Although eosinophilic granulocytes are frequently observed in lymphatic tissue of Hodgkin's patients, no substantial data reveal the prognostic role, if any, of tissue eosinophilia. Thus, eosinophilia was analyzed histologically in 1511 diagnostic biopsy specimens of patients treated under protocol therapy of the German Hodgkin's Lymphoma Study Group between 1988 and 1994. Prominent eosinophilia was seen in 38% of cases, which differed among the histologic types of Hodgkin's disease (HD): none in lymphocyte predominant, 14% in lymphocyte rich classical, 40% in nodular sclerosis grade 1 (NS-1), 55% in nodular sclerosis grade 2, 43% in mixed cellularity (MC), and 54% in lymphocyte depleted. In a multivariate analysis, tissue eosinophilia proved to be the strongest prognostic factor for freedom from treatment failure (P < .001) and overall survival (P < .001) in a stage-stratified model. Among NS-1 patients, the effect was highly significant. In MC, no significant effect of eosinophilia on survival could be demonstrated. Eosinophils secrete CD30 ligand that is capable of binding to CD30 positive HD cells. The activation of TRAF2, followed by NF-kappaB, which occurs on CD30L/CD30 binding, may explain the neoplastic proliferation and apoptosis protection of HD cells. TRAF2 is also activated by EBV-LMP expression, which is detectable in the majority of MC but not NS cases. In addition to the possibility that eosinophils are only passive indicators for other unknown prognostic determinants, it may be concluded that the positive clinical outcome of eosinophilia-negative NS cases could be due to lower NF-kappaB activity.
Hodgkin's disease (HD) is generally considered to have a favorable outcome under modern therapy strategies for many patients, but it remains important for pathologists and oncologists to establish reliable prognostic factors. If a low-risk group could be identified on the basis of histologic features at presentation, these patients would benefit from less aggressive therapy, thus reducing toxic side effects and the rate of therapy-related secondary neoplasias. Conversely, a high-risk population could be used to assess the effectiveness of an intensified therapy in reducing the rate of relapses. Furthermore, prognostic factors may suggest important pathophysiologic mechanisms of HD.
Recently, a large study has established a prognostic score for patients with advanced HD, including the factors serum albumin, hemoglobin, sex, stage IV, age, white cell count, and lymphocyte count into the final model.1 Histomorphologic parameters as prognostic factors have also often been the subject of investigation in HD because of the broad heterogeneity in its histologic appearance. Improvements in treatment have reduced the significant differences in life expectancy between the histologic subgroups as reported elsewhere.2-6
Histology of nodular sclerosis grade 2 (NS-2), as well as CD15 negativity of the malignant cells, are independent prognostic factors for an unfavorable outcome in classical HD.7-10 The relevance of tissue eosinophilia was suggested in an early report.11 In a more recent study presenting 26 cases with prominent eosinophilia, a worse relapse-free interval was suggested, although data about the overall survival were omitted.12 Several other studies failed to show any significance between eosinophilia and survival.13-16 In certain malignancies other than HD, tissue eosinophilia predicts a more favorable outcome.17 18
Interestingly, a study investigating the blood eosinophilia in HD found a worse relapse-free survival, despite reporting a better overall survival for the eosinophilic patients.19
To investigate the role of tissue eosinophilia in patients with HD, we have assessed this parameter in a large series of patients enrolled in the prospective trial of the German Hodgkin's Lymphoma Study Group (GHLSG). The results were correlated with blood eosinophilia, all other clinical data available, and especially freedom from treatment failure (FFTF) and overall survival (SV).
Patients and methods
The patients of this study, aged 15 to 75 years, were recruited between 1988 and 1994, treated according to standard protocols (study generation HD4,5,6) and had a maximal observation period of 120 months. Three treatment groups were defined by stage (Ann-Arbor classification) and additional risk factors:
HD4 (early stages): Ann-Arbor stage I A/B or II A/B without any of the following risk factors: bulky tumor, extranodal involvement, massive spleen involvement, increased erythrocyte sedimentation rate (ESR), or involvement of 3 or more lymph node areas. Staging was ascertained by laparotomy for all patients. Treatment given was radiation only, either with 40 Gy extended field (EF) or 30 Gy EF and 10 Gy involved field (IF). No significant differences were observed between the treatment arms (data not shown).
HD5 (intermediate stages): Ann-Arbor stages I or II with 1 or more of the risk factors mentioned previously and all stage IIIA cases. Treatment given was a combined therapy with 2 × COPP-ABVD (or 2 × COPP-ABV-IMEP), followed by radiation (30 Gy EF + 10 Gy to bulky disease). No significant differences were observed between the treatment arms (data not shown).
HD6 (advanced stages): Ann-Arbor stage IIIB and IV. Treatment given was a combined therapy with 4 × COPP-ABVD (or 4 × COPP-ABV-IMEP), followed by 30 Gy to areas with initial bulky disease, slow response to chemotherapy, or residual disease after chemotherapy. No significant differences were observed between the treatment arms (data not shown).
Histopathology
Paraffin sections of the diagnostic lymph nodes from each patient, collected before any therapy, were reviewed by a panel of 4 histopathologists (R. Fischer, K. Hübner, M.L. Hansmann, and A. Georgii) to give unequivocal diagnosis.20 The diagnoses were supported by immunohistochemical analyses for the large majority of cases.9
All confirmed HD cases with sufficient histologic material were reviewed in this study to evaluate tissue eosinophilia. Clinical data and follow-up were available in 1333 patients from 1511 biopsies reinvestigated here.
Tissue eosinophilia was determined independently by 2 of the authors (R.v.W., S.S.) in paraffin sections stained with hematoxylin and eosin. The amount of eosinophils was tabulated semiquantitatively on a scale similar to former studies.13,14 21 The whole slide was screened on low-power magnification for areas with elevated numbers of infiltrating eosinophils. In these areas, 5 randomly selected high-power fields (HPF; 12.5 × 40) were investigated. First, the total number of cells visible within the HPF was estimated, supported by counting the number of cells lying within the squares of an ocular grid representing 22% of the total HPF area. The respective number was multiplied by 4.54 ( = 100%), giving an estimation of the total cell number. In a second step, the number of eosinophils within the total HPF was counted and the approximate percentage of eosinophils could be calculated. Counting was omitted if clusters of eosinophils (10 or more eosinophils touching each other, regardless whether forming a round or irregular figure) were detectable in an HPF, thus speeding up the procedure.
The groups were “A” (no or a few eosinophils, no clusters), “B 1” (moderate increase of eosinophils, approximately > 5% of all cells or clusters in at least 5 HPFs), and “B 2” (heavy infiltration of eosinophils, approximately > 15% of all cells in at least 5 HPFs).
A statistical analysis demonstrated that the subdivision into group B 1 and B 2 was irrelevant for clinical outcome. In detail, the 5-year survival for cases of group A was 91.9% (standard error: 1.0), for group B1 84.7% (1.9), and for group B2 84.3% (3.8). The pairwise P-values for A versus B1 were < .0001 and for B1 versus B2 P = .74. During all further analysis, therefore, only 2 groups were compared: cases with few eosinophils (group A) against cases with tissue eosinophilia (group B 1 and B 2).
Reproducibility of the counting
The overall interobserver reproducibility for estimating the eosinophils semiquantitatively was 88%. The 12% diverging cases were decided unequivocally on a multiheaded microscope. More than 1 year after having finished the work on the microscope, 1 of the authors reinvestigated 10% of the cases with follow-up to estimate the intraobserver agreement. In 125/133 cases (94%), the results were in concordance with the original grading.
Statistical analysis
FFTF was defined as time from initial staging until progression, lack of complete remission at end of therapy, relapse, death from any cause, or date of last information, whichever occurred earliest. For SV, all deaths from whatever cause were counted as events. SV and FFTF were estimated by the Kaplan-Meier method and compared by the log-rank test. The Cox proportional hazards model (multivariate analysis) was applied to assess the predictive value of eosinophilia, allowing for the effect of the following factors: age, gender, Karnofsky index, extranodal disease, LDH (serum lactate dehydrogenase), ESR, Ann-Arbor stage within the treatment group, B-symptoms, histologic subtype (regarding only 2 groups: LP [lymphocyte predominant], LRcHD [lymphocyte-rich classical HD], NS-1 [nodular sclerosis—grade 1] versus MC [mixed cellularity], NS-2 [nodular sclerosis—grade 2]), large mediastinal mass, hemoglobin, albumin, AP [serum alkaline phosphatase], peripheral blood lymphocytes. All analyses were stratified according to treatment groups (HD4, HD5, HD6: see above).
Two procedures were used to assess the prognostic relevance of eosinophilia, allowing for the effects of other factors. First, the Cox proportional hazard regression model was fitted, including all the listed factors both with and without the inclusion of the factor eosinophilia. By comparing the fit of these 2 models (via the difference in log-likelihood), the significance of the influence of eosinophilia on FFTF and SV was assessed. Because of the relatively large number (n = 15) of factors included, many of which had missing values for some patients, a considerable proportion of cases had to be excluded from this analysis.
Second, a (backward) stepwise selection of significant factors, omitting eosinophilia, was performed. Critical P values for inclusion and exclusion of a factor were .05 and .10, respectively. All those factors that were selected for either the FFTF analysis or the SV analysis (or both) were then refitted, using all cases with full data, for both endpoints. Then the factor eosinophilia was added to the model (along with these selected factors) and the significance of eosinophilia assessed using the change in log-likelihood. Because fewer factors were included in this second procedure, fewer cases had to be excluded due to missing values.
Because of biologic considerations that the effect of the eosinophils could be different for different histologic subtypes of HD, separate analyses were also performed for each main subtype where sample size permitted. All factors found significant in the overall analysis (for SV or for FFTF) were fitted before observing the effect of including the factor eosinophilia. Moreover, a full model, including all initially considered factors, was fitted and the effect of adding eosinophilia was observed.
In addition, separate analyses were performed to determine whether the grading of nodular sclerosis (NS) had prognostic significance, allowing for the factor eosinophilia, and whether CD15 status was associated with tissue eosinophilia.7-9
Results
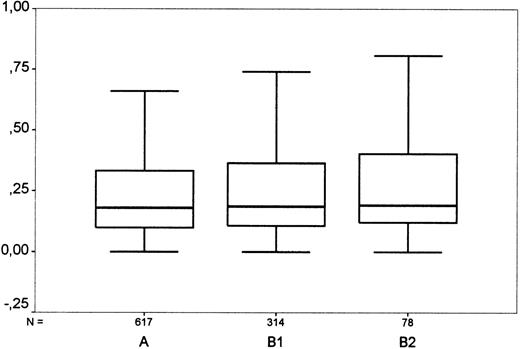
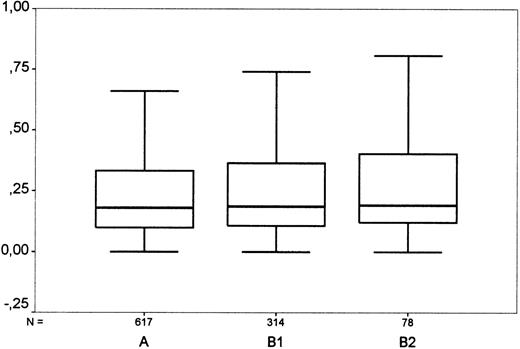
In total, 1511 cases were analyzed for tissue eosinophilia. The prevalence of eosinophilia varied between 0% and 55% among the histologic entities of HD (Table 1). The data suggested, at most, a weak association between blood and tissue eosinophil counts. The Spearman correlation coefficient was r = 0.061 (P = .054) and the nonparametric Kruskal-Wallis test for association between blood and tissue eosinophil counts gave a nonsignificant result (P = .14, comparing tissue eosinophilia groups A with B1 with B2; P = .072, comparing A with [B1+B2]) (Figure 1).
Comparison of blood and tissue eosinophilia in Hodgkin's disease.
“A”: Cases with few eosinophils (< 5% of surrounding cells, no clusters); “B 1”: cases with moderate eosinophilia (5%-15% of surrounding cells or clusters of eosinophils); “B 2”: cases with strong eosinophilia (> 15%). For each group, the diagram shows the highest and lowest values of blood eosinophil count (horizontal bars), the interquartile range (box) in which the middle 50% of values lie, and the median (horizontal line in box). The grouping shows a borderline significance when correlated with the amount of blood eosinophils. Y-axis: Eosinophils in blood (thousand per μL).
Comparison of blood and tissue eosinophilia in Hodgkin's disease.
“A”: Cases with few eosinophils (< 5% of surrounding cells, no clusters); “B 1”: cases with moderate eosinophilia (5%-15% of surrounding cells or clusters of eosinophils); “B 2”: cases with strong eosinophilia (> 15%). For each group, the diagram shows the highest and lowest values of blood eosinophil count (horizontal bars), the interquartile range (box) in which the middle 50% of values lie, and the median (horizontal line in box). The grouping shows a borderline significance when correlated with the amount of blood eosinophils. Y-axis: Eosinophils in blood (thousand per μL).
The analysis of the classical HD cases only showed no significant association between the CD15 status and tissue eosinophilia. Among the CD15 positive cases, 41% showed eosinophilia, and, in the CD15 negative group, the percentage was 34% (chi-square test:P = .09; linear: P = .24). An increased ESR was significantly correlated with tissue eosinophilia as the only clinical factor among the others tested (Table 2).
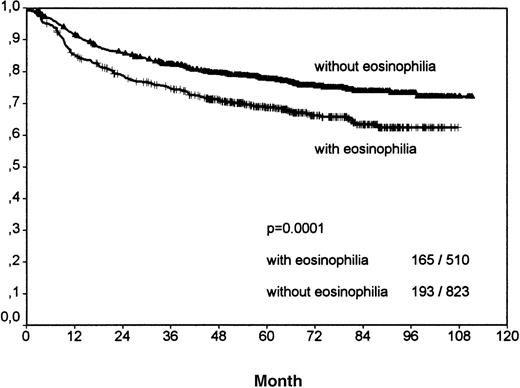
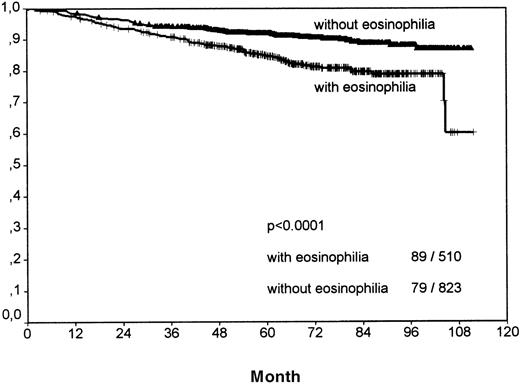
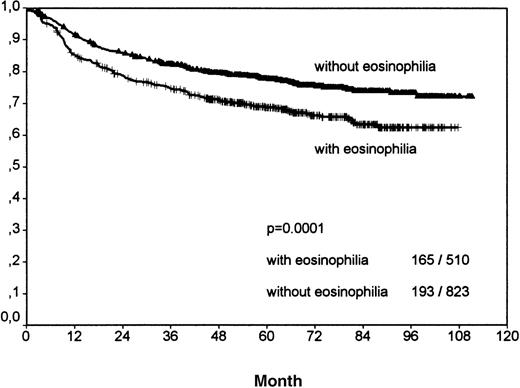
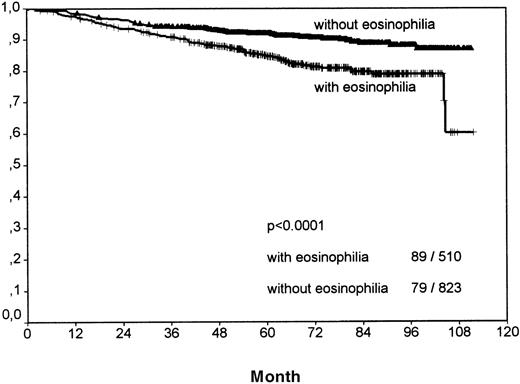
Including all patients and histologic entities, clinical outcome was significantly worse for patients with tissue eosinophilia for both FFTF (P < .001; Figure 2, Table3) and SV (P < .001; Figure3, Table 4). This difference was observed for patients in all stages and for patients above and below 45 years.
Analysis of freedom from treatment failure between all Hodgkin's cases with eosinophilia and without eosinophilia.
The number of events per total number within both groups is indicated in the right bottom. Y-axis: probability.
Analysis of freedom from treatment failure between all Hodgkin's cases with eosinophilia and without eosinophilia.
The number of events per total number within both groups is indicated in the right bottom. Y-axis: probability.
Analysis of overall survival between all Hodgkin's disease cases with eosinophilia and without eosinophilia.
The number of events per total number within both groups is indicated in the right bottom.
Analysis of overall survival between all Hodgkin's disease cases with eosinophilia and without eosinophilia.
The number of events per total number within both groups is indicated in the right bottom.
The British grading of NS cases,7 not including eosinophilia as a criterion in the grading system, showed a significantly better overall survival (P = .0027) for NS-1 versus NS-2 patients in univariate analysis. To test whether this grading had independent prognostic significance from eosinophilia, a comparison of NS-1 and NS-2 stratified for tissue eosinophilia was performed. This testing showed a P-value of .043, indicating that the NS grading seems to have independent prognostic significance from eosinophilia.
Univariate analysis
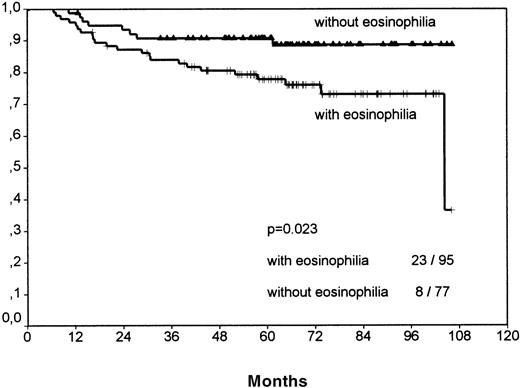
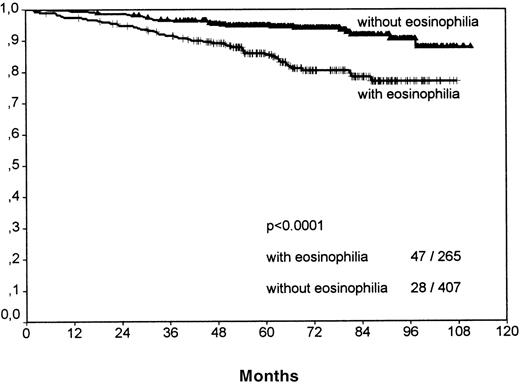
Separate analyses of the histologic subtypes LP, LRcHD, and lymphocyte depleted (LD) were excluded because low numbers would have precluded a meaningful statistical evaluation. Among the 3 most frequent histologic groups of HD, the NS-1 cases showed significant differences for FFTF (P = .0075, no figure shown) and SV (P < .0001, Figure 4) when comparing cases with eosinophilia versus cases without eosinophilia. The smaller subgroup NS-2 revealed for FFTF a borderline value (P = .056, no figure shown) and for SV a significantly worse outcome for patients with eosinophilia (P = .023; Figure5).
Survival analysis for nodular sclerosis grade 1 cases only.
Cases with tissue eosinophilia show a significantly worse outcome.
Survival analysis for nodular sclerosis grade 1 cases only.
Cases with tissue eosinophilia show a significantly worse outcome.
Survival analysis for nodular sclerosis grade 2 cases only.
Cases with eosinophilia show a significantly worse outcome.
Survival analysis for nodular sclerosis grade 2 cases only.
Cases with eosinophilia show a significantly worse outcome.
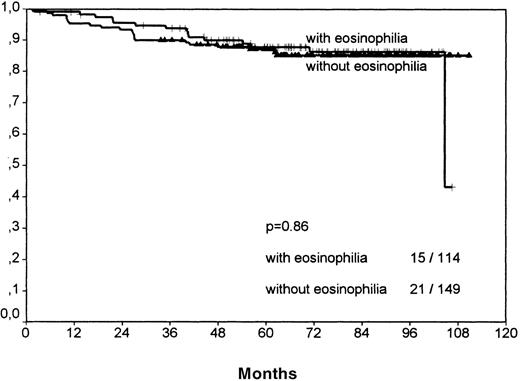
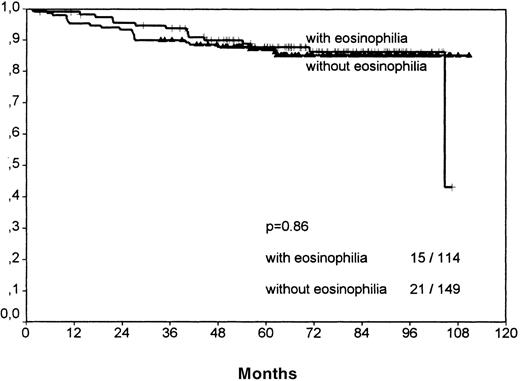
The histologic group MC showed no significant difference between the eosinophilic and the noneosinophilic group, neither for FFTF nor SV (FFTF P = .14, no figure shown; SVP = .86, Figure6).
Survival analysis for mixed cellularity cases only.
No significant difference between cases with and without eosinophilia is observable, in contrast to the results of the nodular sclerosis cases.
Survival analysis for mixed cellularity cases only.
No significant difference between cases with and without eosinophilia is observable, in contrast to the results of the nodular sclerosis cases.
The estimated hazard ratios together with 95% confidence intervals for survival were for NS-1 cases: 2.69 (95%: 1.68-4.29); for NS-2 cases: 2.56 (1.13-5.78); and for MC cases: 1.21 (0.6-2.41). These results suggest that the prognostic effect for survival in MC, if any, is weaker than for that for NS (P = .044).
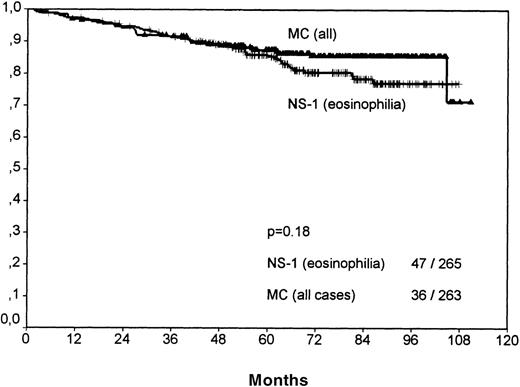
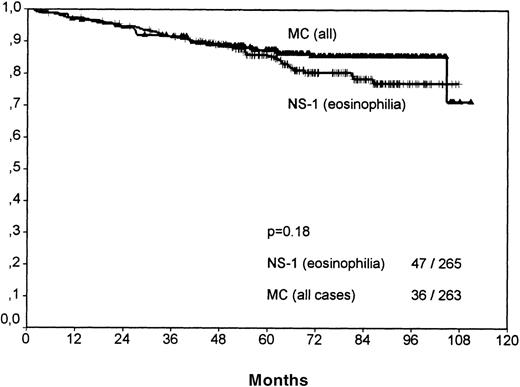
A comparison of NS-1 with MC cases showed that the better clinical outcome of NS-1 patients was based only on those with few eosinophils (SV P = .0033; no figure shown). NS-1 cases with eosinophilia actually showed a very weak trend toward reduced survival when compared with all MC cases; however, this did not reach statistical significance (P = .18, Figure7).
Overall survival for cases of mixed cellularity (all) versus nocular sclerosis grade 1 cases with eosinophilia.
No significant difference is observable. NS-1 cases without eosinophilia have a significantly better outcome than MC cases (P = .003).
Overall survival for cases of mixed cellularity (all) versus nocular sclerosis grade 1 cases with eosinophilia.
No significant difference is observable. NS-1 cases without eosinophilia have a significantly better outcome than MC cases (P = .003).
Multivariate analysis
Adding the factor eosinophilia to the Cox regression model, including all listed factors, significantly improved the model fit for both FFTF and SV (both P < .0001). Because of missing values, only 861 cases could be included in this analysis.
In the second procedure, backward selection (omitting eosinophilia) identified 5 multivariate significant factors (age, gender, Karnofsky index, extranodal disease, and LDH) for FFTF and 3 factors (age, LDH, ESR) for SV. Therefore, for each endpoint, a model, including 6 factors, was fitted to all cases with complete data (1120 cases). Adding the factor eosinophilia to this model significantly improved the model fit for both FFTF and SV (P < .0001). The hazard ratio for FFTF was estimated as 1.60 (95% confidence interval 1.29-1.99) between cases with eosinophilia, compared with those showing no eosinophilia. The corresponding estimated hazard ratio for SV was 1.99 (1.44-2.74). Thus, eosinophilia corresponds to an increase of at least 30% in treatment failure and 40% in mortality per unit time. These results demonstrate that eosinophilia significantly predicts prognosis, allowing for the effects of other prognostic factors.
Table 5 shows summary results of fitting multivariate models to cases with subtype NS and MC, compared with the overall analysis, including all subtypes. For NS cases, eosinophilia is highly significant, allowing for either selected or all other factors, both for FFTF and for SV. For MC cases, eosinophilia has a significant (though not highly significant) effect on FFTF, but the effect on SV is not significant. There were too few cases of the remaining subtypes to model these separately.
Discussion
The results of this histologic analysis present, for the first time, reliable evidence that tissue eosinophilia is a multivariate prognostic factor for the failure-free and overall survival of patients with HD. Tissue eosinophilia in lymph nodes of HD was first noted decades ago but its prognostic relevance remained controversial; whereas most studies addressing this phenomenon did not find a significant correlation with clinical outcome,13-16,22 eosinophilia in peripheral blood was reported to be a positive prognostic indicator.19,23 Some indications of a prognostic role of tissue eosinophilia in HD were discussed by 2 previous reports, both of them saddled with several drawbacks, including small patient numbers (10 and 26 cases, respectively, showing high tissue eosinophilia), omission of a multivariate analysis, or omission of survival data.11 12
Although the reason for tissue eosinophilia is as yet unknown, recent studies have helped to identify multiple factors that act as attracting agents for eosinophils such as IL-5, IgE, PAF, C5a, eotaxin, MCP-2,-3,-4 (monocyte chemotactic proteins), and RANTES.24-26 Among these, IL-5 seems to play an essential role for mobilizing eosinophils from the bone marrow. Moreover, IL-5 synthesis by H/RS cells correlates with eosinophilia,27 an observation that implies that malignant cells may locally promote tissue eosinophilia. Nevertheless, the suggestion of a weak correlation of tissue eosinophilia with elevated blood eosinophil counts (Figure 1) is consistent with the hypothesis that both local and systemic signaling pathways of attraction (by IL-5, eotaxin-1, and others) exist.28-31
There is, however, no clear explanation why only a subset of HD cases shows prominent tissue eosinophilia. The malignant cells may not uniformly produce attracting factors, or unknown cofactors may be responsible for the accumulation. There is evidence to suggest that tissue eosinophilia results from either an increased migration via chemotactic mediators and cytokines/chemokines or by a reduced clearance of eosinophils in the tissue, ie, prolonged life by decreased apoptosis.32,33 IL-5 seems to be an important antideath cytokine responsible for inhibition of programmed eosinophil death.34 35
Our observation that the LP cases did not show eosinophilia is supported by the results of previous studies.36 This finding underlines the point that LP cases of HD represent a distinct entity that is different from classical HD by morphology, immunophenotype, molecular biology, clinical outcome, and presumably chemokine-pathways.5 37-40
Interestingly, although the proportion of eosinophil-rich cases was similar between the NS and MC cases, the clinical significance of eosinophilia was different between these histopathologic categories. Eosinophilia in HD-involved lymph nodes was of prognostic relevance in the NS cases, whereas in MC cases, the effect on survival, if any, was weaker. NS and MC cases together account for more than 80% of HD, and the REAL classification includes them both as “classical HD.” Because these classical types also share an identical immunophenotype, the distinction of histologic classification is presently considered insignificant for clinical purposes. Thus, it is not surprising that most studies investigating eosinophilia in patients with HD failed to detect its prognostic relevance in evaluating aggregated histologic groups of small numbers of cases.13-16 22
It is known, however, that despite many similarities, the histologic subtypes NS and MC differ in several aspects. For example, MC is more frequently associated with detectable Epstein-Barr virus (EBV) infection, the median age of patients with MC is higher, it is more frequently observed in developing countries, and the clinical outcome is supposed to be less favorable than cases classified as NS, especially NS-1.41,42 Moreover, a recent study found eotaxin-1, a potent eosinophil-specific chemoattractant, to be highly expressed only in the NS cases and not in the other morphologic categories of HD.43 TGF-β1-mRNA was found only in cases of NS but not MC, and the major source of TGF-β1, which has an antiproliferative effect on H/RS cells, were the eosinophils.44 This observation has been held responsible for the favorable prognosis of NS compared with MC.44 Our results, showing that NS cases with eosinophilia had a similar prognosis to MC cases, are not consistent with this assumption and show that eosinophilia in NS is not a positive but a negative prognostic indicator (Figures 4 through 7).
Recently, it was found that eosinophils (in addition to other cell types as subsets of activated T cells, neutrophils, and histocyte/macrophages in HD) are an important cellular source of functionally active CD30-ligand for RS/H cells.45,47 The linkage of CD30-ligand to the CD30 antigen, the latter being nearly always present on RS/H cells of the classical types, is known to induce proliferation as well as anti-apoptotic signals.9,47-50CD30 cross-linking mediates a transient activation of predominantly TRAF-2–induced nuclear factor-kappaB (NF-kB).51-53
NF-kB activation is important for cytokine secretion, proliferation, and resistance to apoptosis.54-56 Especially in HD, it plays a decisive role for the proliferation and survival of H/RS cells.57 It is plausible, therefore, that a prominent tissue eosinophilia is capable of influencing clinical outcome or the efficacy of therapy via NF-kB signaling.
This signaling pathway hints also toward a possible explanation for the differences observed between NS and MC cases. Among the nodular sclerosis HD, cases with eosinophilia showed a similar survival as did MC patients (Figure 7). The mechanisms leading to eosinophilia are comparably effective in NS and MC cases, and may thus be similar. The latter histologic subtype, however, is much more often associated with an EBV infection.41,42 Latent-membrane protein of EBV (EBV-LMP1) is also capable of activating NF-kB.52 58-62 In MC cases, therefore, the dysregulation of genes controlling proliferation and resistance to apoptosis may be caused by both EBV infection and eosinophils. This might explain why in MC cases without eosinophilia no favorable outcome was observed (Figure 6). To date, little data about the clinical relevance of EBV infection in HD cases are available, but it could be of interest to investigate separately cases with and without eosinophilia to overcome these competing effects.
Numerous other signaling events within the complicated network of chemokines and cytokines, not all mentioned previously, may also play decisive roles in the host response to HD. The results of this study, however, are supported by recent molecular reports on the role of eosinophils in HD and their possible downstream effects.46
In conclusion, eosinophilia proved to be the strongest unfavorable prognostic factor for survival and freedom from treatment failure in nodular sclerosis HD, allowing for the effects of stage and other prognostic factors. Eosinophils, which are easily identifiable, are not innocent bystander cells in HD. Their histomorphologic identification may help in the effort to find risk-adapted therapies for HD patients.
Supported in part by grant T 1/93/Di 1 from the Deutsche Krebshilfe, Bonn, Germany.
Reprints:Dr Reinhard von Wasielewski, Institut für Pathologie, Medizinische Hochschule Hannover, Carl—Neuberg Straße 1, D-30625 Hannover, Germany; e-mail:wasielewski.reinhard.von@mh-hannover.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.