Persistent therapeutic levels of human factor VIII (hFVIII) would signify a major advance in the treatment of hemophilia A. Here we report sustained expression of hFVIII in immunocompetent mice using recombinant adeno-associated virus (rAAV) vectors. AAV can stably transduce liver cells, the target tissue for efficient hFVIII production. Because of rAAV packaging constraints, we tested 2 constructs using small regulatory elements designed for liver-specific transgene expression linked to B-domain–deleted hFVIII (BDD-hFVIII) cDNA. More than 1012/mL rAAV/BDD-hFVIII virion particles were generated using a transfection scheme that eliminates adenovirus. Coatest and APTT assays confirmed the production of functional BDD-hFVIII protein after transduction of 293 and HepG2 cells. In vivo experiments were performed in C57BL/6 and NOD/scid mice receiving 1010–11 rAAV/hFVIII particles via portal vein injection. All C57BL/6 mice tested developed anti-hFVIII antibody. In contrast, NOD/scid mice expressed hFVIII reaching 27% of normal human plasma levels. As expected, we could not detect hFVIII antigen from plasma samples isolated from control animals receiving equivalent doses of rAAV expressing enhanced green fluorescent protein (EGFP). Transgene mRNA expression was detected primarily in the liver and histologic analysis of the liver revealed no pathologic abnormalities. These results demonstrate a promising approach for treatment of hemophilia A.
Hemophilia A is an inherited sex-linked bleeding disease, resulting from deficiency of coagulation factor VIII (FVIII). Hemophilia A comprises the majority of hemophilia patients (80%) with an incidence of 1 in 5 to 10 000 live males births.1Hemophilia patients suffer from spontaneous bleeding into the large joints and soft tissue, and are at risk for intracranial hemorrhage. Recurrent episodes of joint bleeding are the most frequent manifestation of the disease, leading to crippling arthropathy, particularly in severely affected patients. Currently, treatment for hemophilia A uses the infusion of either plasma-derived or recombinant FVIII protein for bleeding episodes. Prophylactic infusions of FVIII concentrates in children significantly impact the frequency of bleeding episodes and subsequent joint disease.2,3 The short half-life of FVIII (t1/2 12 hours) and the high cost of purified FVIII products make life-long prophylactic FVIII infusion impractical.4 5
Gene therapy is an attractive alternative for the treatment of hemophilia A patients. Persistent expression of hFVIII at therapeutic levels (more than 5% of normal) would make a profound impact on treatment of hemophilia A patients. Both retroviral and adenoviral vectors have been used to deliver FVIII cDNA targeting the liver.6-8 Moloney murine leukemia virus (MoMLv) amphotropic vectors suffer from poor transduction of postmitotic cells.6 Adenovirus carrying the human FVIII cDNA directed to the liver express high-level FVIII in animal models. However, expression wanes with time because of the well-characterized cell-mediated immune response to the vector.8 9
Adeno-associated virus (AAV) is a nonpathogenic defective parvovirus capable of infecting a broad range of mitotic or postmitotic cells.10 Recombinant AAV (rAAV) has been shown capable of targeting liver cells to express functional FIX gene persistently in a large animal model,11 in which FVIII and FIX are synthesized.12,13 A disadvantage of rAAV vectors is restricted packaging capacity.14 Wild-type (wt) AAV is a 4.6 kilobase (kb) linear single strand DNA virus. The transgene size for optimal rAAV packaging is roughly wt size.14 The full-length FVIII cDNA is more than 7 kb, too large for efficient packaging in rAAV. The human FVIII (hFVIII) gene is comprised of a central B domain flanked by the amino A1 and A2 domains and carboxyl A3, C1, and C2 domains. The B domain can be deleted without any significant effect on specific procoagulant activity.15B-domain–deleted hFVIII cDNA (BDD-hFVIII) is 4.4 kb and not thought feasible for testing in rAAV.15 Here we report the generation of high titer rAAV that mediates long-term, therapeutic levels of hFVIII in vivo.
Materials and methods
Vector constructs
rAAV plasmids expressing hFVIII or enhanced green fluorescent protein (EGFP) were constructed. Briefly, pmt2LA15 (gift from D. Pittman, Genetics Institute, Andover, MA) was amplified by polymerase chain reaction (PCR) to generate a 4435 basepair (bp) fragment encoding full sequence of BDD-hFVIII. The 4435 bp BDD-hFVIII cDNA was inserted into a cassette containing either the herpes simplex thymidine kinase (Tk) promoter (pDLZ2) or the Tk promoter linked to Enhancer I of hepatitis B virus (gift from Dr J. H. Ou, University of Southern California, CA) (pDLZ6).16 The BDD-hFVIII cDNA in pDLZ6 was replaced with EGFP cDNA from pTR-EGFP (R. Haberman, UNC Gene Therapy Center, unpublished data) to construct pDLZ8. All constructs use the herpes simplex thymindine kinase polyadenylation signal, and flanked using the rAAV ITRs from pAAV/cFIX.17
Cells and culture
The 293, HeLa, and HepG2 cells were cultured in Dulbecco's modified Eagle's media (DMEM, Gibco/BRL, Gaithersburg, MD) with 10% fetal bovine serum (FBS, Gibco/BRL), with or without antibiotics (penicillin and streptomycin), at 37°C and 5% CO2. FBS was heat inactivated at 55°C for 30 minutes. Under these conditions, FVIII antigen and activity could not be detected in FBS.
Recombinant adeno-associated virus production and purification
rAAV was generated using a 3-plasmid transfection scheme.17 Briefly, subconfluent 293 cells were cotransfected with the rAAV vector plasmid, AAV-helper plasmid pX x 2,18 and adenovirus helper plasmid pX x 6 using calcium phosphate precipitation. Forty-eight hours after transfection, the cells were harvested, lysed by 3-cycles of freeze-thawing, and sonicated to release the rAAV virion particles. After ammonium sulfate precipitation, the virus particles were purified and concentrated by cesium density gradient centrifugation twice. Viral particles were titered by dot blot; the rAAV/hFVIII peak gradient fractions were pooled, dialyzed against phosphate buffer saline (PBS), and stored at −20°C. Wild-type AAV by-production was estimated by dot blot hybridization at the level of 0.3%. rAAV replication assay was performed as described.19
In vitro expression of B-domain–deleted human factor VIII
Two × 105 of 293 or HepG2 cells were plated in each well of 6-well plates. Twenty-four hours after plating, cells were transduced with 1 × 103 rAAV virus particles per cell (MOI = 10), with or without adenovirus (MOI = 1) for 1 hour. The cell media was harvested for analysis and replaced with fresh media every 24 hours after infection. All the media/serum used for assaying hFVIII expression and function were screened free of FVIII.
Antigen, function, and inhibitor assay for human factor VIII
rAAV-originated hFVIII antigen was detected by enzyme-linked immunosorbent assay (ELISA) similar to previously described.17 Briefly, monoclonal sheep anti-hFVIII antibody (Affinity Biological, Hamilton, Ontario, Canada) was used as capture antibody. Peroxidase-conjugated sheep anti-hFVIII antibody (Affinity Biological) was used as a secondary antibody. The FVIII levels were calculated according to the standard curve derived from serial dilution of the pooled normal human plasma (UCRP, Fisher Scientific, Huntersville, NC) diluted either in sodium barbital buffer or C57Bl/6 plasma. The reproducible sensitivity of the ELISA for hFVIII was determined to be 0.3 ng/mL.
Function of the rAAV-originated BDD-hFVIII was tested by the activated partial thromboplastin time (APTT) and Coatest (Chromgenix AB, Milan, Italy). APTT was performed in a similar manner as previously described,17 except using FVIII-deficient plasma rather than FIX-deficient plasma (Pacific Hemostasis, Huntersville, NC). Coatest was performed according to manufacturer's instructions. A serial dilution of pooled normal human plasma was used to generate the standard curve of FVIII activity.
The Bethesda inhibitor assay (BIA)20 was used to detect anti-hFVIII inhibitors in mice serum. Briefly, the mice plasma was incubated at 55°C for 30 minutes to inactivate endogenous murine FVIII. The serial dilution of the treated mice plasma was then mixed with an equal volume of pooled normal human plasma (UCRP, Fisher Scientific) and incubated at 37°C for 2 hours. APTT was performed to determine the residual FVIII activity in the UCRP incubated with the inactivated mice plasma. The anti-hFVIII inhibitor titer was calculated from the residual FVIII activity of each sample, according to the established BIA standard curve.
Animal care and manipulation procedure
The C57BL/6 and non-obese diabetic severe combined immunodeficient (NOD/scid) mice were maintained at the animal facilities at the University of North Carolina at Chapel Hill in accordance with the guidelines of the UNC institutional Animal Care and Use Committee. Each animal was weighed and sedated using a mixture of ketamine (100 mg/kg) and xylanine (5 mg/kg) before virus administration. Under a dissecting microscope, a 1-cm vertical midline abdomen incision was made. 2 × 1010 or 2 × 1011 particles of rAAV/DLZ6 or rAAV/DLZ8 in 200 to 400 μL PBS was injected to liver via portal vein using Harvard Apparatus pump 22 in 2 to 5 minutes. Blood was collected via the retro-orbital plexus, and the plasma was stored at −80°C. Tissues/organs were collected for histology and DNA/RNA analyses from 3 C57BL/6 mice sacrificed at week 30 after injection. Tissues collected included liver, spleen, kidney, testis, heart, brain, spinal cord, intestine, muscle, lymph nodes, and bone marrow. Tissue were either frozen at −80°C (for DNA and RNA isolation) or fixed in 10% neutral-buffered formalin overnight before processing.
DNA isolation and analysis
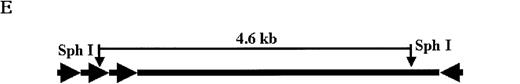
High molecular weight genomic and low molecular weight DNA (Hirt) were isolated and used for Southern blot and DNA PCR as previously described.17 The 29.5 pg, 5.9 pg, 1.18 pg, 0.118 pg, and 0.059 pg of plasmid pDLZ6 were added to 20 μg genomic DNA from control mouse liver produced copy number standard, respectively equivalent to 5, 1, 0.2, 0.02, and 0.01 copies of rAAV/DLZ6 vector genome per murine liver cell. The genomic DNA was digested with restriction enzyme SphI, which cuts the plasmid pDLZ6 internal to each ITR, releasing a 4.6-kb DLZ6 genome, and was separated by agarose gel. The blot was hybridized with 32P-labeled hFVIII probes.
Primer: 5′-AACCTTTACCCCGTTGCTCG-3′ (sense) and 5′-GTCTTTTTGTACACGACTGAGG-3′ (antisense) were used to amplify a 450-bp rAAV/DLZ6 vector unique fragment. The PCR conditions are 95°C for 5 minutes, followed by 30 cycles with 95°C for 2 minutes, 50°C for 1 minute, and 72°C for 1 minute.
RNA extraction, Northern blot, and reverse transcription polymerase chain reaction
Total cellular RNA was extracted from cultured cells or frozen mice tissues were used for Northern blot or reverse transcription (RT)-PCR in a similar manner as previously described.17 The pair of primers (sense primer: 5′-TTCTCCCCAATCCAGCTGG-3′, antisense primer: 5′-GAGTTATTTCCCGTTGATGG-3′) to amplify 534-bp unique hFVIII cDNA fragment were performed at 95°C for 2 minutes, followed with 30 cycles using 95°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute. A pair of β-actin primers was used as an internal control of RT/PCR for each sample.17
Histologic analysis
Formalin-fixed tissues were alcohol-dehydrated and paraffin-embedded. Tissues were sectioned at 6 μm each, deparaffinized in xylene, rehydrated through graded ethanol, and stained with hematoxylin and eosin (H & E) as described previously.17
Results
Packaging of recombinant adeno-associated virus B-domain–deleted human factor VIII
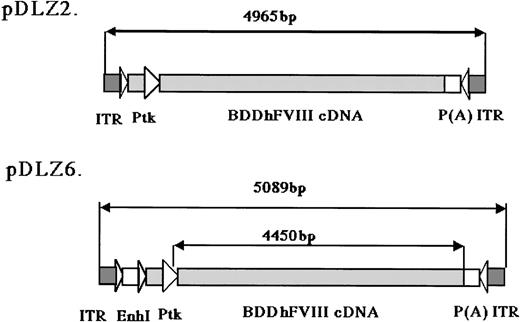
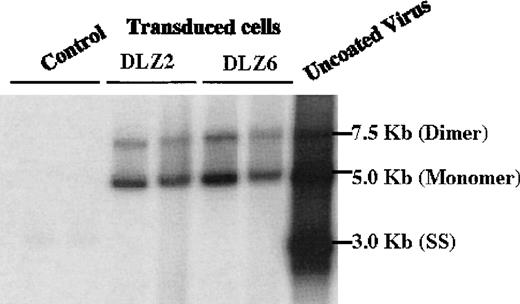
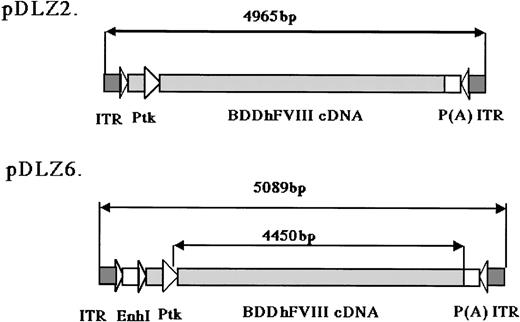
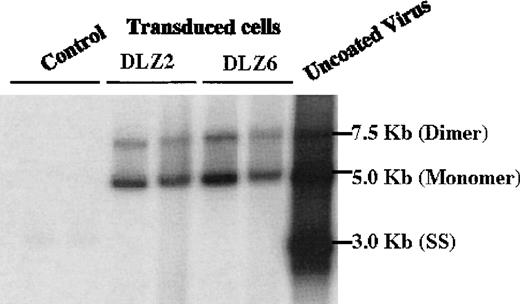
2 rAAV vectors expressing BDD-hFVIII, pDLZ2, and pDLZ6 (Figure1) were constructed to test the use of the herpes TK promoter and hepatitis B virus Enhancer I (EnhI) element. These elements were chosen because of their size (approximately120 bp) and apparent specificity for hepatocyte transcription.16 More than 1012/mL rAAV/DLZ6 or rAAV/DLZ2 particles were produced using triple plasmid transfection and cesium chloride density gradient centrifugation. To confirm the replication of rAAV virions, low molecular weight viral DNA was isolated after transduction of HeLa or HepG2 cells with rAAV (MOI = 10), adenovirus type 5 (MOI = 1), and wt AAV. As shown in Figure 2, the expected monomer and dimer replication forms of rAAV/DLZ6 and rAAV/DLZ2 were detected using a probe specific for the transgene. Isolation of rAAV/DLZ6 virion DNA confirmed that the expected monomer size was packaged (Figure 2). After transduction, rAAV/DLZ6 containing the Tk/EnhI sequence produced a 30-fold increase in mRNA transcript in HeLa and HepG2 compared with rAAV lacking the enhancer element (data not shown). On the basis of these results, we performed FVIII functional assays using vector derived from pDLZ6. hFVIII protein expression was performed by ELISA measurement of FVIII antigen from cell media harvested at 24 hours after transfection and transduction. Assessment of functional hFVIII was performed using APTT and Coatest assays (Table1). Thus, despite its greater than wt size, recombinant virus was efficiently packaged and produced functional BDD-hFVIII. On the basis of these results, rAAV/DLZ6 was used for in vivo analysis.
rAAV/BDD-hFVIII constructs.
The maps for the 2 rAAV constructs expressing BDD-hFVIII are shown: pDLZ2 (4965 bp including 2 ITRs, 107% of wt-AAV) and pDLZ6 (5089 bp including 2 ITRs, 109% of wt-AAV). ITR, AAV inverted terminal repeat; EnhI, Enhancer I of the HBV; Ptk, TK promoter of the HSV; and P(A), herpes simplex thymidine kinase polyadenylation sequence.
rAAV/BDD-hFVIII constructs.
The maps for the 2 rAAV constructs expressing BDD-hFVIII are shown: pDLZ2 (4965 bp including 2 ITRs, 107% of wt-AAV) and pDLZ6 (5089 bp including 2 ITRs, 109% of wt-AAV). ITR, AAV inverted terminal repeat; EnhI, Enhancer I of the HBV; Ptk, TK promoter of the HSV; and P(A), herpes simplex thymidine kinase polyadenylation sequence.
Replication and package of rAAV/BDD-hFVIII.
Low molecular weight DNA (Hirt DNA) was isolated from rAAV/DLZ2, DLZ6 and DLZ8 (control) transduced HeLa and HepG2 cells, separated by agarose gel, and probed with BDD-hFVIII cDNA. From right to left: Control Lane, 1-HepG2+ rAAV/DLZ8; 2-HeLa + rAAV/DLZ8; DLZ2: 1-HeLa + rAAV/DLZ2; 2-HepG2 + rAAV/DLZ2; DLZ6: 1-HeLa + rAAV/DLZ6; 2-HepG2 + rAAV/DLZ6; and uncoated rAAV/DLZ6 virion DNA.
Replication and package of rAAV/BDD-hFVIII.
Low molecular weight DNA (Hirt DNA) was isolated from rAAV/DLZ2, DLZ6 and DLZ8 (control) transduced HeLa and HepG2 cells, separated by agarose gel, and probed with BDD-hFVIII cDNA. From right to left: Control Lane, 1-HepG2+ rAAV/DLZ8; 2-HeLa + rAAV/DLZ8; DLZ2: 1-HeLa + rAAV/DLZ2; 2-HepG2 + rAAV/DLZ2; DLZ6: 1-HeLa + rAAV/DLZ6; 2-HepG2 + rAAV/DLZ6; and uncoated rAAV/DLZ6 virion DNA.
Long-term expression of human factor VIII in mice
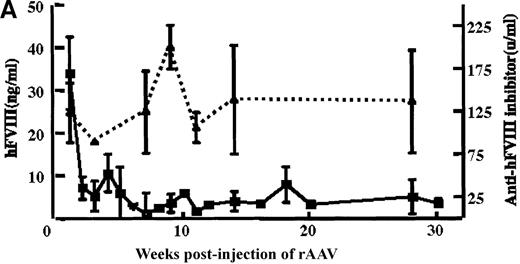
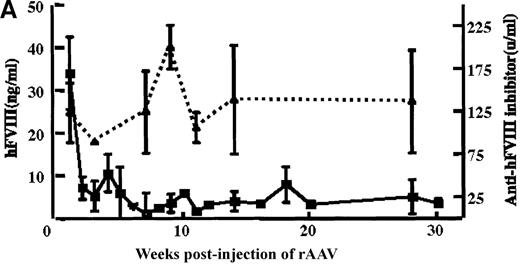
rAAV/DLZ6 was injected into the portal vein of 4-week-old male C57BL/6 mice or 6-week-old NOD/scid mice. Blood samples were collected via the retro-orbit plexus biweekly. BDD-hFVIII antigen was not detected in the plasma of 2 mice receiving 2 × 1010rAAV/DLZ6 until 4 weeks after injection of the AAV(data not shown). Once detected, the hFVIII levels remained at 2% to 3% of normal human levels FVIII (normal hFVIII level = 200 ng/mL) for more than 11 months. In contrast, a mean of 42 ng/mL of BDD-hFVIII or 21% of normal level of human was detected in the plasma of 4 mice receiving 2 × 1011 rAAV/DLZ6 at 1 week after injection (Figure 3A). High titer anti-hFVIII inhibitor was detected in the plasma of all of the mice receiving rAAV/DLZ6 as early as 1 week after injection (Figure 3A). The anti-hFVIII inhibitor titer increased to a maximum titer at 9 to 12 weeks after injection (Figure 3A). The appearance of inhibitor coincided with the decrease in BDD-FVIII plasma antigen. As expected, we could not detect either BDD-hFVIII or anti-hFVIII inhibitor in the plasma of control mice receiving rAAV expressing the EGFP transgene (data not shown).
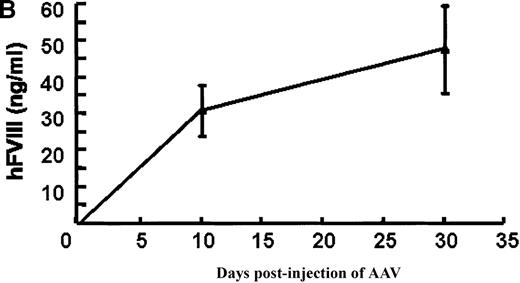
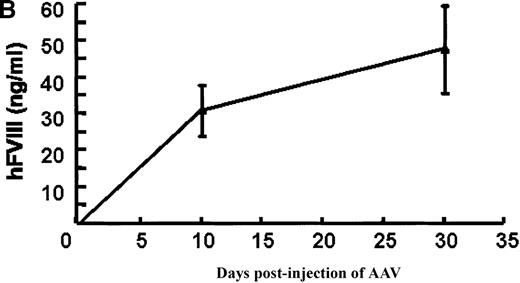
In vivo expression of rAAV/BDD-hFVIII in mice.
Purified rAAV/DLZ6 virus was administered to the C57BL/6 mice via the portal vein. ELISA was used to determine hFVIII level in the plasma and BIA was used to measure anti-hFVIII inhibitor titer. A) BDD hFVIII antigen level and anti-hFVIII inhibitor titer in the plasma of the mice (n = 4) receiving 2 × 1011 rAAV/DLZ6. B) BDD-hFVIII antigen measurement of NOD/scid mice (n = 4) receiving 1.5 × 1011 rAAV/DLZ6. Solid line: hFVIII antigen level; dashed line: anti-BDD-hFVIII inhibitor titer. Human FVIII ELISA standard curve was performed using pooled normal human plasma diluted in either barbital buffered saline or mouse plasma.
In vivo expression of rAAV/BDD-hFVIII in mice.
Purified rAAV/DLZ6 virus was administered to the C57BL/6 mice via the portal vein. ELISA was used to determine hFVIII level in the plasma and BIA was used to measure anti-hFVIII inhibitor titer. A) BDD hFVIII antigen level and anti-hFVIII inhibitor titer in the plasma of the mice (n = 4) receiving 2 × 1011 rAAV/DLZ6. B) BDD-hFVIII antigen measurement of NOD/scid mice (n = 4) receiving 1.5 × 1011 rAAV/DLZ6. Solid line: hFVIII antigen level; dashed line: anti-BDD-hFVIII inhibitor titer. Human FVIII ELISA standard curve was performed using pooled normal human plasma diluted in either barbital buffered saline or mouse plasma.
To adequately assess the expression of BDD-FVIII protein, immuno-incompetent NOD/scid mice received 1.5 × 1011virus via portal vein injection. Plasma levels of BDD-hFVIII determined by ELISA reached 35 ng/mL (17% of normal level) on day 10 after injection and increased to 55 ng/mL (27% of normal level) (Figure 3B). As expected, we did not detect BDD-hFVIII in the plasma of mock infected scid mice (data not shown).
Recombinant adeno-associated virus vector spread and histologic analysis
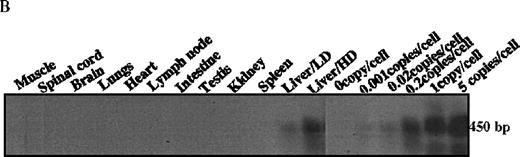
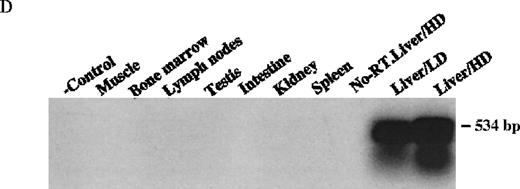
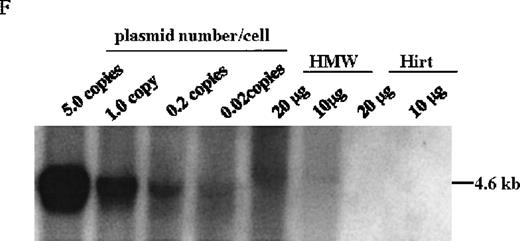
The mice receiving rAAV vector were killed 30 weeks after injection. Peripheral blood, liver, spleen, lymph nodes, kidney, intestine, testis, skin, muscle, heart, lungs, aorta, bone marrow, brain, and spinal cord were analyzed to determine vector spread after intraportal administration. DNA PCR using primer pairs specific for the vector DLZ6 amplified a 450-bp product. Vector genome was detected only from liver samples 30 weeks after portal vein injection (Figure4A and B). RT-PCR used a pair of primers that amplify a 534-bp of BDD-hFVIII cDNA fragment. Only RNA isolated from the liver generated the appropriate PCR product, confirming the DNA PCR result (Figure 4C and D). Amplification of a 250-bp β-actin fragment was used as internal control for RT/PCR. Equal amounts of RNA were used for each sample (data not shown). By using both DNA PCR and Southern blot analysis, we estimate 0.05 copies of rAAV/DLZ6 genome per cell at 30 weeks after transduction in C57Bl/6 animals given 2 × 1011 rAAV particles (Figure 4B and F). This result is in agreement with previous reports.11 21We did not observe any significant pathology in the liver, spleen, gastrointestinal tract, gonads, brain, heart, and lungs (data not shown).
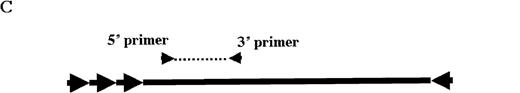
Molecular analysis of the mice receiving injection of rAAV/DLZ6.
A. Diagram of primers designed for detection of rAAV DNA. B. Distribution of rAAV in mice following portal vein injection. A unique 450 bp fragment of rAAV/DLZ6 was amplified to detect distribution of rAAV after hepatic injection. DNA isolated from brain, spinal cord, muscle, bone marrow, heart, lung, testis, lymph node, kidney, intestine, and spleen were analyzed following sacrifice of a mouse receiving high-dose rAAV/DLZ6. Liver/LD and Liver/HD refer to DNA PCR analysis of animals receiving low- and high-dose rAAV/DLZ6. Standard curve 5, 1, 0.2, 0.1, 0.01, and 0 genome copy equivalents of plasmid pDLZ6 diluted with genomic mouse DNA. C. Diagram of the primers designed for RT/PCR. D. RT-PCR analysis of total RNA isolated from control and experimental animals. Primers were designed to amplify a 534-bp BDD-hFVIII specific fragment. –RT control employed RNA isolated from the mouse liver receiving high-dose rAAV/DLZ6. The negative control used RNA isolated from control animal. RNA samples of muscle, brain, lymph nodes, testis, kidney, and spleen were from the mouse receiving high-dose rAAV/DLZ6. LD: liver RNA isolated from mouse receiving low-dose AAV/DLZ6. HD: liver RNA isolated from mouse receiving high-dose rAAV/DLZ6. E. Sph I digestion of Diagram of the restriction digestion using Sph I. F. Southern blot analysis of high molecular weight genomic DNA and Hirt DNA isolated from experimental animals. Standard curve: genomic DNA from control mouse liver with 5, 1, 0.2, and 0.02 genome copy equivalents of plasmid pDLZ6 per cell, respectively. HMW genomic DNA and low molecular wt liver DNA (HIRT) isolated from animals receiving high-dose rAAV/DLZ6.
Molecular analysis of the mice receiving injection of rAAV/DLZ6.
A. Diagram of primers designed for detection of rAAV DNA. B. Distribution of rAAV in mice following portal vein injection. A unique 450 bp fragment of rAAV/DLZ6 was amplified to detect distribution of rAAV after hepatic injection. DNA isolated from brain, spinal cord, muscle, bone marrow, heart, lung, testis, lymph node, kidney, intestine, and spleen were analyzed following sacrifice of a mouse receiving high-dose rAAV/DLZ6. Liver/LD and Liver/HD refer to DNA PCR analysis of animals receiving low- and high-dose rAAV/DLZ6. Standard curve 5, 1, 0.2, 0.1, 0.01, and 0 genome copy equivalents of plasmid pDLZ6 diluted with genomic mouse DNA. C. Diagram of the primers designed for RT/PCR. D. RT-PCR analysis of total RNA isolated from control and experimental animals. Primers were designed to amplify a 534-bp BDD-hFVIII specific fragment. –RT control employed RNA isolated from the mouse liver receiving high-dose rAAV/DLZ6. The negative control used RNA isolated from control animal. RNA samples of muscle, brain, lymph nodes, testis, kidney, and spleen were from the mouse receiving high-dose rAAV/DLZ6. LD: liver RNA isolated from mouse receiving low-dose AAV/DLZ6. HD: liver RNA isolated from mouse receiving high-dose rAAV/DLZ6. E. Sph I digestion of Diagram of the restriction digestion using Sph I. F. Southern blot analysis of high molecular weight genomic DNA and Hirt DNA isolated from experimental animals. Standard curve: genomic DNA from control mouse liver with 5, 1, 0.2, and 0.02 genome copy equivalents of plasmid pDLZ6 per cell, respectively. HMW genomic DNA and low molecular wt liver DNA (HIRT) isolated from animals receiving high-dose rAAV/DLZ6.
Recombinant adeno-associated virus molecular analysis in liver cells
At the time of sacrifice, 30 weeks post-injection, low molecular weight DNA (Hirt DNA) and high molecular weight genomic DNA were isolated from livers of mice receiving rAAV/DLZ6. By using the restriction enzyme Sph I, which cuts internal to each ITR, and Southern blotting, we could detect unrearranged rAAV/DLZ6 genome only in the high molecular weight fraction (Figure 4E and F). Approximately 0.05 vector genome copies per cell were detected in the high molecular weight DNA fraction. DNA PCR confirmed that the rAAV/DLZ6 vector genome signal could not be detected in the Hirt DNA fraction (data not shown). The sensitivity of the PCR assay is 0.001 copies per cell.
Discussion
Recombinant AAV has several unique characteristics making it suitable as a gene delivery vector for hemophilia. rAAV has a broad host cell range that includes the liver,11 the target organ for hemophilia A. Persistent gene expression is achieved most readily in quiescent cells in which the conversion of single- to double-strand AAV forms may remain as episomes, form large repeating concatemers, or integrate into the host cell genome. Purified rAAV preparations are not pathogenic; the host response to rAAV is restricted to a humoral response elicited to AAV capsid proteins.10,17 rAAV vector is capable of transducing primary liver and skeletal muscle cells providing long-term (more than 12 months) FIX expression in vivo.11,17 22
rAAV vectors are limited in terms of the size of the transgene they can package. rAAV packaging and efficiency of transgene expression decreases dramatically when the rAAV/transgene expression cassette reaches 5000 bp or greater.14 This is thought to reflect inefficient packaging of single-strand forms with capsid proteins. Whether this is due to physical limitation of virion DNA insertion into preformed capsids coats is not clear. Despite this potential packaging limitation, we showed that the FVIII cDNA is packaged and expresses functional hFVIII. To do this we used the B-domain–deleted FVIII cDNA previously shown to maintain coagulant activity equivalent to full-length hFVIII. Indeed, the results of BDD-hFVIII in hemophilia A clearly demonstrate procoagulant efficacy equivalent to recombinant factor FVIII.23 Because of AAV packaging size constraints, we used small transcriptional elements previously shown to be liver-specific.16 The use of novel, small liver-specific transcriptional cassettes along with the expansion of rAAV packaging capacity should facilitate a higher level of FVIII production.
To produce high titer rAAV virions for in vivo use, we used a 3-plasmid transient transfection system. The system uses a truncated adenoviral construct that expresses only the ancillary proteins required for AAV replication, a helper plasmid that encodes for AAV rep and cap proteins, and a vector plasmid that encodes for the transgene.18 More than 1012/mL rAAV particles were routinely produced using this system. A functional assay (Coatest) confirmed that the rAAV-transduced cells expressed BDD-hFVIII. As expected, the construct incorporating the hepatitis B EnhI element produced more FVIII per rAAV particle. Previous work described the incorporation of small RNA regulatory elements driving human B-domain FVIII expression; however, the rAAV titer was significantly lower than observed in this report (107 vs 1013particles/mL).24 We believe this relatively low titer virus production highlights the difference in vector packaging schemes. However, we cannot rule out the possibility that differences in packaging are related to intrinsic sequence differences.
rAAV/BDD-hFVIII was injected via the portal vein of C57Bl/6 mice in a dose-dependent fashion. As expected, the amount of detectable BDD-hFVIII in the plasma was increased with the dose. Plasma levels of hFVIII were detected at 4 weeks in the animals receiving 1010 particles and remained constant at a level of 1% to 2% of normal human factor VIII up to 11 months. At a dose of 1011particles, hFVIII could easily be detected by 1 week after injection. A significant drop in circulating FVIII in C57BL/6 plasma coincided with the detection of high titer FVIII inhibitor. This result was expected due to the known development of anti-hFVIII antibodies in C57Bl/6 mice, after repeated administration of hFVIII.25Inhibitor may influence antigen levels either by decreasing or increasing clearance,26 making any assessment of hFVIII production difficult. To delineate hFVIII, expression studies in immunodeficient mice were performed. rAAV-mediated hFVIII protein gradually increases in the plasma with kinetics similar to liver-specific human α-1 antitrypsin21 and human factor IX.27 Plasma FVIII levels in immunodeficient mice reached ∼55 ng/mL, which is equivalent to 27% of normal human factor VIII levels.
The development of antibody to hFVIII protein is variable in FVIII knockout mice.8,28 This suggests that the rate and amount of protein production are important to antibody development. Supratherapeutic levels of hFVIII (5-10 × normal) produced after systemic adenovirus administration may facilitate ‘desensitization’ and immune tolerance.29,30 Because 10% to 20% of severe hemophilia A patients develop inhibitors,31 further examination of inhibitor development after rAAV-mediated FVIII gene transfer in both small and large hemophilia A animal models are necessary.
The rAAV transgene was detected solely in the liver at a level of 5%, consistent with previous reports.11,21 Therefore, improvements in rAAV transduction of liver hepatocytes would be expected to significantly increase production of hFVIII. We found that rAAV-mediated hFVIII secretion from liver occurs in a dose-dependent fashion. The use of a higher vector dose may be sufficient for achieving therapeutic levels needed for clinical trials. However, rAAV interaction with hepatocytes has not been carefully delineated. The onset of circulating hFVIII detected within 1 week may reflect the rapid molecular conversion of single strand AAV to double stranded forms.19,32 33
In conclusion, rAAV can efficiently package and express therapeutic levels of human B-domain–deleted FVIII. Targeted transduction of the liver yields persistent high-level production of circulating factor. Functional assessment of human factor VIII will require further testing in hemophilia A animal models. This data suggests that rAAV can be used for the treatment of hemophilia A.
Acknowledgments
We thank Dr Jing-Hsiung Ou at the University of Southern California for providing us with the plasmid puc-HBV1 containing the Enh I of the human hepatitis B virus, and Ms Debra Pittman at Genetic Institute Inc for providing plasmid pmt2LA.
Supported by NIH RO1 DK54,419. C.E.W. is a recipient of the Lucille Markey Trust.
Reprints:Christopher Walsh, UNC Gene Therapy Center, Room 7101 Thurston Building, CB #7352, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: cwalsh@med.unc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.