP transgene after transduction and expansion of human CD34+ cells. Cellular extracts equivalent to 5000 cells (upper panel) were amplified as described in “Materials and methods” with GFP-specific primers, together with IL-2–specific primers as internal controls. Sizes of the corresponding PCR products are indicated. Lanes are as follows: M, molecular weight marker; HeLa (negative control for GFP, positive control for IL-2); 4.5, a clone of HeLa containing 1 copy of HIV-CMV-GFP vector; 0, untransduced CD34+ cells; MLV-CMV-PGK-EF1, CD34+ cells transduced with the corresponding vectors (see A); CMV-EPO, CD34+ cells transduced with HIV-CMV vector (lane CMV) and analyzed after expansion and differentiation into erythroid cells (see text); CMV-GM, same as CMV-EPO but analyzed after expansion and differentiation into monocytic cells (see text); PGK-EPO and PGK-GM, same as CMV-EPO and CMV-GM but from CD34+ cells transduced with HIV-CMV vector (lane PGK). To ensure proportionality, cellular extracts equivalent to 1700 cells (lower panel) were amplified separately.
PCR analysis after transduction of CD34+ cells
Cells (5 × 105) were lysed in 18 μL of 50-mmol/L Tris HCl, pH 8.0; 20-mmol/L NaCl; 1-mmol/L ethylenediaminetetraacetic acid; and 1% sodium dodecyl sulfate. Proteinase K (2 μL at 10 mg/mL) was added, and samples were incubated for 30 minutes at 55°C. After digestion, 200 μL of water was added, and samples were boiled for 10 minutes to inactivate proteinase K. PCR amplification was performed using HotStarTaq (Qiagen GmbH, Hilden, Germany) in a final volume of 50 μL and 2 μL or 0.7 μL of extract with the following cycles: 95°C, 15 minutes; 40 cycles 94°C, 30 minutes; 60°C, 1 minute; 72°C, 1 minute; plus a 10-minute extension phase at 72°C. Sequences of the PCR primers were as follows: 5′ human interleukin-2 (hIL-2) GCAACTCCTGTCTTGCATTG; 3′ hIL-2 AATGTGAGCATCCTGGTGAG; 5′ GFP GTGAGCAAGGGCGAGGAGC; and 3′ GFP CTTGATGCCGTTCTTCTGCTTGT. Conditions were adjusted to observe a decrease in the final PCR product yield when diluting samples by a factor of 3 (Figure 1A).
Purification and transduction of primary human T cells
Peripheral blood mononuclear cells were purified from buffy coats of healthy donors over a Ficoll-Paque gradient. Macrophages were removed by plastic adherence for 2 hours at 37°C, and the remaining cells were incubated with a cocktail of monoclonal antibodies against HLA-DR, CD25, CD69, CD19, CD16, CD11b, and CD14 (Pharmingen, San Diego, CA). After a 30-minute incubation on ice, cells were washed twice and incubated with magnetic beads (Dynabeads) conjugated with goat antimouse immunoglobulin (Ig) G at a 1:4 target:bead ratio. Bead-bound cells were removed 30 minutes later using a magnet. Remaining cells were further purified through 2 more rounds at an increased target:bead ratio (1:10). This purification protocol typically resulted in a 99.5% pure population of resting T cells as determined by flow cytometry analysis with antibodies against the activation markers HLA-DR, CD25, and CD69. Cells maintained in RPMI 1640 supplemented with 10% fetal calf serum, 5-mmol/L penicillin and streptomycin (GIBCO BRL), and 2-mmol/L glutamine (GIBCO BRL) were activated with phytohemagglutinin (PHA; Sigma, St Louis, MO) at 3 μg/mL for 48 hours and subsequently cultured in RPMI 1640 containing 10% fetal calf serum and recombinant IL-2 (Sigma) at 10 U/mL. A total of 5 × 105 cells were transduced with various lentiviral vectors at a multiplicity of infection (MOI) of 5 HeLa-TU per cell in 24-well plates in the presence of PHA, in a final volume of 500 μL. Flow cytometry analysis of GFP expression was performed 5 days after transduction.
Cytokines
All cytokines were recombinant human material. Granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax) from Essex Chimie & Sandoz (Basel, Switzerland) was used at 20 ng/mL, G-CSF (Neupogen) from Roche (Basel, Switzerland) at 10 ng/mL, and erythropoietin (Eprex) from Cilag (Schaffhausen, Switzerland) at 2 U/mL. Other cytokines were purchased from Peprotech EC (London, England) and used at the following concentrations: FLT3-L 25 ng/mL, TPO 10 U/mL, SCF 20 ng/mL, IL-3 10 ng/mL, tumor necrosis factor (TNF) 40 ng/mL, and IL-4 20 ng/mL.
Antibodies and immunoreactants
Phycoerythrin-conjugated monoclonal antibodies (mAbs) were the following: anti-CD34 (mIgG1 clone 8G12) from Becton Dickinson (Mountain View, CA), anti-glycophorin-A (mIgG1, clone JC 159), anti-CD42b (mIgG2a, clone AN51), and isotypic controls from Dako (Glostrup, Denmark). Biotin-labeled mAbs included anti-CD14 (mIgG2a, clone UCHM1) from Ancell Corp (Bayport, MN), anti-CD15 (mIgM cloneDU-HL60-3) from Sigma, and isotypic controls from Ancell. Allophycocyanin-labeled streptavidin was from Pharmingen. Anti-CD34 mIgG-coated M450 Dynabeads were from Dynal.
In vitro differentiation
Megakaryocytic differentiation was evaluated after 10 days of expansion culture with FLT3-L, TPO, and SCF. For the other lineages, cells were washed after 9 to 11 days of expansion culture with FLT3-L, TPO, and SCF and incubated with erythropoietin (EPO), IL-3, and SCF for erythroid differentiation, GM-CSF and SCF for monocytic differentiation, and G-CSF and SCF for granulocytic differentiation. Differentiation into dendritic cells (DCs) was performed as previously described.26 Briefly, transduced CD34+ cells were expanded for 14 to 28 days with FLT3-L, TPO, and SCF. Cells were then induced into mature DCs by exposure to GM-CSF and IL-4 for 3 days, followed by GM-CSF, IL-4, and TNF for 3 more days. The amplification ratio was between 100 and 1000, depending on the experiment and the cell lineage.
Flow cytometry analysis
Cells were analyzed as described26 on a FACSCalibur (Becton Dickinson) with slight modifications. FL-1 was used for GFP, FL-2 for phycoerythrin-labeled mAbs, FL-3 for identification of living cells with the nonpermeant DNA dye 7-amino-actinomycin D (Sigma),27 and FL-4 for biotinylated mAbs indirectly labeled with streptavidin-allophycocyanin. Cell suspensions were adjusted to 0.5% paraformaldehyde prior to analysis. Data were analyzed using WINMDI software written by J. Trotter at Scripps Institute (La Jolla, CA) and CellQuest software (Becton Dickinson).
Results
Transduction of human hematopoietic progenitors with MLV vectors and HIV-based lentivectors: the EF1α promoter induces high transgene expression in lentivector-transduced CD34+cells
Human cord blood CD34+ cells were purified by a single round of positive selection with magnetic beads, resulting in preparations approximately 90% pure. Cells were maintained overnight in serum-free medium supplemented with TPO and were exposed for 24 hours to MLV- or HIV-based GFP-expressing vectors pseudotyped with VSV G prepared by transient transfection of 293T cells as previously described.11,22 The lentiviral packaging system used in these experiments was of a so-called second generation22and included the HIV-1 gag, pol, tat,and rev genes. The MOI was 10 HeLa-TU per CD34+cell. After transduction, cells were incubated for another 3 days in medium containing fetal calf serum, FLT3-L, TPO, and SCF and analyzed by flow cytometry for GFP (Figure 1) and CD34 expression (not illustrated) and lysed for PCR analysis. The relative potencies of 3 internal transcription units were compared within the context of lentivectors: the CMV, the PGK, and the EF1α promoters.
As shown in Figure 1A, a sharp subpopulation of GFP+hematopoietic progenitors could only be seen when cells were transduced with HIV vectors containing the PGK or the EF1α promoters. Cells transduced with MLV vector or HIV vector containing the CMV promoter displayed only a small percentage of GFP+ cells together with a high heterogeneity in GFP expression. A side-by-side comparison of GFP expression after transduction of HeLa and CD34+cells revealed that the promoters examined behaved quite differently in these 2 cell types. The PGK promoter was weak in HeLa cells (overlap between GFP+ and GFP− cells) and strong in HPCs. The EF1α promoter was intermediate in HeLa cells and very potent in HPCs, with a mean value typically 100 times higher in transduced than in control HPCs. The CMV promoter, whether in an MLV or an HIV vector, was equally strong in HeLa cells, contrasting with its low activity in transduced HPCs. A PCR-based quantification performed at the time of the flow cytometry analysis (Figure 1B) indicated that the low expression from MLV-CMV vector in HPCs was due at least in part to poor gene transfer in these cells, a consequence of the limited stimulation of CD34+ cells during transduction. In contrast, the low expression in HPCs exposed to the HIV-CMV vector (Figure 1A) could not be explained by a low frequency of transduction, because equivalent amounts of transgene DNA were detected in cells transduced with all 3 HIV vectors (Figure 1B). This indicates that the CMV promoter does not govern efficient expression in hematopoietic progenitors, a finding in accordance with previous reports.17,18,20 We also ascertained that GFP expression originated from integrated proviruses. For this, we performed a PCR analysis following 3 weeks of expansion of the transduced CD34+ cells. During this period, the cell numbers increased by a factor of 900 to 1800, depending on conditions and experiments. In the particular experiment illustrated here (Figure 1B), the percentage of cells expressing GFP from the PGK promoter decreases by a factor of approximately 2 after expansion and differentiation into erythroid or monocytoid cells, which paralleled a slight decrease in GFP DNA copy number. It confirms that the bulk of transgene DNA remains in the cells after massive expansion, excluding its presence as unintegrated forms. It also correlates the previous demonstration that long-term transgene expression cannot be detected after transduction with integrase-deficient HIV vectors.12
Transduction of human CD34+ cells as a function of increasing vector concentration
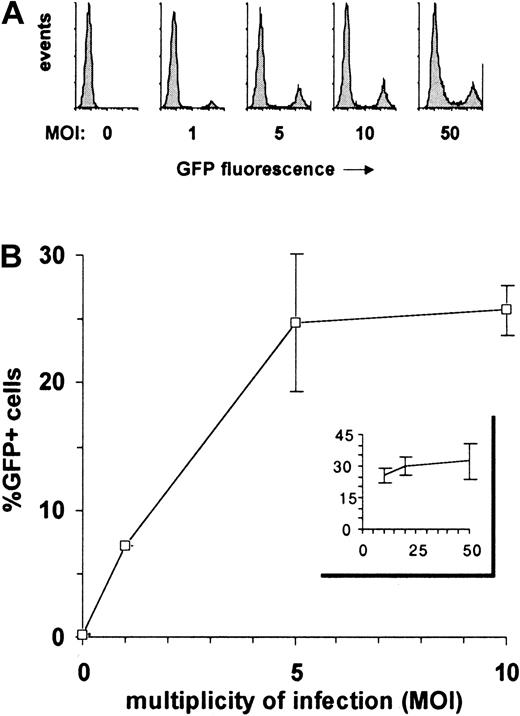
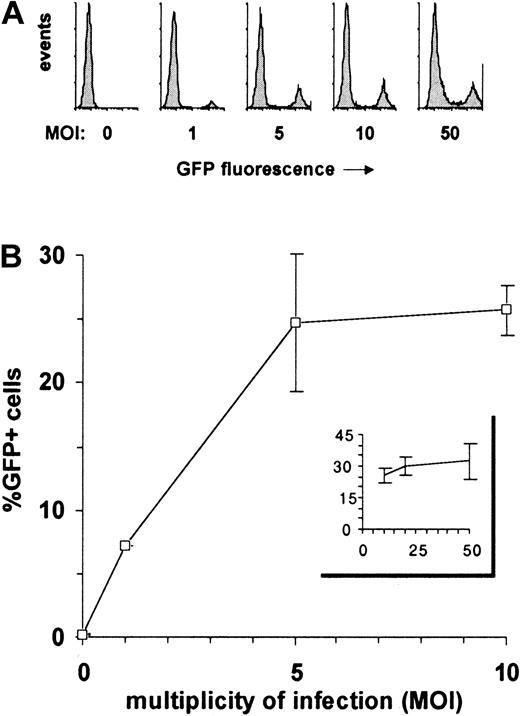
The high levels of GFP expression induced by the EF1α-containing HIV-derived vector allowed for a reliable determination of gene transfer efficiency because even low numbers of transduced cells were easily detected. This vector was thus used to evaluate the influence of the MOI on the transduction efficiency of CD34+ cell (Figure 2). Although the percentage of GFP+ cells initially increased as a direct function of the MOI, the curve flattened starting at an MOI of 5, reaching a maximum of 25% GFP+ cells (± 5% according to experiments) at MOIs 20 and above. In the absence of optimal cytokine-induced proliferation, only a fraction of human CD34+ cells is thus permissive to lentivector-mediated transduction, as previously suggested.28
Effect of MOI on transduction efficiency of lentivectors in human CD34
+ cells. A total of 105CD34+ cells were transduced with various doses (1, 2, 5, 10, 20, and 50 × 105 TU) of EF1α-GFP lentiviral vector, allowing MOIs ranging from 1 to 50. GFP expression was analyzed by flow cytometry after 4 days. (A) Data from one representative experiment are shown as frequency histograms of GFP fluorescence intensity versus cell number (events). Gates for GFP+ cells were set up according to untransfected cells (MOI 0). (B) Mean percentages of GFP+ cells obtained with MOIs of 0 to 10 and 10 to 50 are represented in the main panel and in the insert, respectively. These data are from 3 independent experiments for the 0-to-10 MOI range and from 2 independent experiments for the 10-to-50 MOI range. Error bars represent SD.
Effect of MOI on transduction efficiency of lentivectors in human CD34
+ cells. A total of 105CD34+ cells were transduced with various doses (1, 2, 5, 10, 20, and 50 × 105 TU) of EF1α-GFP lentiviral vector, allowing MOIs ranging from 1 to 50. GFP expression was analyzed by flow cytometry after 4 days. (A) Data from one representative experiment are shown as frequency histograms of GFP fluorescence intensity versus cell number (events). Gates for GFP+ cells were set up according to untransfected cells (MOI 0). (B) Mean percentages of GFP+ cells obtained with MOIs of 0 to 10 and 10 to 50 are represented in the main panel and in the insert, respectively. These data are from 3 independent experiments for the 0-to-10 MOI range and from 2 independent experiments for the 10-to-50 MOI range. Error bars represent SD.
Transgene expression in hematopoietic lineages after transduction of CD34+ cells with HIV vectors containing PGK and EF1α promoters
A critical issue for the genetic treatment of a variety of lymphohematologic disorders will be the levels of expression of the putatively therapeutic transgene in the appropriate subset of differentiated cells. In that respect, recent reports using HIV-based vectors with an internal expression cassette driven by the CMV promoter have documented the incapacity of this transcriptional element to induce strong transgene expression in precursors as well as mature blood cells.17 18 With the hope of identifying more suitable alternatives, hematopoietic progenitors transduced with HIV-based vectors containing the PGK or the EF1α promoters were differentiated in vitro to compare the relative potency of these vectors in various hematopoietic lineages. For this, transduced CD34+ cells were first expanded for 7 to 14 days in FLT3-L/TPO/SCF and then incubated in various differentiating media for an additional 5- to 10-day period, allowing the generation of erythroid cells, granulocytes, and monocytes (see “Materials and methods”). Megakaryocytes were induced in FLT3-L/TPO/SCF exclusively. For DCs, transduced CD34+ cells were expanded for 14 to 28 days with FLT3-L/TPO/SCF. Cells were then induced into mature DCs by exposure to GM-CSF/IL-4 for 3 days, followed by exposure to GM-CSF/IL-4/TNF for 3 more days.
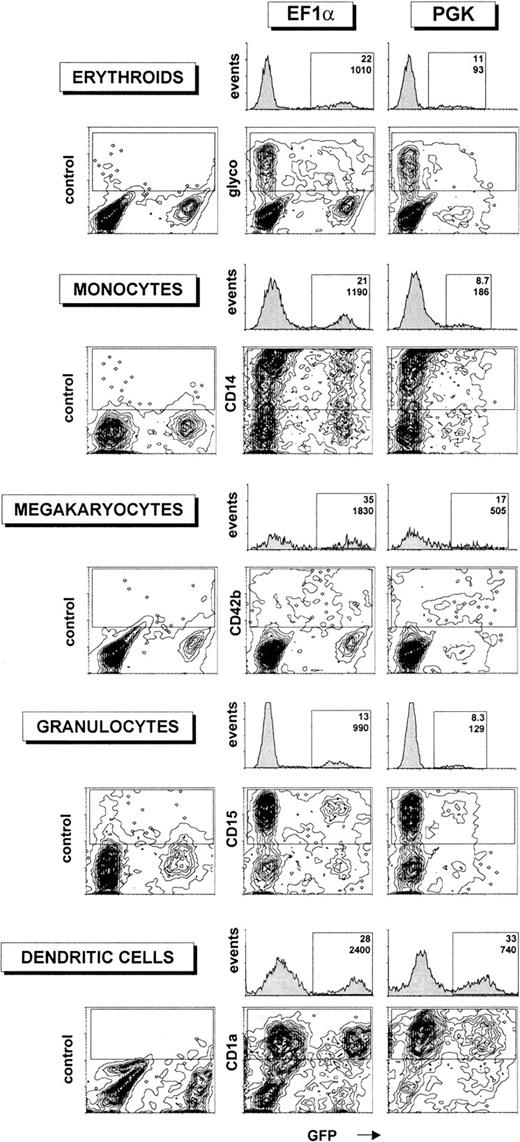
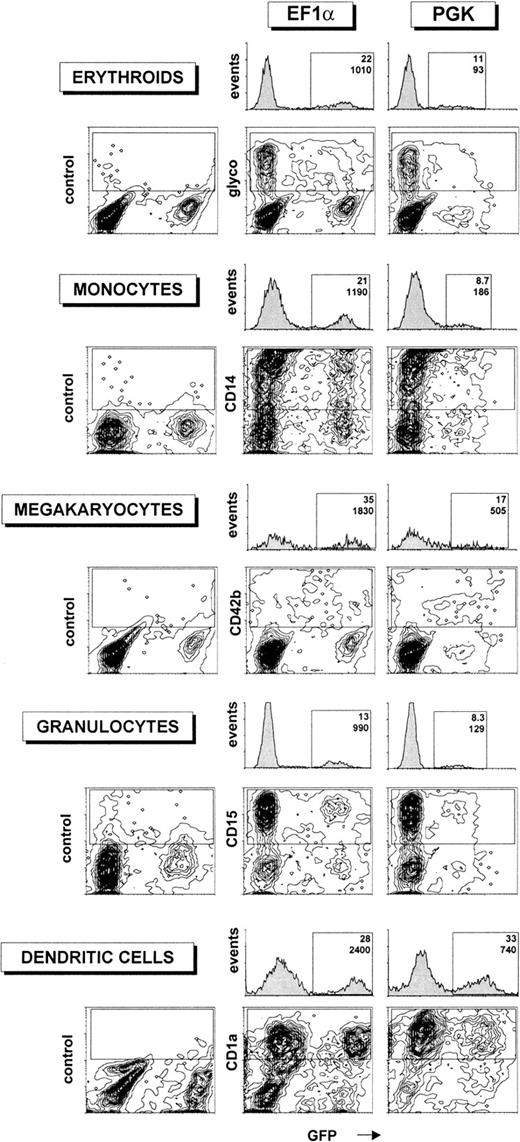
The differentiated cells were then analyzed by flow cytometry to determine the percentage of lineage-specific marker+/GFP+ cells as well as the relative levels of GFP expression in the differentiated populations (Figure3). Transgene expression was high in all lineages examined after transduction of precursors with the EF1α-containing lentiviral vector, with a signal-to-noise ratio (mean of fluorescence intensity of GFP+ cells divided by mean of fluorescence intensity of GFP− cells) between 150 and 200, depending on the cell type. The PGK promoter induced lower and more variable levels of GFP expression in differentiated cells. Expression of GFP under the control of the PGK promoter was highest in DCs, where it was only 3.5-fold less potent than that driven from the EF1α promoter, and worst in erythroid cells (glycophorin+), where it was 11-fold weaker than its EF1α counterpart. However, even in this case, the signal-to-noise ratio was still high enough to permit a clear discrimination between GFP+ and GFP− cells, allowing for the reliable analysis of transgene expression.
Transgene expression in differentiated hematopoietic lineages after transduction of human CD34+ cells with HIV-derived vectors.
Human CD34+ cells from umbilical cord blood (105 cells) were transduced with 106 TU of HIV vectors (corresponding to an MOI of 10) containing either the EF1α or the PGK promoters and differentiated into various hematopoietic lineages as described in “Materials and methods.” After differentiation, HIV-EF1α– (first and second column) or HIV-PGK–transduced cells (third column) were analyzed by flow cytometry for both GFP (x-axis) and lineage-specific marker expression (glycophorin, CD14, CD42b, CD15, and CD1a, respectively, for erythroids, monocytes, megakaryocytes, granulocytes, and DCs). Isotype control antibodies were used in the first column. For each promoter, cell populations expressing high levels of lineage-specific markers were gated (upper rectangle in 2-dimensional plots), and monoparametric frequency histograms of GFP expression for these cells were generated (located above the 2-dimensional plots). The percentage of GFP+ cells (upper number) and median of fluorescence intensity of GFP (lower number) was determined. For the experiment generating erythroids, monocytes, megakaryocytes, and granulocytes, the fraction of GFP+ CD34+ cells before differentiation was 22% for EF1 and 16% for PGK. For the experiment generating DCs, the fraction of GFP+ precursors before differentiation was 32% for EF1 and 29% for PGK. These data are representative of 4 independent experiments.
Transgene expression in differentiated hematopoietic lineages after transduction of human CD34+ cells with HIV-derived vectors.
Human CD34+ cells from umbilical cord blood (105 cells) were transduced with 106 TU of HIV vectors (corresponding to an MOI of 10) containing either the EF1α or the PGK promoters and differentiated into various hematopoietic lineages as described in “Materials and methods.” After differentiation, HIV-EF1α– (first and second column) or HIV-PGK–transduced cells (third column) were analyzed by flow cytometry for both GFP (x-axis) and lineage-specific marker expression (glycophorin, CD14, CD42b, CD15, and CD1a, respectively, for erythroids, monocytes, megakaryocytes, granulocytes, and DCs). Isotype control antibodies were used in the first column. For each promoter, cell populations expressing high levels of lineage-specific markers were gated (upper rectangle in 2-dimensional plots), and monoparametric frequency histograms of GFP expression for these cells were generated (located above the 2-dimensional plots). The percentage of GFP+ cells (upper number) and median of fluorescence intensity of GFP (lower number) was determined. For the experiment generating erythroids, monocytes, megakaryocytes, and granulocytes, the fraction of GFP+ CD34+ cells before differentiation was 22% for EF1 and 16% for PGK. For the experiment generating DCs, the fraction of GFP+ precursors before differentiation was 32% for EF1 and 29% for PGK. These data are representative of 4 independent experiments.
Taken together, these data indicate that the transduction of human CD34+ cells with HIV-based vectors containing internal promoters derived from the EF1α and, to a lesser extent, the PGK gene results in high levels of transgene expression in several hematopoietic lineages.
EF1α promoter-based lentiviral vectors induce high levels of transgene expression in primary T lymphocytes
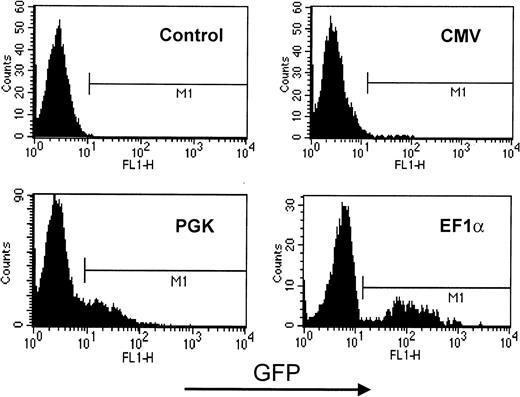
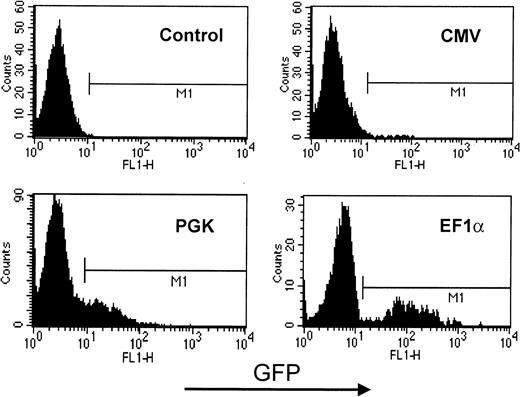
The system used in these experiments did not allow for the easy differentiation of CD34+ precursors into cells of the lymphocytic lineage. Therefore, to compare the relative value of CMV-, PGK-, and EF1α-containing HIV-derived vectors in these cells, mature T lymphocytes purified from the peripheral blood were directly used as transduction targets (Figure 4). The results revealed the same hierarchy as observed in HPCs and in the various other blood cell lineages, with the EF1α promoter inducing levels of GFP expression significantly higher than those yielded by the PGK promoter, whereas cells transduced with CMV-based vectors exhibited levels of GFP expression that were incompatible with proper detection by flow cytometry. Of note, preliminary experiments in human primary B cells show that the CMV promoter is very active in these cells (not illustrated). This indicates that the poor activity of the CMV promoter is not universal in human hematopoietic cells.
GFP expression in lentivector-transduced primary human T lymphocytes.
CMV-, PGK-, or EF1α-containing HIV-derived vectors were used to infect PHA-activated primary T lymphocytes. Cells were maintained for 5 days in culture medium supplemented with IL-2 before analyzing GFP expression by flow cytometry. Two-dimensional plot represents cell number as a function of GFP levels. Results are representative of at least 5 independent experiments.
GFP expression in lentivector-transduced primary human T lymphocytes.
CMV-, PGK-, or EF1α-containing HIV-derived vectors were used to infect PHA-activated primary T lymphocytes. Cells were maintained for 5 days in culture medium supplemented with IL-2 before analyzing GFP expression by flow cytometry. Two-dimensional plot represents cell number as a function of GFP levels. Results are representative of at least 5 independent experiments.
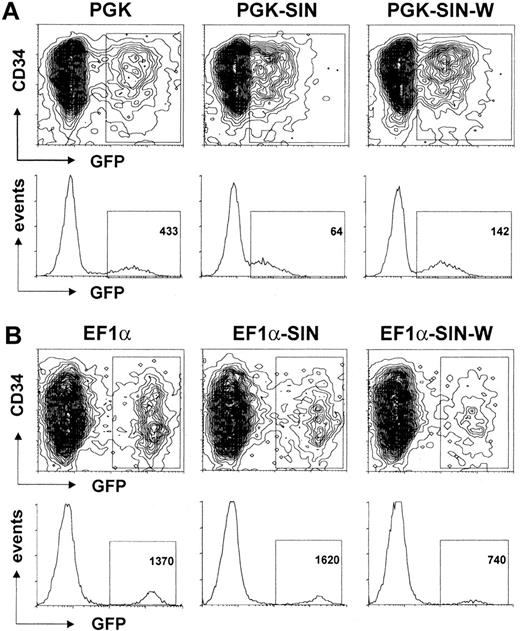
Influence of SIN design and WPRE on transgene expression
The use of SIN 3′ LTR U3-deleted HIV vectors increases the biosafety of this system and avoids a possible interference between the viral LTR and the vector's internal promoter.23 EF1α- and PGK-containing HIV-derived SIN vectors were therefore tested for their ability to induce high levels of transgene expression in hematopoietic precursors (Figure 5). Most interestingly, the SIN design was accompanied by a dramatic decrease (6-fold) in GFP expression within the context of the PGK vector, whereas it instead had a slightly yet reproducibly positive effect when introduced in the EF1α vector. Inserting the sequence for the WPRE 29 upstream of the 3′ LTR has been shown to promote transgene expression from HIV-derived vectors in some targets.24 It indeed stimulated GFP production from the PGK promoter by a factor of 2.15 in hematopoietic precursors. In contrast, it exerted a negative effect on expression from the highly active EF1α promoter, with a decrease by a factor of 1.85. Taken together, these indicate that the strong EF1α promoter is less sensitive than the PGK promoter to a SIN configuration, making it a better candidate for gene expression in hematopoietic cells.
Effect of SIN design and WPRE addition on transgene expression in human CD34+ cells.
A total of 105 CD34+ cells were transduced with 106 HeLa-TU of the indicated GFP-expressing HIV-derived vectors and analyzed by flow cytometry 4 days later. Vectors were as follows: (A) PGK (2 intact LTRs); PGK-SIN (3′ SIN-LTR deletion, see “Materials and methods”); PGK-SIN-W (addition of WPRE upstream of the 3′ SIN-LTR). (B) Same analysis as in A but with EF1α promoter instead of PGK promoter. Results are represented as contour graphs of CD34 expression versus GFP expression (4-log scale). For each condition, GFP expression is also displayed as histograms of GFP fluorescence intensity (x-axis, 4-log scale) versus number of cells (y-axis, linear). Number in square represents median of fluorescence intensity of GFP+ cells.
Effect of SIN design and WPRE addition on transgene expression in human CD34+ cells.
A total of 105 CD34+ cells were transduced with 106 HeLa-TU of the indicated GFP-expressing HIV-derived vectors and analyzed by flow cytometry 4 days later. Vectors were as follows: (A) PGK (2 intact LTRs); PGK-SIN (3′ SIN-LTR deletion, see “Materials and methods”); PGK-SIN-W (addition of WPRE upstream of the 3′ SIN-LTR). (B) Same analysis as in A but with EF1α promoter instead of PGK promoter. Results are represented as contour graphs of CD34 expression versus GFP expression (4-log scale). For each condition, GFP expression is also displayed as histograms of GFP fluorescence intensity (x-axis, 4-log scale) versus number of cells (y-axis, linear). Number in square represents median of fluorescence intensity of GFP+ cells.
Discussion
This work describes the transduction of human CD34+cells with improved HIV-derived vectors aimed at increasing gene expression as well as biosafety. Our results indicate that these vectors can efficiently transduce CD34+ cells using conditions under which MLV-based vectors are inefficient. Furthermore, our study demonstrates that an HIV-derived vector containing an internal EF1α promoter induces high levels of transgene expression in both hematopoietic precursors and in vitro differentiated blood lineages, and in primary T cells. Using this vector, we also found that human CD34+ cells can be efficiently transduced at a relatively low MOI but that the efficacy of gene transfer saturates at about 20% to 30% of transduced cells. Moreover, the HIV-derived vector containing an internal EF1α promoter fully tolerates a SIN design.
Lentiviral vectors represent a very attractive tool for gene therapy of hematologic disorders because of their ability to transduce pluripotent hHSCs.14-18 Two essential issues will need, however, to be addressed prior to envisaging the use of HIV-based vectors for therapeutic purposes. The first issue is biosafety, to which the use of multiply attenuated packaging constructs, stable producer cell lines, and SIN vectors will significantly reduce the risk that replication-competent recombinants emerge from the vector-manufacturing system. The second issue is efficiency, ie, the levels of transgene expression in the therapeutically relevant mature blood cell. Indeed, for instance, with hematologic disorders resulting from an enzymatic deficiency or with anemias secondary to defects in hemoglobin synthesis, function will not be restored without achieving significantly high levels of expression of the curative gene. In that respect, GFP represents a marker of choice to study promoter activity in target cells because it permits a direct quantification of the percentage of transduced cells as well as of the level of transgene expression in each individual cell.
CMV promoter-containing HIV-derived vectors can induce high levels of transgene expression in the central nervous system11-13and have allowed the initial demonstration that pluripotent hematopoietic precursors can be efficiently transduced by this gene delivery tool. CMV-based vectors, however, are largely useless for transferring therapeutic genes into most lymphohematopoietic cells because in these targets their transcriptional activity is prohibitively low.17,18 20 We thus observed that CMV-driven GFP production was insufficient to determine reliably either the percentage of transduced cells or the level of transgene expression in both CD34+ cells and several differentiated hematopoietic derivatives (Figures 1 and 4, and data not shown). In sharp contrast, vectors in which GFP was transcribed from the EF1α or, to a lesser extent, the PGK promoter were highly active in both categories of cells.
The EF1α-GFP lentivector was used to assess the dose responsiveness of CD34+ cell transduction because GFP+ cells could easily be discriminated from GFP− cells even when present in very low amounts. Surprisingly, under conditions of limited stimulation, we found that increasing the MOI above 10 did not increase significantly the fraction of transduced cells, which plateaued between 20% to 30%, depending on the experiments. A similar saturation of the fraction of transducible CD34+ cells was previously noted and attributed to the fact that lentivectors can transfer genes into cells that are in the G1 but not in the G0phase of the cycle.28 Still, lentivector-transduced CD34+ cells are pluripotential.17,19It remains to be determined whether the fraction of permissive hHSCs can be augmented, without triggering cell division, by the use of specific cocktails of cytokines, as recently described for the transduction of resting human T cells with HIV vectors.30Alternatively, transduction of human CD34+ cells could also be improved under conditions promoting cell division, as recently reported.7 10
The MOI of 10 used here to achieve optimal transduction is significantly lower than the ones described in previous studies, where it ranged between 60 to 300 and 1000 to 3000.17 18 This difference may be partly artifactual, because we define the titer of HIV vector stocks in HeLa cells, which are approximately 4 times less sensitive to transduction than 293T cells, another commonly used target for lentivector titration (data not shown). Nevertheless, it is also worth emphasizing that we expose the CD34+ cells to the vector particles in a small volume (105 cells in 200 μL) and during 24 hours—2 factors that could enhance the vector-target meeting probability.
Both HIV-PGK and HIV-EF1α vectors were analyzed for transgene expression in differentiated hematopoietic cells after transduction of CD34+ cells. GFP could be readily detected in all lineages analyzed, after transduction with both types of vectors. However, transgene expression in lineage marker–positive cells was consistently higher with the EF1α promoter than with its PGK counterpart. Also, there was a lower degree of interlineage variation with the EF1α promoter. It is remarkable that, with both of these promoters, the percentage of GFP+ cells remained fairly constant as the CD34+ cells proliferated (global increase in cell number was between 100 and 1000; Figure 3) and differentiated into their various hematopoietic derivatives. This indicates that there was neither silencing of the promoters nor preferential loss of transduced cells during the expansion and maturation phases of the culture. Engraftment and repopulation assays in NOD/SCID will be performed with both HIV-PGK and HIV-EF1α vectors to confirm the stability of expression from these promoters in vivo.
Lastly, we studied the effect of 2 modifications in the HIV vector backbone designed to improve its utility as a therapeutic tool. One is the deletion of a major part of U3 in the 3′ LTR of the vector plasmid, leading to a SIN configuration.23 This deletion reduces the potential for generation of replication-competent viruses and prevents potential interference between the LTR and internal promoter. In some circumstances, however, it can negatively affect transgene expression, apparently by decreasing the efficiency of polyadenylation.31-36 We found that the SIN design exerted a dramatically negative impact on GFP expression in CD34+cells transduced with a PGK-based lentivector, while it did not decrease transcription efficiency from the EF1α promoter. It is possible that the SIN deletion may be deleterious only in the context of low levels of transcription.
The SIN-induced decrease in PGK-driven transgene expression could be partly rescued by inserting the WPRE in the vector—immediately upstream of the deleted 3′ LTR. This confirms our recent demonstration that WPRE can stimulate gene expression by a factor of 3 to 6 in epithelial cells.24 However, the addition of WPRE did not stimulate GFP expression in CD34+ cells transduced with an EF1α promoter-based vector, having instead a slight yet reproducible negative effect. Although our lack of understanding of the mechanism of action of WPRE makes it difficult to explain this difference, it could be that the high amounts of transcripts generated by the EF1α promoter clog the pathway targeted by this post-transcriptional regulatory element.
In conclusion, the present work indicates that EF1α promoter-containing HIV-derived vectors are both highly efficient and very potent for expressing transgenes in human hematopoietic progenitor cells as well as in all of their blood cell derivatives, even in a SIN configuration. These vectors thus represent attractive tools for the genetic treatment of lymphohematologic disorders and for the study of hematopoiesis via the lentivector-mediated modification of human HSCs.
Acknowledgments
We thank D. Wohlwend, C. Cudre-Mauroux, and M. Loche for their help with the flow cytometry analyses, the cloning, and the artwork, respectively.
Supported by grants from the Swiss National Foundation to D.T. and R.H.Z., from the Clayton Foundation, the Fondation Gabriella Giorgi Cavaglieri and Fondation Dubois-Ferrière Dinu-Lipatti to D.T., and from the Fondation pour la lutte contre le cancer et pour les recherches médico-biologiques to R.H.Z.
P.S. and V.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Didier Trono, Department of Genetics and Microbiology, C.M.U., 1 rue Michel-Servet, CH-1211 Geneva 4, Switzerland; e-mail:didier.trono@medecine.unige.ch.