One major obstacle to the effective treatment of cancer is to distinguish between tumor cells and normal cells. The chimeric molecules created by cancer-associated chromosomal abnormalities are ideal therapeutic targets because they are unique to the disease. We describe the use of a novel approach based on the catalytic RNA subunit of RNase P to destroy specifically the tumor-specific fusion genes created as a result of chromosome abnormalities. Using as a target model the abnormal BCR-ABL p190 and p210 products, we constructed M1-RNA with guide sequences that recognized the oncogenic messengers at the fusion point (M1-p190-GS and M1-p210-GS). To test the effectiveness and the specificity of M1-p190-GS and M1-p210-GS, we studied in vitro and in vivo effects of these RNA enzymes againstBCR-ABLp190 andBCR-ABLp210, bearing in mind that both fusion genes share the ABL sequence but differ in the sequence coming from the BCR gene. We showed that M1-p190-GS and M1-p210-GS can act as sequence-specific endonucleases and can exclusively cleave target RNA that forms a base pair with the guide sequence (GS). We also demonstrated that when M1-p190-GS and M1-p210-GS were expressed in proper mammalian cell models, they abolished the effect of BCR-ABL by specifically decreasing the amount of the target BCR-ABL mRNA and preventing the function of theBCR-ABL oncogenes. These data clearly demonstrate the usefulness of the catalytic activity of M1-GS RNA to cleave specifically the chimeric molecules created by chromosomal abnormalities in human cancer and to represent a novel approach to cancer treatment.
A key difficulty in the effective treatment of patients with cancer is distinguishing between tumor cells and normal cells. This is why current treatments for cancer are often ineffective. There have been remarkable advances in our understanding of the molecular biology of cancer that suggest new mechanisms for the selective destruction of tumors.1-3 The molecular characterization of tumor-specific chromosomal abnormalities has revealed that fusion proteins are involved in most types of cancer.4-6 These fusion proteins result from chimeric genes created by the translocation and production of chimeric mRNA species that contain exons from each gene involved in the translocation. Chimeric molecules are ideal therapeutic targets because they are unique to the disease; they only exist in the tumor cells, not in the patient's normal cells.1-3 Inhibition of chimeric gene expression by antitumor agents specifically kills leukemic cells without affecting normal cells.
As therapeutic agents, zinc-finger proteins,3 antisense RNA,1,2,7 or hammerhead-based ribozymes8 9 have been used. All these methods have some limitations. Zinc-finger proteins must act at the DNA level, interacting with the desired sequence and blocking transcription. However, gene fusions at the DNA level occur within introns, and this implies that a new zinc-finger has to be designed for every patient. Thus, directing the strategy at the mRNA level seems to be more practical. Antisense molecules, either oligodeoxynucleotides or antisense RNA, act in a 1:1 stoichiometric relationship. This problem can be overcome with the use of ribozymes that, because of catalytic activity, process and destroy a higher number of target molecules per molecule of ribozyme. However, hammerhead ribozymes, in turn, require presence of specific nucleotide sequences in the target RNA to be cut, and these requirements cannot always be fulfilled. These data imply that new therapeutic tools would be desirable to allow the inactivation of any chimeric fusion gene product.
M1 RNA is the catalytic RNA subunit of RNase P from Escherichia coli. RNase P is a ribonucleoprotein complex that catalyzes the hydrolysis reaction that removes a 5′ leader sequence from tRNA precursors and several other small RNAs of similar structure. Studies of substrate recognition by M1-RNA and RNase P10-12 have led to the development of a general strategy of gene targeting in which M1 RNA can be used as a tool to cleave any specific mRNA sequence simply by the 3′ terminal addition to the ribozyme sequence of a so-called guide sequence (M1-GS) complementary to the target mRNA, that forms a base-pair with it and leaves a 5′-ACCAC-3′ unpaired stretch needed for the M1-GS RNA to recognize and cleave this artificially created substrate13 (Figure1C). Thus, M1-GS RNA, apart from some requirements to improve its cleavage efficiency,14 can be specifically directed to cut any mRNA sequences. We have taken advantage of this property to destroy the tumor-specific fusion genes created as a result of chromosomal translocations.
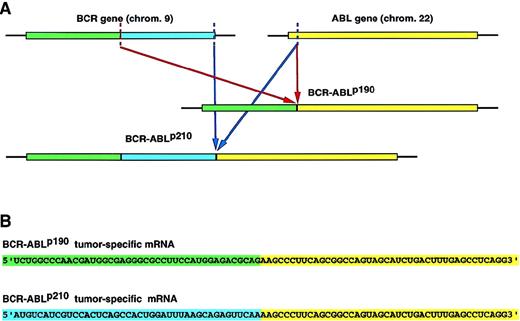
Overview of the strategy used to target the BCR-ABL mRNA by RNase P. (A) The translocation t(9:22)(q34;11) in Ph1+-leukemia.
The BCR-ABL oncogene is created by the translocation of sequences from the ABL gene on chromosome 9 to the BCRgene on chromosome 22. The same cytogenetic aberration can generate alternative chimeric mRNA and proteins, depending on the precise breakpoint within the BCR gene.BCR-ABLp190 and p210 oncogenes contain identical ABL-derived sequences but differ in the number of BCR nucleotides. (B) Nucleotide sequences of the fusion point between BCR and ABL sequences inBCR-ABLp190 andBCR-ABLp210 cDNA. The chimeric molecules generated by the recurrent chromosomal rearrangements represent ideal therapeutic targets because they are unique to the disease state. (C) Inhibition of the tumor-specific product by the specific cleavage of the tumor-specific BCR-ABLp190 andBCR-ABLp210 products by RNase P.
Overview of the strategy used to target the BCR-ABL mRNA by RNase P. (A) The translocation t(9:22)(q34;11) in Ph1+-leukemia.
The BCR-ABL oncogene is created by the translocation of sequences from the ABL gene on chromosome 9 to the BCRgene on chromosome 22. The same cytogenetic aberration can generate alternative chimeric mRNA and proteins, depending on the precise breakpoint within the BCR gene.BCR-ABLp190 and p210 oncogenes contain identical ABL-derived sequences but differ in the number of BCR nucleotides. (B) Nucleotide sequences of the fusion point between BCR and ABL sequences inBCR-ABLp190 andBCR-ABLp210 cDNA. The chimeric molecules generated by the recurrent chromosomal rearrangements represent ideal therapeutic targets because they are unique to the disease state. (C) Inhibition of the tumor-specific product by the specific cleavage of the tumor-specific BCR-ABLp190 andBCR-ABLp210 products by RNase P.
We have used as a model target, a well-characterized example in the hematopoietic system that involves the rearrangement of the BCRand ABL genes in Philadelphia chromosome-positive (Ph1+) chronic myelogenous leukemia and acute lymphoblastic leukemias. This translocation results in the formation of chimeric BCR-ABL oncogenes.15-24 Depending of the precise breakpoint within the BCR gene, fusion proteins of 210 kd (p210) or 190 kd (p190) are produced that have deregulated ABL tyrosine kinase activity. p210 and p190 BCR-ABL oncogenes contain identical ABL-derived sequences, but they differ in numbers of BCR-encoded amino acid residues16-19,21,25-28(Figure 1A). We have previously shown that the BCR-ABLoncogenes inhibit apoptosis by a Bcl-2 pathway as a part of their oncogenic phenotype.29 Inhibition of BCR-ABL expression in Ba/F3+p190 cells reverses this phenotype, and cells die by apoptosis.3 29
For the design of an M1-GS RNA that can disrupt a chimeric RNA created by cancer-associated chromosomal translocations, it is necessary to target the junction sequence. Otherwise, normal mRNA that shares part of the chimeric RNA sequence will also be cleaved by the M1-GS RNA, with resultant damage to host cells. In the case of theBCR-ABLp190 and p210 sequences, they contain identical ABL-derived sequences, but they differ in the number of BCR-encoded sequences (BCR-ABLp190 consists of BCR exon 1 and ABL exon 2, and BCR-ABLp210consists of BCR exon 3 and ABL exon 2) (Figure 1A). We have constructed M1-RNA with guide sequences that recognize the oncogenic messengers at the fusion point (9 nucleotides at each side of the breakpoint), which we call M1-p190-GS and M1-p210-GS. To test the effectiveness and specificity of M1-p190-GS and M1-p210-GS, we studied the in vitro and in vivo effects of these RNA enzymes against bothBCR-ABLp190 andBCR-ABLp210, bearing in mind the already mentioned fact that both fusion genes share the same ABLsequence but differ in that part coming from theBCR gene. We showed that M1-p190-GS and M1-p210-GS can act as sequence-specific endonucleases and can exclusively cleave target RNA that forms base pairs with the guide sequence (GS). The current study demonstrated that the artificially created ribozyme is specific not only in vitro but also in cultured cells. This study represents, to our knowledge, the first gene therapy approach that makes use of the catalytic activity of M1-RNA to destroy mRNA derived from the tumor-specific oncogenes created as a result of chromosomal translocations. The data presented here define a new therapeutic tool for the treatment of cancer.
Materials and methods
Plasmids
pTK117, a pUC19 derivative in which the DNA sequence coding forE. coli M1RNA is under the control of the T7 RNA polymerase promoter, was a generous gift from Sidney Altman (Yale University, New Haven, CT). Moloney murine leukemia virus- based MFG retroviral vector was a gift from Richard Mulligan.
Enzymes and chemicals
T4 DNA ligase, T4 polynucleotide kinase, and restriction endonucleases were purchased from New England Biolabs; Taq DNA polymerase, T7 RNA polymerase, RNasin (ribonuclease inhibitor) and ribonucleoside-triphosphates were obtained from Promega (Madrid, Spain); deoxynucleoside triphosphates were from Pharmacia; RNase-free DNase I was purchased from HT Biotechnology (Cambridge, UK); radiolabeled chemicals and T7 Sequenase kit were purchased from Amersham (Madrid, Spain); DNA polymerase I Klenow fragment was from Boehringer Mannheim (Madrid, Spain); and DNA oligonucleotides were purchased from Isogen (Netherlands).
Ribozyme synthesis
The DNA templates for M1RNA with the anti–BCR-ABLp190 or the anti– BCR-ABLp210–directed guide sequences were constructed by the polymerase chain reaction (PCR) with the gene for M1RNA as found in plasmid pTK117 as a template.13 The 5′ primer, OliT7 5′-gcgattcTAATACGACTCACTATAG-3′, was common to all the ribozymes constructed, annealing with the T7 promoter and providing a 5′ EcoRI site for cloning. The 3′ primers contained the appropriate guide sequences and were Olip190 5′-gcgtcgacGTGGTGAGACGCAGAAGCCCTTCTATGACCATG-3′) and Olip210 5′-gcgtcgacGTGGTAGAGTTCAAAAGCCCTTCTATGAC.
CATG-3′
The 3′ proximal sequences of 10 nucleotides serve as primers for the PCR with the pUC19 sequence. The underlined sequences, the bold sequences, and the lowercase ones correspond, respectively, to the ACCAC-3′ sequence, the guide sequences, and the SalI restriction site. The 2 different PCR products (M1-190-GS and M1-210-GS) were cloned in pUC19 and were completely sequenced to exclude any mutation during the PCR reaction. Plasmids pUC19-M1-190-GS and pUC19-M1-210-GS were linearized with SalI and transcribed in vitro with T7 RNA polymerase according to manufacturer's instructions. Transcribed ribozymes were phenol-chloroform extracted and precipitated with ethanol, and their integrity was checked either by acrylamide/urea or agarose electrophoresis.
Construction of artificial substrates
The fusion points of BCR-ABLp190 and BCR-ABLp210 mRNA were created by annealing the oligonucleotides Oli-190-sense 5′-AGCTTGAGGGCGCCTTCCATGGAGACGCAGAAGCCCTTCAGCGGCCAGTAGCATCG-3′) with Oli-190-antisense 5′-AATTCGATGCTACTGGCCGCTGAAGGGCTTCTGCGTCTCCATGGAAGGCGCCCTCA-3′) and Oli-210-sense 5′-AGCTTGCCACTGGATTTAAGCAGAGTTCAAAAGCCTTCAGCGGCCAGTAGCATCG-3′) with Oli-210-antisense 5′-AATTCGATGCTACTGGCCGCTGAAGGGCTTTTGAACTCTGCTTAAATCCAGTGGCA-3′), respectively. These phosphorylated annealed products, containing 50 nucleotides spanning at the fusion point of BCR-ABLp190 and BCR-ABLp210(25 nucleotides at each side of the corresponding fusion point) were cloned to the HindIII/EcoRI site of pcDNA3. Once cloned, plasmids were linearized with EcoRI, and artificial substrates were transcribed from the pcDNA3 T7 promoter in the presence of [32P]-GTP and then subjected to purification on 10% polyacrylamide/8 mol/L urea gels. Acrylamide slices containing the transcripts were eluted, ethanol-precipitated, resuspended in water, and were ready for use as targets in the in vitro ribozyme catalytic reaction.
Assays for cleavage by M1-GS RNA
RNA enzymes and uniformly labeled substrates synthesized as described above were incubated for 4 hours at 50°C in 25 mmol/L Tris, pH 7.5, 50 mmol/L NH4Cl, and 50 mmol/L MgCl buffer in a final reaction volume of 20 μL. Reactions were stopped by the addition of 6 μL of a solution containing 95% formamide, 20 mmol/L EDTA, 0.05% bromophenol blue, and 0.05% xylene cyanol FF boiled for 5 minutes and chilled on ice. The reaction products were analyzed on 10% polyacrylamide/8 mol/L urea gels.
Construction of plasmids for in vivo studies
To clone M1RNA-GS to the MFG retroviral vector, plasmids pUC19-M1-190-GS and pUC19-M1-210-GS were digested withEcoRI/SalI and blunt-ended with DNA polymerase I Klenow fragment. The fragment corresponding to the M1-RNA-GS was gel purified and ligated to the NcoI site (blunted) of the pMFG vector. The authenticity of the MFGM1-190-GS and MFG-M1-210-GS constructs was confirmed by DNA sequencing.
Cell culture
Cell transfection
Ba/F3 + p190 and Ba/F3 + p210 cells were transfected by electroporation (960 μF, 220 V) with 20 μg MFG-M1-190-GS and MFG-M1-210-GS, respectively, along with 1 μg MC1-puro expression vector. Cell lines were analyzed by Northern blotting for MFG-M1-190-GS and MFG-M1-210-GS expression. Cells were screened for resistance to IL-3 withdrawal and level of BCR-ABL and Bcl-2expression by Northern blotting. Cell viability was determined by trypan blue exclusion.
RNA analysis
Total cytoplasmic RNA (10 μg) was glyoxylated and fractionated in 1.4% agarose gels in 10 mmol/L Na2HPO4 buffer (pH 7). After electrophoresis, the gel was blotted onto Hybond-N (Amersham), ultraviolet light-cross-linked, and hybridized to32P-labeled probes. Loading was monitored by reprobing the filters with mouse β-actin cDNA.
Results
Cleavage of model BCR-ABL substrates by M1-GS RNA in vitro
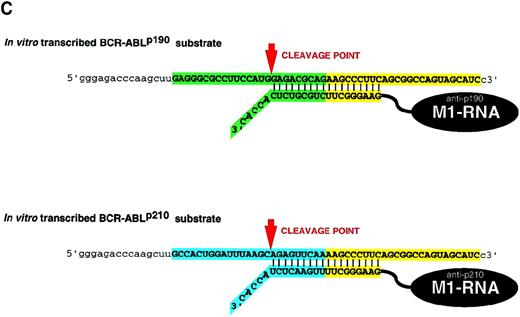
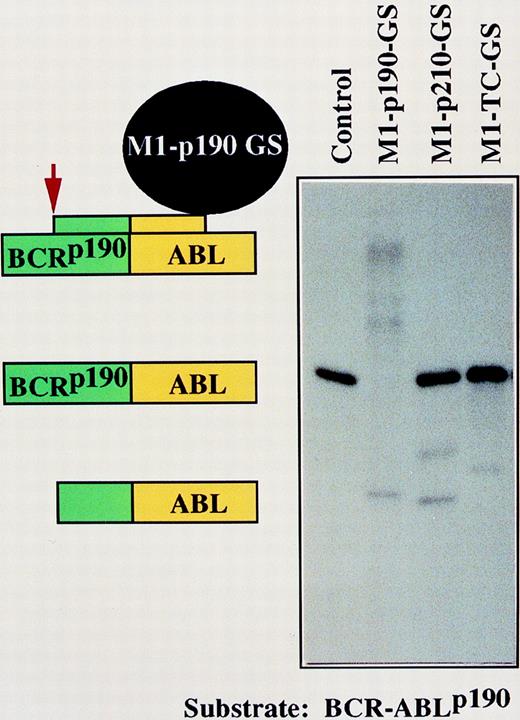
The BCR-ABL genes have been well characterized.15-28 These gene products are essential for the viability of tumor cells.3,29 32 Because they are so well studied, we used them as model targets for gene inactivation by M1-GS RNA. DNA encoding GS that contained a sequence of 18 nucleotides complementary to the fusion regions ofBCR-ABLp190 andBCR-ABLp210 mRNA was covalently linked to the 3′ end of DNA that encoded M1 RNA (Figure 1). To prove the specificity of the constructs M1-p190-GS and M1-p210-GS, we prepared short 50-nucleotide BCR-ABL p190 and p210substrates that corresponded to the target site indicated by capital letters in Figure 1B. The specificity was tested by incubating the in vitro transcribed M1-p190-GS and M1-p210-GS with the substrates. The RNA transcripts of these constructs, M1-p190-GS and M1-p210-GS, cleaved target RNA that contained 50 nucleotides of the related fusion gene sequence (Figures 2 and 3). Cleavage in the target occurred at the predetermined position. Our M1-GS RNA designed against the product of the BCR-ABL genes only cleaved substrates complementary to the GS. As shown in Figures 2and 3, M1-p190-GS could only cleave substrateBCR-ABLp190 but not substrateBCR-ABLp210, which contained a sequence of 9 nucleotides identical to the p210 fusion point (Figure1B). Conversely, M1-p210-GS, which contained a sequence complementary to BCR-ABLp210 mRNA, could efficiently cleave p210 but not p190. Therefore, M1-GS RNA appeared to act as a sequence-specific endonuclease, recognizing substrates through specific base-pairing between the GS and the target sequence in vitro. Moreover, an M1-RNA linked to an unrelated BCR-ABL guide sequence (M1-TC-GS) was unable to cleave p190 or p210 substrates (Figures 2and 3).
Sequence-specific cleavage ofBCR-ABLp190 substrate by M1-p190-GS.
Autoradiograph of cleavage products generated by M1-GS constructs.BCR-ABLp190 substrate was incubated either alone (lane 1), with M1-p190-GS (lane 2), with M1-p210-GS (lane 3), or with M1-TC-GS (lane 4). Schematic representation of specific complexes and products formed by M1-p190-GS and substrates (lane 2) are indicated on the left. However, an inappropriate cleavage was generated under the conditions used in lanes 3 and 4. The nature of these products and of the apparent miscleavages has not been determined.
Sequence-specific cleavage ofBCR-ABLp190 substrate by M1-p190-GS.
Autoradiograph of cleavage products generated by M1-GS constructs.BCR-ABLp190 substrate was incubated either alone (lane 1), with M1-p190-GS (lane 2), with M1-p210-GS (lane 3), or with M1-TC-GS (lane 4). Schematic representation of specific complexes and products formed by M1-p190-GS and substrates (lane 2) are indicated on the left. However, an inappropriate cleavage was generated under the conditions used in lanes 3 and 4. The nature of these products and of the apparent miscleavages has not been determined.
Sequence-specific cleavage ofBCR-ABLp210 substrate by M1-p210-GS.
Autoradiograph of cleavage products generated by M1GS constructs.BCR-ABLp210 substrate was incubated either alone (lane 1), with M1-p190-GS (lane 2), with M1-p210-GS (lane 3), or with M1-TC-GS (lane 4). Schematic representation of specific complexes and products formed by M1-p210-GS and substrates (lane 3) are indicated on the left. Total digestion ofBCR-ABLp210 substrate can be obtained by M1-p210-GS if the time of incubation is longer (data not shown).
Sequence-specific cleavage ofBCR-ABLp210 substrate by M1-p210-GS.
Autoradiograph of cleavage products generated by M1GS constructs.BCR-ABLp210 substrate was incubated either alone (lane 1), with M1-p190-GS (lane 2), with M1-p210-GS (lane 3), or with M1-TC-GS (lane 4). Schematic representation of specific complexes and products formed by M1-p210-GS and substrates (lane 3) are indicated on the left. Total digestion ofBCR-ABLp210 substrate can be obtained by M1-p210-GS if the time of incubation is longer (data not shown).
Generation of Ba/F3+p190 and Ba/F3+p210 cell lines transduced with M1-p190-GS and M1-p210-GS RNA
Because M1-GS RNA against BCR-ABLp190 and p210 junction sequences acted efficiently and specifically against the related substrates in vitro, we decided to examine the activity of the M1-GS RNA against an endogenous BCR-ABL target. We had previously established murine cell lines, Ba/F3 + p190 and Ba/F3 + p210, that express human p190 and p210 mRNA constitutively by integrating a plasmid construct that express p190 and p210, respectively.3,29,32 Although the parental mouse Ba/F3 cell line is an IL-3–dependent hematopoietic cell line, the transformed Ba/F3 + p190 and Ba/F3 + p210 cells are IL-3–independent because of the activity of p190 and p210 oncogenes.29-31 However, if the expression of p190 and p210 is inhibited, Ba/F3 + p190 and Ba/F3 + p210 cells should become IL-3 dependent and, in the absence of IL-3, they should die.3,29 32 Therefore, during the selection of M1-GS RNA-transduced Ba/F3 + p190 and Ba/F3 + p210 cells, we used 10% WEHI-conditioned DMEM medium as a source of IL-3.
For the potential application of a novel M1-GS RNA to gene therapy for the treatment of cancer, it is important to express the M1-GS RNA constitutively and under the control of a strong promoter in vivo. High-level expression under the control of the LTR promoter would be advantageous if M1-GS RNA were to be used as a therapeutic agent for cancer treatment. The specific design of the construct was based on our previous success in inserting an antisense sequence into the MFG vector.32 This strategy yielded antisense molecules that were active in cultured cells.32 To express M1-GS RNA in mammalian cells, expression vectors MFG-M1-p190-GS and MFG-M1-p210-GS were generated by cloning the respective genes into the MFG vector under the control of the LTR promoter. The specifically designed sequences, shown in Figure 1C (M1-p190-GS and M1-p210-GS), should be active only in the presence of the complementary BCR-ABL junction and should remain inactive in the presence on the unrelatedBCR-ABLp210 and p190 sequences, respectively.
In the presence of IL-3, Ba/F3 + p190 and Ba/F3 + p210, cells were transfected separately with plasmids MFG-M1-p190-GS and MFG-M1-p210-GS along with a gene that encodes resistance to puromycin. To generate p190 and p210 cells that had been stably transduced with M1-GS RNA constructs, the medium was replaced 24 hours after transfection with DMEM supplemented with 10% fetal calf serum and 3 μg/mL puromycin. The transduced cells were cultured for another 72 hours, and IL-3 was removed from the medium for assays of subsequent viability (see below).
In earlier studies about the in vivo specificity of anti–BCR-ABL hammerhead ribozymes, the lack of activity against cellular c-ABL is considered proof of ribozyme selectivity.9 But if the cell lines used in these studies are of mouse origin and the target BCR-ABL is the human oncogene, it is possible that the differences between human and mousec-ABL genes may be responsible for the lack of activity of the antisense molecules against the endogenous abl product. To examine the specificity of our M1-GS RNA, we also used Ba/F3 + p210 cells as a control for the activity of the M1-p190-RNA, and we used Ba/F3 + p190 cells as a control for the activity of the M1-p210-RNA. Stably transduced p190 and p210 cells that harbored an M1-GS RNA construct were generated using the respective plasmids (described above). The various lines of transduced cells allowed us to examine the activity and specificity of the M1-GS RNA against the respective BCR-ABL target genes.
Efficient expression of the M1-GS RNAs in mammalian cells
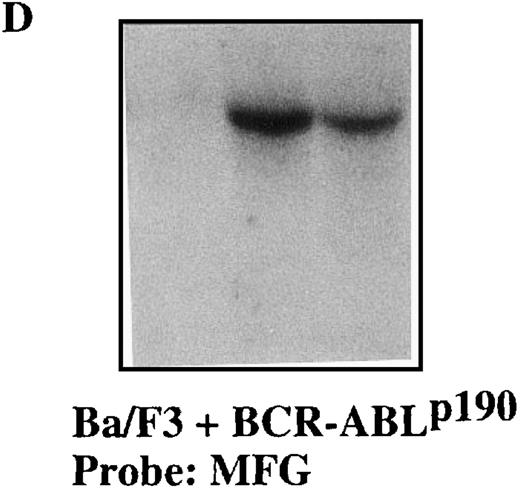
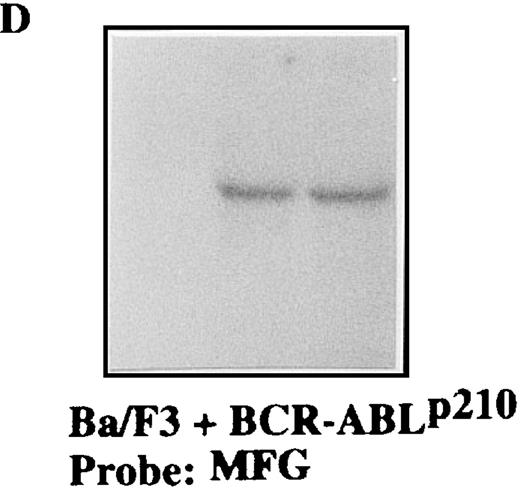
To confirm the stable expression of the M1-GS RNA in p190 and p210 cells, we performed Northern blot analysis with a probe that was complementary to the 3′-untranslated region of the MFG vector (Figures 4 and5). Total RNA from p190 and p210 cells that had been transfected with the various plasmids was extracted after transfection. The results in Figures 4D and 5D clearly demonstrate that each M1-GS RNA was expressed at significant levels and that the transcripts were obviously stable. The finding that M1-GS RNA transcripts in stable transfectants were stable serves to underlie the potential usefulness of our expression system for future gene therapy.
Sequence-specific cleavage ofBCR-ABLp190 mRNA by M1-p190-GS RNA in Ba/F3+p190 cells transfected with the MFG-M1-p190-RNA vector.
Total RNA was isolated from Ba/F3 + p190 cells (lane 1), Ba/F3 + p190 cells transfected with the MFG-M1-p190-GS expression vector (lane 2), and Ba/F3 + p190 cells transfected with the MFG-M1-p210-GS expression vector (lane 3). Cellular RNA was hybridized to a human ABL probe (A), to a mouse β-actin cDNA (B), to a human Bcl-2 probe (C), and to a 3′-untranslated MFG probe (D). Autoradiography was for 9 hours at −70°C.
Sequence-specific cleavage ofBCR-ABLp190 mRNA by M1-p190-GS RNA in Ba/F3+p190 cells transfected with the MFG-M1-p190-RNA vector.
Total RNA was isolated from Ba/F3 + p190 cells (lane 1), Ba/F3 + p190 cells transfected with the MFG-M1-p190-GS expression vector (lane 2), and Ba/F3 + p190 cells transfected with the MFG-M1-p210-GS expression vector (lane 3). Cellular RNA was hybridized to a human ABL probe (A), to a mouse β-actin cDNA (B), to a human Bcl-2 probe (C), and to a 3′-untranslated MFG probe (D). Autoradiography was for 9 hours at −70°C.
Specific suppression ofBCR-ABLp210 mRNA transcripts in Ba/F3 + p210 cells transfected with the MFG-M1-p210-RNA vector.
Total RNA was isolated from Ba/F3 + p210 cells (lane 1), Ba/F3 + p210 cells transfected with the MFG-M1-p190-GS expression vector (lane 2), and Ba/F3 + p210 cells transfected with the MFG-M1-p210-GS expression vector (lane 3). Cellular RNA was hybridized to a human ABL probe (A), to a mouse β-actin cDNA (B), to a human Bcl-2 probe (C), and to a 3′-untranslated MFG probe (D). Autoradiography was for 9 hours at −70°C.
Specific suppression ofBCR-ABLp210 mRNA transcripts in Ba/F3 + p210 cells transfected with the MFG-M1-p210-RNA vector.
Total RNA was isolated from Ba/F3 + p210 cells (lane 1), Ba/F3 + p210 cells transfected with the MFG-M1-p190-GS expression vector (lane 2), and Ba/F3 + p210 cells transfected with the MFG-M1-p210-GS expression vector (lane 3). Cellular RNA was hybridized to a human ABL probe (A), to a mouse β-actin cDNA (B), to a human Bcl-2 probe (C), and to a 3′-untranslated MFG probe (D). Autoradiography was for 9 hours at −70°C.
Activity and specificity of the M1-GS RNA against the related endogenous BCR-ABL cellular target
We transfected mouse Ba/F3 cells that stably expressed humanBCR-ABLp190 and p210 mRNA with plasmid that encoded the M1-GS RNA, and we selected cells by exposure to puromycin 24 hours after transfection. Puromycin-resistant cells were cultured in medium without IL-3, and cell viability was assessed in terms of the ability to exclude trypan blue dye. As shown in Table1, Ba/F3 + p190 cells that expressed M1-p190-GS died, whereas the control Ba/F3 + p190 cells remained alive 24 hours after the withdrawal of IL-3. Moreover, M1-p190-GS did not kill any Ba/F3 cells that expressed the unrelatedBCR-ABLp210 in spite of the fact that both oncogenes contained identical ABL-derived sequences but differed in the number of BCR-nucleotides. This result demonstrated the high specificity of the M1-p190-GS for targeting the chimeric p190 gene. As indicated in Table 1, similar results were obtained with the M1-p210-GS RNA that specifically interfered with the viability of Ba/F3 + p210 cells without affecting the survival of cells expressing the BCR-ABLp190oncogene.
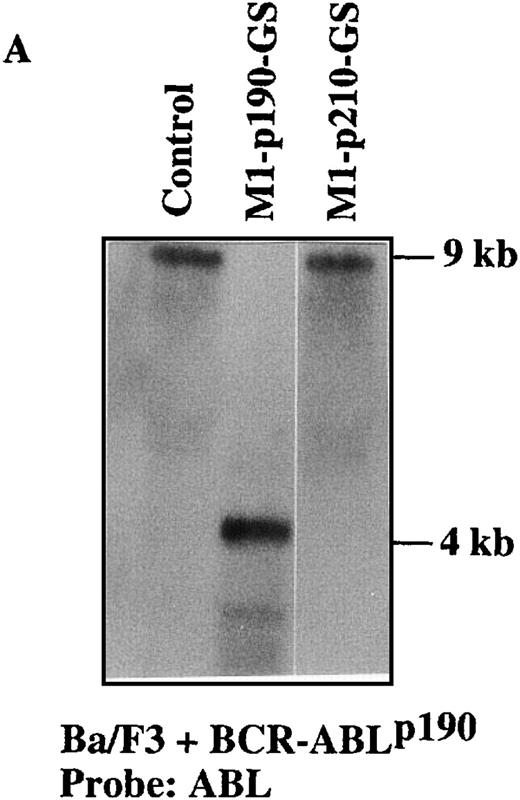
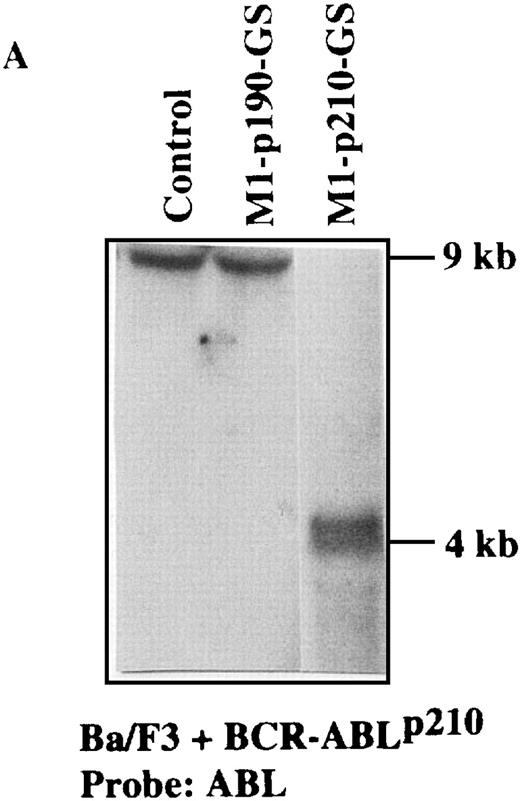
Because the M1-GS RNA overcame the BCR-ABL–mediated IL-3 independence in Ba/F3 cells, we tried to detect the anticipated cleavage product directly by Northern blot analysis (Figures 4A and5A). Total RNA from M1-GS–transduced p190 and p210 cells was extracted in the presence of IL-3. The levels of BCR-ABL mRNA were determined by hybridization of the Northern blots with a probe specific for the ABL gene. As illustrated in Figures 4A and 5A, the length of the cleavage products was exactly as anticipated (approximately 4 kb). No reduction in the level of expressedBCR-ABL mRNA was observed in the controls (Ba/F3 + p190 cells transduced with M1-p210-GS and Ba/F3 + p210 cells transduced with M1-p190-GS). Detection of the cleavage fragment proved that M1-GS RNA was catalytically active and cleaved specifically the appropriate target mRNA in cultured cells.
We have previously shown that the BCR-ABL oncogenes inhibit apoptosis by a Bcl-2 pathway as part of their oncogenic phenotype.29 Therefore, we investigated further whether the effect of our M1-GS RNA might indeed involve the inhibition ofBcl-2 expression. We asked whether the specific depletion ofBCR-ABLp190 and p210 by the related M1-GS might lead to the down-regulation of Bcl-2. The level of Bcl-2 mRNA was analyzed by Northern blot analysis in Ba/F3 + p190 and Ba/F3 + p210 cells transduced with M1-GS RNA. As illustrated in Figures 4C and 5C, there was a specific depletion ofBcl-2 RNA levels in Ba/F3 + p190 cells transduced with M1-p190-GS and Ba/F3 + p210 cells transduced with M1-p210-GS RNA, respectively. Bcl-2 mRNA production was not affected in the control cells (Ba/F3 + p190 cells transduced with M1-p210-GS and Ba/F3 + p210 cells transduced with M1-p190-GS). To compare directly the effect of our M1-GS RNA with those produced by molecules containing only antisense sequences, total RNA from Ba/F3 + p190 cells transfected either with the M1-p190-GS or with an identical vector carrying the antisense sequence against the BCR-ABLp190fusion32 was extracted in the presence of IL-3. The level of BCR-ABLp190 mRNA was determined by Northern blot analysis. As illustrated in Figure6, it was possible to detect some BCR-ABLp190 mRNA in cells transfected with the vector carrying the antisense sequence against the BCR-ABLp190 fusion32 but not in cells transfected with the M1-p190-GS.
Comparison of suppression ofBCR-ABLp190 mRNA transcripts.
Direct comparison of suppression ofBCR-ABLp190 mRNA transcripts in Ba/F3 + p190 cells transfected either with the MFG-M1-p190-RNA vector or with the same vector carrying only theBCR-ABLp190 antisense sequences. Total RNA was isolated from Ba/F3 + p190 cells transfected with the MFG-M1-p190-GS expression vector (lanes 1-3), control Ba/F3 + p190 cells (lane 4), and Ba/F3 + p190 cells transfected with the MFG-p190-antisense expression vector32 (lane 5). Cellular RNA was hybridized to a human ABL probe. RNA integrity was demonstrated by hybridizing the same filter with a mouse β-actin cDNA probe. Autoradiography was for 9 hours at −70°C.
Comparison of suppression ofBCR-ABLp190 mRNA transcripts.
Direct comparison of suppression ofBCR-ABLp190 mRNA transcripts in Ba/F3 + p190 cells transfected either with the MFG-M1-p190-RNA vector or with the same vector carrying only theBCR-ABLp190 antisense sequences. Total RNA was isolated from Ba/F3 + p190 cells transfected with the MFG-M1-p190-GS expression vector (lanes 1-3), control Ba/F3 + p190 cells (lane 4), and Ba/F3 + p190 cells transfected with the MFG-p190-antisense expression vector32 (lane 5). Cellular RNA was hybridized to a human ABL probe. RNA integrity was demonstrated by hybridizing the same filter with a mouse β-actin cDNA probe. Autoradiography was for 9 hours at −70°C.
These data showed that M1-GS RNA specifically prevented function of theBCR-ABLp190 andBCR-ABLp210 RNA and proteins, respectively, and clearly demonstrated that the artificially created ribozymes were specific not only in vitro but also in cultured cells. This showed the usefulness of the catalytic activity of M1-RNA to destroy the tumor-specific oncogenes created as a result of chromosomal translocations. These data defined a new therapeutic tool of gene inactivation of fusion genes created by chromosomal abnormalities for the treatment of cancer.
Discussion
Gene therapy promises to be an effective strategy for the treatment of cancer. The molecular characterization of tumor-specific chromosomal abnormalities has revealed that fusion proteins are the consequence in most forms of cancer. These fusion proteins are encoded by chimeric genes generated by chromosomal rearrangements. The presence of the chimeric molecules is necessary for the persistence of the tumor cell.3,29,32 These chimeric molecules represent ideal therapeutic targets because they are unique to the disease state and they exist in the tumor cells but not in the normal cells of the patient. Inhibition of chimeric gene expression by antitumor agents specifically kills the cancer cells without affecting the normal cells.3,32 As therapeutic agents, zinc-finger proteins,3 antisense RNA,1,2,7 or hammerhead-based ribozymes8 9 have been used. However, all these methods present limitations, implying that new therapeutic tools are required as gene-inactivating agents that should be able to inhibit any chimeric fusion gene product.
We have presented a novel strategy to target tumor-associated fusion proteins using M1-GS-RNA targeted against the BCR-ABL fusion mRNA. If it was successfully achieved in Ph1-ALL–derived cells, this inhibition might have caused the cells that integrated the M1-GS-RNA sequences to die, thus preventing us from verifying our hypothesis. This is why we chose as a first target the Ba/F3 + p190 and Ba/F3 + p210 cell lines, previously rendered IL-3 independent on expression of human p190- and p210-coding sequences, respectively.3,29 32 If p190 and p210 expression had been inhibited, which would have led to cell death in a culture medium deprived of IL-3, the cells could have been rescued by the addition of exogenous IL-3. Therefore, Ba/F3 + p190 and Ba/F3 + p210 cells were transfected by constructs carrying M1-GS-RNA designed against theBCR-ABL fusion junction and were proved to be specific in vitro. The M1-p190-GS and M1-p210-GS transcripts generated under the control of the LTR promoter specifically killed Ba/F3 + p190 and Ba/F3 + p210 cells, respectively, in the absence of IL-3. This effect was specific because control Ba/F3 + p210 cells transfected with the M1-p190-GS construct and Ba/F3 + p190 cells transfected with the M1-p210-GS construct did not die after IL-3 withdrawal. The levels ofBCR-ABLp190 andBCR-ABLp210 mRNA were specifically reduced in selected Ba/F3 + p190 + M1-p190-GS and Ba/F3 + p210 + M1-p210-GS cells, respectively (BCR-ABLp190 andBCR-ABLp210 oncogenes share 50% homology because the ABL sequences involved in the gene fusion are the same). We conclude that this approach can successfully achieve suppression of the product of the BCR-ABL oncogenes used as target models because the M1-p190-GS transcript is specific forBCR-ABLp190 and not forBCR-ABLp210, and the M1-p210-GS transcript is specific for BCR-ABLp210and not for BCR-ABLp190.
We have shown that the targeting of BCR-ABL gene products by M1-GS RNA results in virtually complete inhibition of BCR-ABL function in tissue culture cells within the constraints of the methods we have used. Results with M1-GS RNA were clearly better than those with antisense in the cells used (Figure 6). These differences provided strong evidence that the inhibitory effects on BCR-ABL function were GS mediated. The experiments also showed that the inhibitory effect was not the result of an antisense phenomenon because M1-p190-GS and M1-p210-GS formed a specific hydrogen-bound complex with the target RNA.
Although the creation of artificial M1-GS RNA is of great current interest, no such enzyme has yet been created against the chimeric products created by chromosomal translocations in human cancer and has been tested in animal cells. Our novel M1-GS RNA cleavedBCR-ABL mRNA specifically. In past efforts to destroyBCR-ABL mRNA by antisense or ribozyme molecules, it was difficult to demonstrate specificity.1,2,7-9 Therefore, in this kind of investigation, it is important to confirm that cell death does indeed originate from specific suppression by the therapeutic agent. Because the efficacy was considerable, our M1-GS RNA was very specific, and the length of the guide sequence could have varied and easily could have been adjusted. M1-GS RNA in general should be considered a novel class of potentially powerful gene-inactivating agents that should be able to cleave other chimeric mRNA created by chromosomal abnormalities in cancer. The general methodology we describe here is effective and is a further demonstration of the validity of M1-GS RNA methodology for the reduction of expression of targeted genes.13 These results might provide the basis for future gene therapy for the treatment of cancer.
The goal of the experiments described in this article was to demonstrate the general usefulness of the RNase P technology for the inhibition of chimeric gene products created by chromosomal abnormalities using a BCR-ABL system as a model. This methodology may itself lead to the production of an antitumor therapy. However, the efficiency of the agents must be evaluated in proper animal models, and the efficiency of the delivery process is still a major problem to be investigated and solved. Nevertheless, these advances provide new therapeutic tools for the treatment of cancer, and they hold some promise for more selective, nontoxic therapy in the future.
Acknowledgment
We thank S. Altman for providing the plasmid pTK117.
Supported by the European Commission (BMH4-CT96-0375); the DGCYT (UE96-0041, PB96-0816, and 1FD97-0360); the Fundación Cientı́fica of the AECC; the Junta de Castilla y León (CS12/99); the FIS (99/0935); and the National Institutes of Health (1 R01 CA79955-01). C.C. is a fellow of the Fundación Ferrer Investigación.
Reprints:I. Sánchez-Garcı́a, Departamento de Proliferación y Diferenciación Celular, Instituto de Microbiologı́a Bioquı́mica, CSIC/Universidad de Salamanca, Edificio Departamental, Avenida del Campo Charro s/n, 37007-Salamanca, Spain; e-mail: isg@gugu.usal.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.