Protein tyrosine phosphorylation is an integral part of cytokine-induced proliferation and differentiation of hematopoietic cells. The authors previously reported cloning and characterization of the receptor tyrosine kinase Tif, also termed Tyro3. Using the yeast 2-hybrid technology, they recently identified that the p85 subunit of phosphatidylinositol 3-kinase (PI3 kinase) interacted with the cytoplasmic domain of Tyro3. On treatment with epidermal growth factor (EGF), NIH3T3 cells expressed EGFR/Tyro3 (a fusion receptor with the extracellular domain from epidermal growth factor receptor and the transmembrane and cytoplasmic domains from Tyro3), and EGFR/Tyro3 was rapidly phosphorylated on tyrosine residues. The interaction between Tyro3 and p85 was also confirmed by glutathione S-transferase (GST) pull-down experiments. Co-immunoprecipitation followed by Western blot analysis revealed that PI3 kinase was associated with and phosphorylated by the activated Tyro3. Tyro3-associated PI3 kinase exhibited an enhanced kinase activity. In addition, EGF treatment of EGFR/Tyro3-expressing cells led to enhanced phosphorylation of Akt, a downstream component of PI3 kinase. Treatment of NIH3T3 cells expressing a full length of rat Tyro-3, but not NIH3T3 cells, with protein S also resulted in phosphorylation of Akt. Soft agar colony assays showed that the addition of EGF to EGFR/Tyro3-transfected cells, but not to the parental NIH3T3 cells, resulted in a concentration-dependent increase in the formation of anchorage-independent colonies. Tyro3-mediated transformation of NIH3T3 cells was significantly blocked by wortmannin, a PI3 kinase-specific inhibitor. Results of these combined studies strongly suggested that the oncogenic transforming ability of Tyro3 was mediated at least in part by the PI3 kinase pathway.
Receptor tyrosine kinases (RTK) play an essential role in cell growth, differentiation, and metabolism.1 These receptors have an extracellular ligand-binding domain, a single transmembrane domain, and a cytoplasmic domain. After treatment with the appropriate ligands, RTKs usually dimerize, which results in autophosphorylation on specific tyrosine residues and their activation. These autophosphorylated tyrosine residues are often the binding sites for many cytoplasmic proteins containing SH2 domain(s). The interaction between receptors and cytoplasmic proteins initiates a cascade of signal transduction processes, ultimately resulting in a variety of cellular responses and activities.
The Axl subfamily of RTK consists of 3 known members.2 They include Axl (alternatively termed Ufo or Ark), Tyro3 (alternatively termed Tif, Sky, Rse, Dtk), and Mer (alternatively termed Eyk).2 The product of growth arrest-specific gene 6 (Gas6) has been identified as the ligand for this family of RTKs.3,4 On the other hand, the anticoagulant factor protein S, which shares structural homology with Gas6, also has certain affinity with some of the receptors and is capable of receptor activation in a heterologous system.5Overexpression of Axl and Sky genes is associated with tumorigenesis.6,7 In fact, Axl, the founding member of the subfamily kinases, was originally cloned from human chronic myelogenous leukemic cells,6 and an elevated level ofAxl expression is detected in this type of leukemia.6 We reported previously that Tif was cloned from K562, a cell line of myelogenous leukemic origin.8 Our subsequent studies showed that TifmRNA expression was downregulated during terminal differentiation of HEL and K562 leukemia cell lines.9 Gas6, in conjunction with heregulin and forskolin, is capable of stimulating maximal proliferation of human Schwann cells,10 suggesting that Gas6 is a growth factor. In addition, Taylor et al7 have shown that mouse mammary tumors expressed elevated levels ofSky mRNA. When transfected into Rat1a fibroblasts, mouseSky is capable of morphologic transformation, enhancement of colony formation in soft agar culture, and stimulation of tumor growth in nude mice.7
Using gene knockout approaches, Lu et al11 recently showed that Tyro3 family receptors were essential for mammalian development. Mice lacking any single receptor, or any combination of 2 receptors, were viable and capable of producing apparently healthy offspring. However, mice with null mutations in Tyro3, Axl, and Mer produced no mature sperm because of the progressive death of differentiating germ cells.11 In addition, these receptors appeared to be essential for the development of mature nervous, immune, and reproductive systems.11
Biochemically, Sky is capable of dimerization,12 and the purified form of Tif has an autophosphorylation activity.8It has been demonstrated that Src family kinases, including Src, Fyn, and Yes, may be associated with Sky.13 Using the yeast 2-hybrid system, we have demonstrated that a p85 (a subunit of PI3 kinase) peptide fragment containing an SH2 domain interacts with the cytoplasmic domain of Tyro3. This interaction was confirmed in vivo using the NIH3T3 cell line, which expresses the transfected Tyro3 protein. The association between p85 and Tyro3 is ligand-dependent. Tyro3 activation also leads to an enhanced phosphorylation of Akt, a downstream component of PI3-kinase. In addition, soft agar assays have demonstrated that Tyro3 activation results in an increase of anchorage-independent colonies.
Materials and methods
Yeast 2-hybrid system
Yeast 2-hybrid screening was performed using a kit purchased from Clontech (Palo Alto, CA) and following the protocol provided by the supplier. Briefly, the bait used for the 2-hybrid system was the entire cytoplasmic domain of Tyro3 (amino acids 453-891) cloned in frame into the pAS2-1 plasmid vector. The resultant plasmid was named pAS2-Tyro3-Cyto. The yeast strain (CG-1945) was first transformed with pAS2-1-Tyro3-Cyto and then with pools of kidney cDNA cloned in pACT-2 plasmid. Colonies capable of growth on the minimal synthetic dropout plates without amino acids of His, Trp, and Leu and with compound 3-amino-triazole (3AT, 10 mmol/L) were selected for further analysis for β-galactosidase (β-Gal) activities using a protocol provided by the supplier (see also reference 14). Approximately 4.5 × 105 independent cDNA clones were screened. A replica filter assay and a liquid assay were used for determination of β-gal activities. The liquid assay results were presented. Cell pellets were suspended in Z buffer (Na2HPO4, 60 mmol/L; NaH2PO4, 40 mmol/L; KCl 10, mmol/L; MgSO4, 1 mmol/L), and cells were lysed by repeated freezing/thawing. Equal amounts of cell lysates were analyzed for β-Gal activity after the addition of ONPG (2.2 mmol/L) and β-mercaptoethanol (100 mmol/L). The reaction mixtures were incubated at 30°C, and the optical density of each sample at 420 nm (OD420) was determined.
The kinase domain of Tyro3 (amino acids 464-866) was expressed as a GST fusion protein using the plasmid pGEX-2T (Pharmacia Biotech, Piscataway, NJ). GST-Tyro3 and GST fusion proteins were induced by isopropylthiogalactose and purified through affinity chromatography according to the protocol provided by the supplier.
Cell lines
NIH3T3 cells were cultured in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C with 5% CO2. NIH3T3 cell derivatives include those stably expressing a full-length rat Tyro3, a chimeric receptor of EGFR/Tyro3 (the extracellular domain from human EGFR and the transmembrane/cytoplasmic domains from rat Tyro3), or a chimeric receptor of Tyro3/TrkC (the extracellular domain from rat Tyro3 and the transmembrane/cytoplasmic domains from rat TrkC). The pSRα expression vector as described5 was used for the expression of EGFR/Tyro3, Tyro3, and Tyro3/TrkC. These stably transfected cell clones were cultured in DMEM with 10% FBS and G418 (0.2 mg/mL). The cells were split when they reached 80% confluence. To activate EGFR/Tyro3 or Tyro3 receptor, cells were first cultured in DMEM with 2% FBS for 24 hours, and this was followed by serum starvation for 3 hours. For various time periods, EGF (100 ng/mL) was added to NIH3T3-EGFR/Tyro3 (or 5 μmol/L protein S to NIH3T3-Tyro3 cells) and to NIH3T3 as a control. The PI3 kinase inhibitor wortmannin (200 nmol/L) was added to some cells 30 minutes before EGF treatment. The treated cells were lysed in a lysis buffer (50 mmol/L Tris HCl, pH 7.4; 150 mmol/L NaCl; 1% [vol/vol] Triton X-100, 1 mmol/L EDTA; 1 mmol/L Na4VO3; 1 mmol/L PMSF; 10 μg/mL aprotinin; 10 μg/mL leupeptin; 10 μg/mL soy bean trypsin inhibitor; 10 mmol/L NaF). Cell lysates were stored at −70°C for subsequent analyses.
Western blot analysis
Cells treated with EGF or protein S for various times were collected and lysed. Equal amounts of proteins were analyzed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and then by Western blotting. Protein blots were first probed with anti-Tyro3, anti-p85, anti-Akt, anti-phospho-Akt, or antiphosphotyrosine (anti-PY) IgG and then with a second antibody conjugated with horseradish peroxidase. Specific signals on the blot were detected with an enhanced chemiluminescent method. The anti-Tyro3 (recognizing only the cytoplasmic domain of Tyro3) and phosphotyrosine IgG were purchased from Santa Cruz (Santa Cruz, CA). The anti-p85 was from Upstate Biotechnology (Lake Placid, NY). Akt and phospho-Akt (AktSer473) antibodies were purchased from New England Biolab (Beverly, MA). The anti-PY IgG (PY99) was purchased from Santa Cruz.
Immunoprecipitation
Equal amounts of protein lysates (2 mg) were supplemented with an anti-Tyro3 antibody and incubated at room temperature for 2 hours or overnight at 4°C. Protein A/G agarose beads (25 μL) were then added to each immunoprecipitation mixture, and the incubation continued at room temperature for 1 hour or at 4°C for 2 hours. Immunoprecipitates were collected and washed 3 times with the lysis buffer as described above. Immunoprecipitates were either analyzed by SDS-PAGE followed by Western blotting or were assayed for PI3 kinase activities.
Soft agar assay
Soft agar assays were performed essentially as described.15 Briefly, a solution of 1.2% Bactoagar mixed 1:1 with 2 × DMEM was supplemented with FBS at a final concentration of 2%. In addition, EGF of various final concentrations (0, 10, 20, 50, and 100 ng/mL) was added to the mixture. The complete medium was layered onto 6-well plates. NIH3T3-EGFR/Tyro3 cells, as well as NIH3T3 cells, were mixed in a 0.36% Bactoagar solution containing EGF and FBS. An additional set of EGF-treated NIH3T3-EGFR/Tyro3 cells was cultured in the presence of wortmannin (200 nmol/L). Cell suspensions were seeded at a density of 1 × 104 on top of 6-well bottom agar plates. After 2 weeks' incubation at 37°C and 5% CO2, colonies in each well were counted. Results were summarized from 4 independent experiments.
PI3 kinase assays
PI3 kinase assays were performed essentially as described.16 Tyro3 immunoprecipitates were resuspended in 30 μL of a reaction mixture (10 mmol/L Tris HCl, pH 7.4; 100 mmol/L NaCl; 3 mmol/L EDTA; 7 mmol/L MgCl2). The reaction was initiated by the addition of γ-32P-ATP (10 μCi; New England Nuclear; Boston, MA) and the substrate of phosphatidylinositol (PI) 4,5-biphosphate (Sigma, St Louis, MO). The reaction mixtures were incubated on a rotator at room temperature for 10 minutes and were stopped by the addition of 100 μL 1 N HCl, and PI lipids were sequentially extracted in 300 μL chloroform/methanol 1:1 and in 100 μL dH2O. The organic phase was dried under vacuum, and PI lipids, resuspended in 20 μL chloroform/methanol, were resolved by thin-layer chromatography and then by autoradiography.
Results
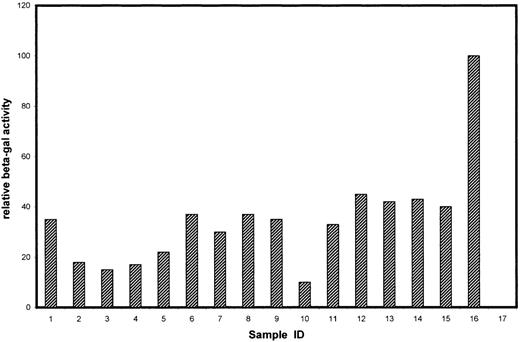
To identify cytoplasmic components that mediate the biologic function of Tyro3, we screened a human kidney cDNA library using the yeast 2-hybrid system for proteins directly interacting with the cytoplasmic domain of Tyro3. Initial screening of approximately 4.5 × 105 gross transformants resulted in approximately 3 dozen yeast clones that were capable of growth on selection plates. Additional screening by assaying their β-Gal activity narrowed the number down to 15 yeast clones (Figure1). Partial DNA sequence analyses revealed that these clones contained fragments of various lengths, corresponding to 15 individual genes. These genes could be separated into 3 groups (Table). Group A corresponded to those known or implicated in signal transduction in various systems. Group B corresponded to the genes not fully characterized or with an unknown function. Group C consisted of genes that were apparently not involved in signal transduction. Some of the group C genes have been reported to activate, by an unknown mechanism, the Gal4 transcription activity in the yeast 2-hybrid system (www.fccc.edu/research/labs/golemis).
β-Gal activities of positive clones isolated by the yeast 2-hybrid system.
The 16 clones correspond to those genes described in Table 1. Samples 1-7, 8-12, and 13-15 belong to groups A, B, and C, respectively. Sample 16 is a positive control for the β-Gal assay using a full-length Gal4 expression plasmid (pCL1; Clontech), and sample 17 is a negative control (empty vector-transformed) for the assay.
β-Gal activities of positive clones isolated by the yeast 2-hybrid system.
The 16 clones correspond to those genes described in Table 1. Samples 1-7, 8-12, and 13-15 belong to groups A, B, and C, respectively. Sample 16 is a positive control for the β-Gal assay using a full-length Gal4 expression plasmid (pCL1; Clontech), and sample 17 is a negative control (empty vector-transformed) for the assay.
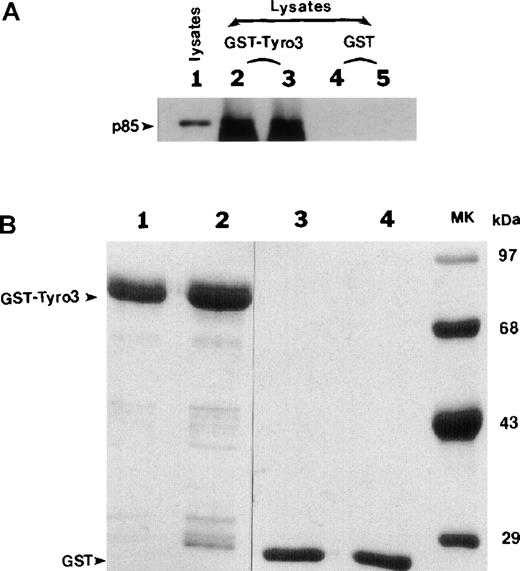
The #1 clone (Figure 1; Table 1), encoding the p85 subunit of PI3 kinase, stood out because PI3 kinase interacts through p85 with many receptor tyrosine kinases and mediates receptor function.17Complete DNA sequencing revealed that the p85 cDNA fragment was fused in frame to the Gal4 DNA-binding domain and that the fragment contained the region encoding an SH2 domain (data not shown) known to bind phosphorylated tyrosine residues. We first used the GST pull-down approach to confirm the potential interaction between Tyro3 and PI3 kinase. The intracellular domain of Tyro3 was expressed as a GST-fusion protein. GST-Tyro3, as well as GST, was purified by affinity chromatography. SDS-PAGE analysis of purified GST-Tyro3 (lanes 1 and 2) and GST (lanes 3 and 4) was shown in Figure2B, and partial degradation of GST-Tyro3 was observed. The purified GST-Tyro3 and GST, immobilized onto glutathione resins, were incubated with NIH3T3 cell lysates. After thorough washes, proteins associated with GST-Tyro3 or GST alone were analyzed by SDS-PAGE and then by Western blotting using an anti-p85 antibody. Figure 2A shows that GST-Tyro3 (lanes 2 and 3), but not GST alone (lanes 4 and 5) pulled down a p85-specific antigen that migrates to the same position as the cellular 1 (lane 1), indicating that p85 interacts with Tyro3 kinase domain.
The p85 subunit of PI3-kianse interacts with Tyro3 kinase domain.
(A) Purified GST-Tyro3 and GST alone used as a control were immobilized onto glutathione Sepharose beads and were incubated with NIH3T3 cell lysates. After washes, the proteins associated with GST-Tyro3 and GST beads were analyzed by SDS-PAGE and Western blotting using an anti-p85 antibody. Lane 1 is NIH3T3 total cell lysates. Lanes 2 and 3 are replicates of proteins bound to GST-Tyro3 beads. Lanes 4 and 5 are replicates of proteins bound to GST beads. (B) Purified GST-Tyro3 (lanes 1 and 2) and GST (lanes 3 and 4) were analyzed by SDS-PAGE. Lane 5 consists of protein standards.
The p85 subunit of PI3-kianse interacts with Tyro3 kinase domain.
(A) Purified GST-Tyro3 and GST alone used as a control were immobilized onto glutathione Sepharose beads and were incubated with NIH3T3 cell lysates. After washes, the proteins associated with GST-Tyro3 and GST beads were analyzed by SDS-PAGE and Western blotting using an anti-p85 antibody. Lane 1 is NIH3T3 total cell lysates. Lanes 2 and 3 are replicates of proteins bound to GST-Tyro3 beads. Lanes 4 and 5 are replicates of proteins bound to GST beads. (B) Purified GST-Tyro3 (lanes 1 and 2) and GST (lanes 3 and 4) were analyzed by SDS-PAGE. Lane 5 consists of protein standards.
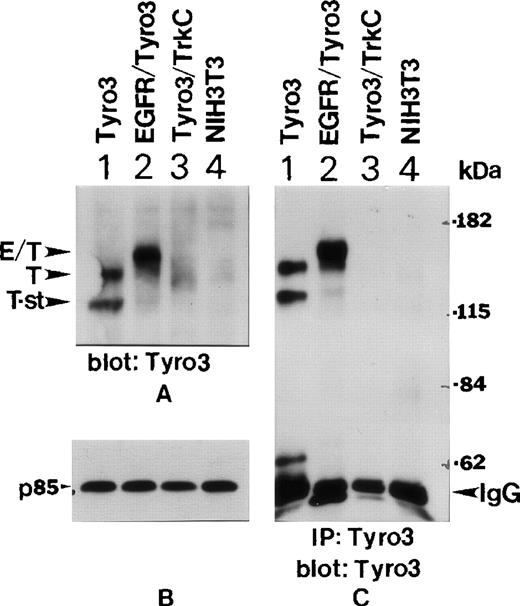
To study whether PI3 kinase interacts with Tyro3 in vivo, we obtained NIH3T3 cell lines expressing transfected Tyro3, EGFR-Tyro3, or Tyro3/Trk-C as previously described.5 Western blot analyses using the anti-Tyro3 antibody showed (Figure3A) that abundant Tyro3 (T) and EGFR/Tyro3 (E/T) were expressed in Tyro3 (lane 1) and EGFR/Tyro3 (lane 2) transfected cell lines, but not in parental NIH3T3 (lane 4) and Tyro3/Trk-C (lane 3) cell lines. The anti-Tyro3 antibody did not recognize Tyro3/TrkC because the fusion receptor contained the extracellular domain of Tyro3. Interestingly, there existed a short form of Tyro3 (T-st) in Tyro3-transfected cells (Figure 3A, lane 1) and it might have been a degradation product of Tyro3. Figure 3B shows the same blot was probed with an anti-p85 antibody as a loading control. To confirm further the specificity of expressed receptor molecules, we immunoprecipitated various cell lysates using the anti-Tyro3 antibody. The immunoprecipitates were blotted for Tyro3 antigen. Figure 3C shows that both forms of Tyro3 (lane 1, T and T-st) and EGFR-Tyro3 (lane 2, E/T) were efficiently immunoprecipitated by the anti-Tyro3 antibody.
Ectopic expression of EGFR-Tyro3 and Tyro3.
(A) NIH3T3 cells transfected with a full-length Tyro3 (lane 1), an EGFR/Tyro3 fusion receptor (lane 2), and a Tyro3/Trk-C fusion receptor (lane 3) were lysed, and equal amounts of cell lysates were analyzed for Tyro3 expression using an antibody recognizing the C-terminal part of the receptor. E/T denotes the EGFR/Tyro3 fusion receptor, and T denotes the Tyro3 receptor. T-st indicates a short form of Tyro3. (B) As a loading control, equal amounts of the cell lysates, as described in Figure 3A, were analyzed by SDS-PAGE and by Western blotting using an anti-P85 antibody. (C) Various cell lysates were immunoprecipitated with the anti-Tyro3 antibody, and immunoprecipitates were analyzed for Tyro3 expression.
Ectopic expression of EGFR-Tyro3 and Tyro3.
(A) NIH3T3 cells transfected with a full-length Tyro3 (lane 1), an EGFR/Tyro3 fusion receptor (lane 2), and a Tyro3/Trk-C fusion receptor (lane 3) were lysed, and equal amounts of cell lysates were analyzed for Tyro3 expression using an antibody recognizing the C-terminal part of the receptor. E/T denotes the EGFR/Tyro3 fusion receptor, and T denotes the Tyro3 receptor. T-st indicates a short form of Tyro3. (B) As a loading control, equal amounts of the cell lysates, as described in Figure 3A, were analyzed by SDS-PAGE and by Western blotting using an anti-P85 antibody. (C) Various cell lysates were immunoprecipitated with the anti-Tyro3 antibody, and immunoprecipitates were analyzed for Tyro3 expression.
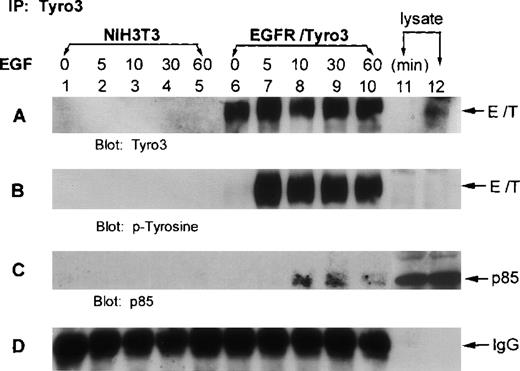
To examine whether p85 interacted with Tyro3 in vivo, we treated EGFR-Tyro3 and parental NIH3T3 cells with EGF for various times. Cell lysates were immunoprecipitated with the anti-Tyro3 antibody. The immunoprecipitates were first blotted for Tyro3. Western blot analyses showed (Figure 4A) that Tyro3 antigen was present in EGFR/Tyro3 cells (lanes 6-10) but not in NIH3T3 cells (lanes 1-5). Tyro3 was phosphorylated on tyrosine residues 5 minutes after EGF treatment (Figure 4B, lane 7), and phosphorylation persisted for at least 1 hour (lane 10). Reprobing the same blot using an anti-p85 antibody showed that p85 was co-immunoprecipitated by the anti-Tyro3 antibody in cells treated with EGF (Figure 4C, lanes 8-10), indicating that p85 was associated with the tyrosine-phosphorylated receptor. In addition, phosphotyrosine blot analyses indicated that p85 was significantly phosphorylated 10 minutes after EGF treatment (data not shown).
The p85 subunit of PI3 kinase interacts with phosphorylated EGFR/Tyro3.
NIH3T3 cells transfected with EGFR/Tyro3 (lanes 6-10) and the parental cells (lanes 1-5) were treated with EGF for various times. Equal amounts of cell lysates were immunoprecipitated with the anti-Tyro3 antibody. Immunoprecipitates were then blotted for (A) Tyro3, (B) PY-Tyro3, or (C) P85. (D) IgG portion of the gel was shown as a loading control. Lanes 11 and 12 were nontreated lysates from NIH3T3 and NIH3T3-EGFR/Tyro3, respectively. EGF, epithelial growth factor.
The p85 subunit of PI3 kinase interacts with phosphorylated EGFR/Tyro3.
NIH3T3 cells transfected with EGFR/Tyro3 (lanes 6-10) and the parental cells (lanes 1-5) were treated with EGF for various times. Equal amounts of cell lysates were immunoprecipitated with the anti-Tyro3 antibody. Immunoprecipitates were then blotted for (A) Tyro3, (B) PY-Tyro3, or (C) P85. (D) IgG portion of the gel was shown as a loading control. Lanes 11 and 12 were nontreated lysates from NIH3T3 and NIH3T3-EGFR/Tyro3, respectively. EGF, epithelial growth factor.
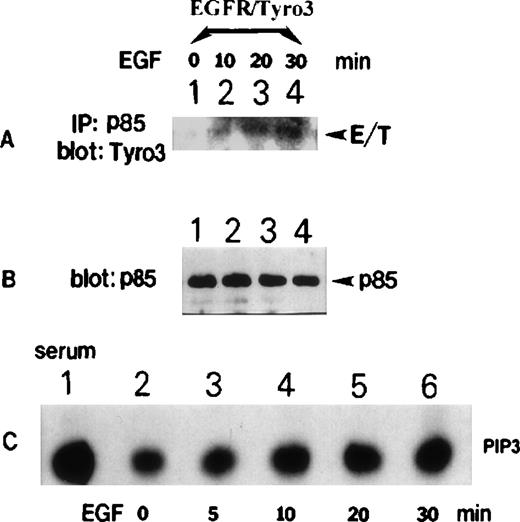
To confirm the observed interaction between p85 and Tyro3, reciprocal experiments were performed in that EGF-treated, as well as the vehicle-treated, control. EGFR/Tyro3 cell lysates were immunoprecipitated with the anti-p85 antibody. Western blot analyses revealed (Figure 5A) the presence of significant amounts of Tyro3 in p85 immunoprecipitates from EGF-treated cell lysates (lanes 2-4). Little Tyro3 was detected in vehicle-treated cells (Figure 5A, lane 1). Figure 5B shows the presence of approximately equal amounts of p85 protein in immunoprecipitates of all treatments. To determine whether PI3 kinase was activated in EGFR/Tyro3 cells after EGF treatment, Tyro3 immunoprecipitates from cells treated with EGF for various times were assayed for the PI3 kinase activity. Figure 5C shows that there was a basal level of PI3 kinase activity (only the PIP3 portion was shown) associated with Tyro3, and the PI3 kinase activity was increased after the addition of EGF (lanes 3-6). As a positive control, PI3 kinase immunoprecipitates from serum-stimulated cells exhibited high levels of PI3 kinase activity (Figure 5C, lane 1).
EGFR/Tyro3 activation is associated with an increased PI3 kinase activity.
NIH3T3-EGFR/Tyro3 cells treated with EGF for various times were lysed. Equal amounts of cell lysates were immunoprecipitated with the anti-p85 antibody. Immunoprecipitates were blotted for (A) Tyro3 and (B) p85. (C) NIH3T3-EGFR/Tyro3 cells treated with EGF for various times were immunoprecipitated with the anti-Tyro3 antibody. Immunoprecipitates were analyzed for the PI3 kinase activity, as described in “Materials and methods.” The PIP3 portion of the thin-layer chromatography data was shown. Lane 1 is the PI3 kinase assay results (a positive control) using p85-immunoprecipitates from serum-stimulated cell lysates. EGF, epithelial growth factor.
EGFR/Tyro3 activation is associated with an increased PI3 kinase activity.
NIH3T3-EGFR/Tyro3 cells treated with EGF for various times were lysed. Equal amounts of cell lysates were immunoprecipitated with the anti-p85 antibody. Immunoprecipitates were blotted for (A) Tyro3 and (B) p85. (C) NIH3T3-EGFR/Tyro3 cells treated with EGF for various times were immunoprecipitated with the anti-Tyro3 antibody. Immunoprecipitates were analyzed for the PI3 kinase activity, as described in “Materials and methods.” The PIP3 portion of the thin-layer chromatography data was shown. Lane 1 is the PI3 kinase assay results (a positive control) using p85-immunoprecipitates from serum-stimulated cell lysates. EGF, epithelial growth factor.
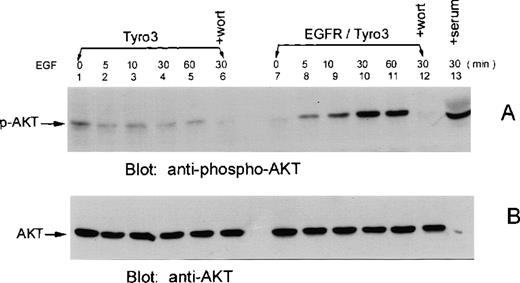
Akt is a downstream component of the PI3 kinase signaling pathway, and the phosphorylation of Akt is tightly associated with its activation.18 To study whether Tyro3 activation induces Akt activation, EGFR/Tyro3 and parental cells were treated with EGF, and cell lysates were blotted for the presence of phosphorylated Akt (p-Akt). Figure 6A shows that a small amount of p-Akt was detected in EGFR/Tyro3 cells in the absence of EGF (lane 7) or in parental cells in the presence (lanes 2-5) or absence (lane 1) of EGF. On the other hand, the level of p-Akt was significantly increased in EGFR/Tyro3 cells 5 minutes after EGF treatment (Figure 6A, lane 8), reaching the peak level approximately 30 minutes after treatment. The enhanced phosphorylation of Akt lasted for at least 30 additional minutes (Figure 6A, lane 11). In the presence of wortmannin, both basal and ligand-induced Akt phosphorylation was completely blocked, indicating that Akt phosphorylation is a consequence of PI3 kinase activation. As a positive control, serum-starved EGFR/Tyro3 cells were again fed with 20% serum and analyzed for phosphorylated Akt levels (lane 13). The blot was stripped and reprobed with an anti-Akt antibody recognizing all forms of Akt. The results are shown in Figure 6B as a loading control.
EGF induces Akt phosphorylation in EGFR/Tyro3 cells.
NIH3T3-EGFR/Tyro3 (lanes 7-12) and NIH3T3 cells (lanes 1-6) were treated with vehicle (lanes 1 and 7) or EGF (lanes 2-6 and 8-12) for various times. (A) Equal amounts of cell lysates were blotted for the presence of phosphorylated Akt (p-Akt) using a phosphospecific anti-Akt antibody. Lanes 6 and 12 were EGF-treated (30 minutes) cells cultured in the presence of wortmannin. Lane 13 represents the serum-starved NIH3T3-EGFR/Tyro3 cells fed again the serum for 30 minutes. (B) The same blot was stripped and reprobed with an antibody recognizing all forms of Akt. EGF, epithelial growth factor.
EGF induces Akt phosphorylation in EGFR/Tyro3 cells.
NIH3T3-EGFR/Tyro3 (lanes 7-12) and NIH3T3 cells (lanes 1-6) were treated with vehicle (lanes 1 and 7) or EGF (lanes 2-6 and 8-12) for various times. (A) Equal amounts of cell lysates were blotted for the presence of phosphorylated Akt (p-Akt) using a phosphospecific anti-Akt antibody. Lanes 6 and 12 were EGF-treated (30 minutes) cells cultured in the presence of wortmannin. Lane 13 represents the serum-starved NIH3T3-EGFR/Tyro3 cells fed again the serum for 30 minutes. (B) The same blot was stripped and reprobed with an antibody recognizing all forms of Akt. EGF, epithelial growth factor.
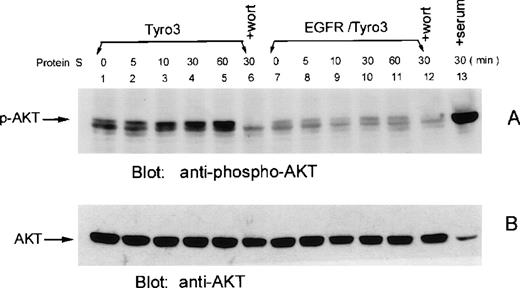
To examine whether Akt phosphorylation also occurred in rat Tyro3-transfected cells after the addition of ligand, we treated Tyro3 cells and EGFR/Tyro3 cells with human protein S for various times. Western blot analyses showed (Figure 7A) that a basal level of Akt phosphorylation was detectable in untreated control cells (lane 1). Akt phosphorylation started to increase 10 minutes after protein S treatment (Figure 7A, lane 3), and the enhanced phosphorylation persisted for at least 55 minutes (Figure 7A, lanes 5). No enhanced Akt phosphorylation was observed when EGFR/Tyro3 cells were treated with (Figure 7A, lanes 8-11) or without (lane 7) protein S treatment. Again, the basal and protein S-induced Akt phosphorylation was blocked by wortmannin (lanes 6 and 12). As a positive control, serum-starved Tyro3 cells treated with 20% serum for 30 minutes were analyzed for Akt phosphorylation (Figure 7A, lane 13). Figure 7B shows all forms of Akt protein as a loading control.
Human protein S induces Akt phosphorylation in Tyro3 cells.
NIH3T3-Tyro3 cells (lanes 1-6) and NIH3T3 cells (lanes 7-12) were treated with the vehicle (lanes 1 and 7) and human protein S (lanes 2-6 and 8-12) for various times. Cell lysates were blotted for phospho-Akt (A). Lanes 6 and 12 consist of protein S–treated (30 minutes) cells cultured in the presence of wortmannin. Lane 13 represents serum-starved NIH3T3-Tyro3 cells stimulated with the serum for 30 minutes. The same blot was stripped and reprobed with the antibody recognizing all forms of Akt (B).
Human protein S induces Akt phosphorylation in Tyro3 cells.
NIH3T3-Tyro3 cells (lanes 1-6) and NIH3T3 cells (lanes 7-12) were treated with the vehicle (lanes 1 and 7) and human protein S (lanes 2-6 and 8-12) for various times. Cell lysates were blotted for phospho-Akt (A). Lanes 6 and 12 consist of protein S–treated (30 minutes) cells cultured in the presence of wortmannin. Lane 13 represents serum-starved NIH3T3-Tyro3 cells stimulated with the serum for 30 minutes. The same blot was stripped and reprobed with the antibody recognizing all forms of Akt (B).
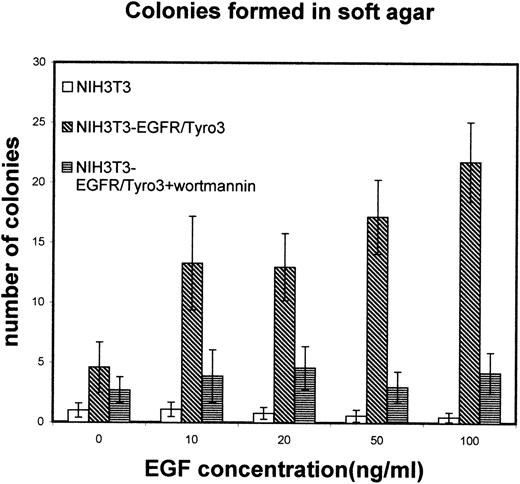
To determine whether Tyro3 receptor activation was correlated with its transforming ability, EGFR/Tyro3 and the parental cells were cultured in soft agar plates supplemented with various concentrations of EGF in the presence or absence of wortmannin. Figure8 shows that in the absence of EGF, the number of anchorage-independent (transforming) colonies of EGFR/Tyro3 cells was similar to that of NIH3T3 cells. However, in the presence of EGF (10 ng/mL) the number was significantly increased in EGFR/Tyro3 cells but not in NIH3T3 cells. The increase in transforming colonies was concentration-dependent on the ligand and significantly inhibited by wortmannin. These observations indicate that the PI3 kinase pathway largely mediates Tyro3-induced transformation.
Tyro3 activation results in transformation.
NIH3T3-EGFR/Tyro3 and NIH3T3 cells were cultured in the presence of various concentrations of EGF and analyzed for their ability to form anchorage-independent colonies in soft-agar assays. As controls, wortmannin was supplemented to EGF-treated EGFR/Tyro3 cells. EGF, epithelial growth factor.
Tyro3 activation results in transformation.
NIH3T3-EGFR/Tyro3 and NIH3T3 cells were cultured in the presence of various concentrations of EGF and analyzed for their ability to form anchorage-independent colonies in soft-agar assays. As controls, wortmannin was supplemented to EGF-treated EGFR/Tyro3 cells. EGF, epithelial growth factor.
Discussion
Using yeast 2-hybrid technology, we demonstrated the interaction between p85 and the cytoplasmic domain of Tyro3. This in vitro interaction has been confirmed by GST pull-down experiments and by our in vivo studies using NIH3T3 cell lines expressing a fusion receptor protein with its extracellular domain from EGF receptor and its transmembrane in addition to cytoplasmic domains from Tyro3. The p85 association with Tyro3 was dependent on tyrosine phosphorylation of Tyro3. Apparently, the SH2 domain of p85 was involved in the protein–protein interaction because the cDNA fragment of p85 isolated from the positive yeast clone contained the region encoding an SH2 domain (data not shown). It has been shown that the chicken C-terminal region of Rek, most likely a Tyro3 ortholog, has 2 consensus SH2-binding motifs.19 The first motif (YDLM) is predicted to bind PI3 kinase, whereas the second one (YVNI) is predicted to bind Grb2/Sem5.19 The same YDLM motif is conserved in rat Tyro3 (amino acids 752-75520) and thus, may be the site for PI3 kinase interaction. In addition, the deduced amino acid sequence of rat Tyro3 shows 97% identity to a mouse counterpart. The high degree of structural conservation indicates that the signaling pathway of Tyro3 between these 2 species should be also conserved. One may question whether Tyro3 protein expressed in bacteria is phosphorylated because it captures p85. Western blot analysis using an anti-PY antibody demonstrated that there existed some tyrosine-phosphorylated GST-Tyro3 (data not shown), presumably as a result of autophosphorylation of the overexpressed protein.
Overexpression of many PTK genes alone is often sufficient for their functional activation and transformation.1 Taylor et al7 have shown that overexpression of Sky receptor tyrosine kinase, either at the cell surface or inside the cell, results in ligand-independent activation. Our observation that EGF is required for increased colony formation in soft agar assays suggests that overexpression of Tyro3 alone may not result in maximal activation of the receptor. Considering that PI3 kinase activation is dependent on ligand-induced Tyro3 phosphorylation (Figure 5) and that PI3 kinase and Akt activation are usually associated with cell proliferation,17 18 it is reasonable to conclude that the transforming activity of Tyro3 is mediated at least in part by the PI3 kinase pathway. In fact, the addition of wortmannin drastically reduced the number of transforming colonies in EGF-treated EGFR/Tyro3 cells (Figure 8).
Protein S was originally identified as a ligand for Tyro3.5Subsequent studies by Godowski et al3 have shown that human Gas6, but not human protein S, is the true ligand for Tyro3 because human protein S is unable to activate human Tyro3. Although several studies have confirmed that Gas6 is a ligand for Tyro3, Axl, and Mer receptor tyrosine kinases,4 the status of protein S remains unresolved. Recently, it has also been demonstrated that bovine protein S is capable of stimulating Sky tyrosine phosphorylation,21 suggesting that protein S may function as a Tyro3 ligand at least in some species. Our current study also confirmed that interspecies ligand-receptor interaction occurs between protein S and Tyro3 (Figure 7).
The physiological role of Gas6 remains unclear. Gas6, in conjunction with heregulin and forskolin, stimulates maximal proliferation of human Schwann cells.10 It has been shown that Sky and its ligand Gas6 regulate proliferation and differentiation of primordial germ cells22 and that Dtk is involved in early hematopoiesis.2 Recently, it was observed that Gas6 and its receptor Tyro3 support the function of osteoclasts,23suggesting a role of the vitamin K-dependent ligand in bone resorption. Interestingly, it has been demonstrated that cross-linking of protein S, an anticoagulator, to lymphocyte cell surface promotes cell aggregation and inhibits their proliferation,24 suggesting that the clotting pathway may modulate wound-related inflammatory responses. Recent gene knockout studies have shown that Tyro3 family receptors are essential for development of the reproductive system.11 These receptors appear to be essential for the development of mature nervous and immune systems.11
Acknowledgment
The authors thank Dr Trevor Stitt of Regeneron Inc for providing Tyro3-expressing cell lines.
Supported by Public Service Award CA59985.
Drs Lan and Wu contributed equally to the work reported here.
Reprints:Wei Dai, Division of Hematology-Oncology, Department of Internal Medicine, University of Cincinnati College of Medicine, K-Pavilion, ML-508, 231 Bethesda Avenue, Cincinnati, OH 45267; e-mail:wei.dai@uc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.