The authors have shown accelerated endothelialization on polyethylene terephthalate (PET) grafts preclotted with autologous bone marrow. Bone marrow cells have a subset of early progenitor cells that express the CD34 antigen on their surfaces. A recent in vitro study has shown that CD34+ cells can differentiate into endothelial cells. The current study was designed to determine whether CD34+ progenitor cells would enhance vascular graft healing in a canine model. The authors used composite grafts implanted in the dog's descending thoracic aorta (DTA) for 4 weeks. The 8-mm × 12-cm composite grafts had a 4-cm PET graft in the center and 4-cm standard ePTFE grafts at each end. The entire composite was coated with silicone rubber to make it impervious; thus, the PET segment was shielded from perigraft and pannus ingrowth. There were 5 study grafts and 5 control grafts. On the day before surgery, 120 mL bone marrow was aspirated, and CD34+ cells were enriched using an immunomagnetic bead technique, yielding an average of 11.4 ± 5.3 × 106. During surgery, these cells were mixed with venous blood and seeded onto the PET segment of composite study grafts; the control grafts were treated with venous blood only. Hematoxylin and eosin, immunocytochemical, and AgNO3staining demonstrated significant increases of surface endothelialization on the seeded grafts (92% ± 3.4% vs 26.6% ± 7.6%; P = .0001) with markedly increased microvessels in the neointima, graft wall, and external area compared with controls. In dogs, CD34+ cell seeding enhances vascular graft endothelialization; this suggests practical therapeutic applications.
Improved healing and function of vascular grafts can be achieved with a proper seeding technique and an appropriate cell source. In 1978 Herring et al1 were the first to show the benefits of seeding vascular grafts with endothelial cells. Zilla et al2,3 modified the seeding method using mass culture techniques with homologous serum, and they demonstrated a significantly improved patency rate in grafts seeded with the cultured endothelial cells. Several studies have shown that seeding grafts with endothelial cells obtained from external jugular vein, saphenous vein, or adipose tissue also led to improved graft healing.4 5
We have demonstrated that cells in circulating blood can contribute to the surface endothelialization of polyethylene terephthalate (PET) vascular grafts and have named this phenomenon fallout healing.6 Mature endothelial cells have been shown to circulate in fresh peripheral blood.7 These cells, detached from the lining of the cardiovascular tree, may be a source of fallout healing to endothelialize flow surfaces of impervious PET grafts.6 On the other hand, polymerase chain reaction analysis on endothelial cells harvested from the surface of impervious grafts implanted in irradiated dogs transplanted with bone marrow from dog leukocyte antigen mismatched, unrelated donors showed a pure donor genotype,8,9 suggesting that the cellular source of fallout healing was predominantly from endothelial precursor cells in the bone marrow. Based on this finding, Fujita et al10 used bone marrow blood to preclot/seed PET grafts, and they observed an extensively accelerated endothelialization as early as 1 week after implantation, but the unwanted side effect of microcalcification was found at approximately 4 weeks after implantation.
Bone marrow contains pluripotent CD34+ cells, which are known to give rise to hematopoietic cells. In vitro studies show that they can differentiate into mature endothelial cells.9,11 12 The purpose of this study was to determine whether canine bone marrow-derived CD34+ cells have the in vivo potential to enhance vascular graft endothelialization with an effect comparable to treatment with bone marrow blood when seeded onto impervious composite grafts implanted in the descending thoracic aorta (DTA) of dogs, but avoiding the unwanted early microcalcification.
Materials and methods
Ten healthy young mongrel dogs were used. This study was approved by the Animal Care and Use Committee of The Hope Heart Institute. Care and use of all animals complied with the Guide for the Care and Use of Laboratory Animals.
CD34+ cell separation
One day before surgery, the dogs were anesthetized and 120 mL bone marrow was aspirated from the humeri and mixed with heparin at a dose of 10 IU/mL.13 The marrow was then taken to the Fred Hutchinson Cancer Research Center for separation of CD34+cells. The marrow cells were Ficoll separated (specific gravity, 1.074) and washed twice in phosphate-buffered saline (PBS)/2% horse serum. Cells were incubated at 1 × 108/mL with mouse antidog monoclonal antibody 1 H614 at 10 μg/mL for 30 minutes at 4°C. They were then washed in PBS/2% horse serum and were incubated with immunomagnetic rat antimouse IgG1 microbeads (Miltenyi Biotech, Auburn, CA). The cell bead mixture was run through a magnetic column, and the column was washed several times to remove nonspecifically bound cells. It was then released from the magnet, and the positively selected cells were eluted from the column.15
The separated CD34+ cells were kept in approximately 1 mL PBS with 2% horse serum overnight at 4°C in a slow rotation chamber. They were then washed free of proteins, and their viability was assessed using trypan blue stain. Cells that stained blue were considered nonviable because the dye would have penetrated these cells and caused the cytoplasm to stain. The number of cells that did not stain was determined using a counting chamber. An aliquot of these cells was further analyzed using a fluorescent activated cell sorter (FACS). Samples were run on a FACScan (Becton-Dickinson, Mountain View, CA) and list mode data analyzed using WINLIST (Verity Software House, Topsham, ME).
Graft preparation and seeding
A three-component composite, impervious, tandem graft was constructed by suturing 4-cm long segments of 8-mm diameter standard ePTFE to each end of a 4-cm long, 8-mm diameter crimped knitted PET graft. This PET segment, including end suture lines, was wrapped in a silicone rubber sheet, and then the entire tandem composite graft was externally coated with an adherent, 2-mm–thick silicone rubber layer (Figure 1A). Thus, the seeding effect could be strictly evaluated in the central PET segment because the ePTFE segments at the ends prevented pannus extension from the native aorta, and transmural tissue ingrowth from the perigraft areas was blocked by the external coating.
Scheme of experimental model.
(A) Design of composite graft. (B) Implantation in the descending thoracic aorta.
Scheme of experimental model.
(A) Design of composite graft. (B) Implantation in the descending thoracic aorta.
The central PET segment of the composite graft was seeded using a modified four-step preclot method. In the first step, 1 mL CD34+ cell suspension was mixed with 3 mL peripheral venous blood and pressurized into the lumen and the space between the PET graft wall and its external silicone rubber sheet wrap, with Fogarty vascular clamps in place at each end of the PET segment. The graft was rotated for approximately 5 minutes to ensure adequate spread. The excess mixture of cell suspension and blood from this step was removed from the lumen and was saved for later use in the fourth step. In the second and the third steps, 5 mL peripheral venous blood was injected into the lumen to form a layer of fibrin coagulum in the PET graft wall and its surface, and a 4F Fogarty balloon catheter was used to remove excess thrombus from the graft lumen and surface. In the fourth step, the remaining mixture of blood and CD34+ cells from step 1 was mixed with 5 mL heparinized blood (4 mL blood and 1 mL heparin) to activate antithrombin III to neutralize the excessive thrombin remaining on and in the graft surface and wall and to decrease the graft's thrombogenicity.16-18 This mixture was pressurized into the lumen. The seeded graft was then kept in a moist sponge at room temperature for approximately 20 minutes while another team was preparing to expose and clamp the DTA and make it ready for implantation.
The control graft, which was the same composite used for seeded grafts, was treated with venous blood without seeding.
Graft implantation
The prepared graft was then placed in the canine DTA (Figure 1B) with the establishment of a carotid-femoral shunt during aortic cross clamping. The surgical techniques are described in our previous publications.6 8 Both the seeded and the control grafts were implanted using the same technique.
This study included 5 grafts in each of the seeded and control groups, and all grafts were studied after 4 weeks of implantation.
Specimen evaluation
At the end of the study, after induction of deep anesthesia, 10 000 IU heparin was given intravenously, and the dog was exsanguinated. The specimen was removed and then gently flushed with Dulbecco's phosphate-buffered saline solution, opened longitudinally, rinsed again, pinned flat, and photographed.
Three sets of full-wall thickness longitudinal tissue samples were taken from each of the ePTFE-PET anastomotic areas and from the center of the PET graft. Additional samples were taken from areas of interest in the PET graft. Each set of tissue samples, including 4 adjacent tissue blocks (approximately 1 cm × 0.5 cm each), was studied as follows: (1) resin-embedded sections stained with hematoxylin and eosin for general observations; (2) wax-embedded sections for immunocytochemical studies, including monoclonal mouse antihuman Factor VIII/von Willebrand factor (FVIII/vWF) antibody (code M-616; DAKO, Carpinteria, CA) and canine CD34+ polyclonal antibody for endothelial cells15 and monoclonal mouse antihuman smooth muscle α-actin antibody (code M-851; DAKO) for smooth muscle cells; (3) scanning electron microscopy; and (4) transmission electron microscopy.
The flow surface was then stained with 0.5% silver nitrate and viewed under a 65× stereomicroscope to assess the endothelial-like cell coverage (ELCC) score, expressed as the percentage of the flow surface covered by polygonal endothelial-like cells (ELCs). The most representative thickness of the graft surface lining, determined by careful examination of each of the hematoxylin and eosin-stained sections, was measured with a micrometer. The average value with standard deviation was then calculated for each group.
Results
Separation of CD34+ cells
The average number of CD34+ cells isolated in the 5 grafts in each group was 11.4 4 ± 5.3 × 106 (Table1), and viability was nearly 100%, as judged by trypan blue exclusion just before seeding.
General observations
All grafts were patent at the time of retrieval, and there was no sign of infection. In both the seeded and the control groups, composite grafts were well encapsulated with surrounding pleural tissue, though it was not firmly attached because of the nature of the silicone rubber coating. There was a thin rim of dark red blood clot in the space between the PET and the silicone rubber wrap in test and control grafts. There was no indication of infarction in the kidneys or other abdominal organs in any of the 10 dogs studied.
Graft flow surface study
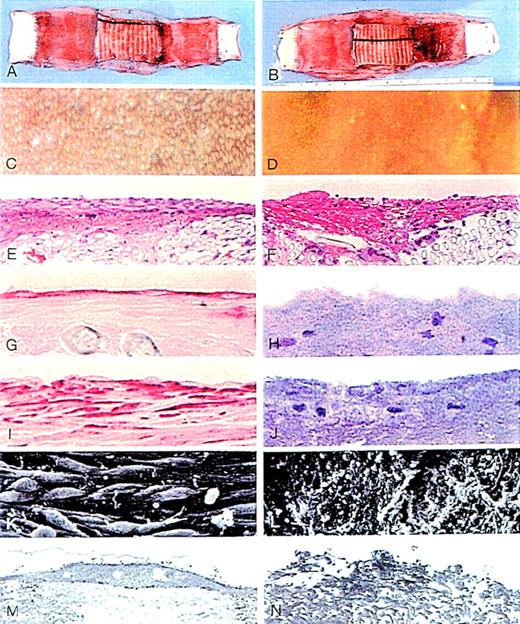
Seeded and control grafts looked similar to the naked eye. However, under the stereomicroscope, the seeded graft flow surface was seen to be extensively covered with a thin layer of white or pink glistening tissue, whereas the control graft surface was covered with a thin layer of translucent fibrinlike material with some thrombus (Figures2A, 2B). Silver nitrate staining made it possible to distinguish quantitatively the areas covered with polygonal ELCs on the flow surface (Figures 2C, 2D). Table1 shows the ELCC scores for each of the grafts in the seeded and the control groups; the average score was 92% ± 3.4% on the seeded graft flow surfaces and only 26.6% ± 7.6% on the control grafts (P = .00001; Student ttest). There was no significant correlation between the number of separated CD34+ cells and the degree of ELCC on the seeded grafts.
Comparisons of representative seeded and control grafts.
Panels on left show seeded grafts, and panels on right, control grafts. (A,B) Gross specimens. (C,D) Silver nitrate-stained flow surfaces (×85). (E,F) Hematoxylin and eosin stain (×145). (G,H) CD34 stain (×580). (I,J) Smooth muscle α-actin stain (×560). (K,L) Scanning electron microscopy (×1000). (M,N) Transmission electron microscopy (×5400).
Comparisons of representative seeded and control grafts.
Panels on left show seeded grafts, and panels on right, control grafts. (A,B) Gross specimens. (C,D) Silver nitrate-stained flow surfaces (×85). (E,F) Hematoxylin and eosin stain (×145). (G,H) CD34 stain (×580). (I,J) Smooth muscle α-actin stain (×560). (K,L) Scanning electron microscopy (×1000). (M,N) Transmission electron microscopy (×5400).
Silver nitrate staining also showed that the surface of the ePTFE segments in both the seeded and control grafts had no ELC coverage, except on the pannus zones to the extent of a few millimeters inward from the anastomotic line. There were rare instances where a small, isolated ELC patch was found in the middle of the surfaces of the ePTFE segments of the composite grafts in both control and seeded grafts.
Histologic evaluation
There were no osteocytes, osteoblasts, or microcalcification in the 4-week seeded grafts. Immunocytochemical staining with FVIII/vWF and CD34 antibody demonstrated that the ELCs delineated by silver nitrate staining had the nature of endothelial cells.
On the seeded grafts, there was a layer of neointima approximately 174 ± 53 μm thick consisting of a single layer of endothelial cells shown to be positive with FVIII/vWF and CD34+ staining on the surface, and there were varying amounts of positive α-actin staining cells and some inflammatory cells. Most of the control graft surfaces were covered with a thin layer of pseudointima approximately 124 ± 94 μm thick. There were no endothelial cells on the pseudointima, which was largely composed of a fibrin coagulum with some red cells, macrophages, neutrophils, giant cells, and occasional α-actin positive cells (Figures 2E-2J). Patches of neointima similar to those covering the seeded grafts were observed occasionally on the control graft surface.
Scanning and transmission electron microscopic studies showed a contiguous layer of endothelial cells on the seeded graft surfaces. Cell morphology varied from a spindle-shape to a fish-scale appearance. Most of the areas on the control grafts were covered with a film of proteinlike fibrin material mixed with blood cells (Figures 2K-2N).
In seeded and control groups, the graft wall and the space between the wall and the external silicone rubber wrap were filled primarily with a fibrinous matrix infiltrated with red blood cells and some inflammatory cells. The major difference was that in the seeded grafts, microvessels of varying shapes and sizes, which were positively delineated by FVIII/vWF and CD34 staining, frequently appeared in the neointima, the graft wall, and the space between the wall and the external wrap. Several microvessels showed a tendency to connect with each other in these areas (Figures 3A-3C). The microvessels in the externally wrapped space appeared to be relatively larger than in the graft wall or intima. In comparison, similar microvessels were less often found in the control grafts.
Antibodies to endothelial cell CD34 stain delineate the enhanced microvessel formation in seeded grafts.
(A) Inside the neointima and external space of the graft (×75). (B) Microvessel (arrow) and representative view of continuous linear staining pattern of a microvessel traversing the graft wall (arrowheads) (×75). (C) Enlargement of area in B (arrowheads) (×300).
Antibodies to endothelial cell CD34 stain delineate the enhanced microvessel formation in seeded grafts.
(A) Inside the neointima and external space of the graft (×75). (B) Microvessel (arrow) and representative view of continuous linear staining pattern of a microvessel traversing the graft wall (arrowheads) (×75). (C) Enlargement of area in B (arrowheads) (×300).
Discussion
CD34+ cells include pluripotent hematopoietic progenitor cells and are defined by the expression of their surface antigen, which is a mucin-like cell surface glycoprotein. Angioblasts and hematopoietic stem cells share certain antigenic determinants, including Flk-1, TIE-2, and CD34.19 During embryologic development, blood islands arise from splanchnopleural mesoderm. Peripherally located cells are precursors of endothelial cells called angioblasts, whereas the centrally located cells are hematopoietic precursor cells. They are thought to come from a common precursor called the hemangioblast. This is supported by the observation that hematopoietic cells such as QH 1 and MB 1 in the quail and CD34 and PECAM-1 in the mouse19 20 also express many molecules in the endothelium.
Asahara et al11 isolated putative endothelial cell progenitors from peripheral blood by magnetic bead selection on the basis of cell surface CD34+ antigen expression that became spindle-shaped endothelial cells and proliferated for 4 weeks. Rafii et al9 successfully cultured CD34+ cells and demonstrated that they can differentiate into endothelial cells in vitro. Shi et al in 19946 and Kouchi et al in 199821 demonstrated, in different dog models, definite endothelialization from fallout sources on graft surfaces shielded from external ingrowth. Subsequent studies by Shi et al8,9further demonstrated that the fallout endothelial cells are derived from bone marrow. This led to the study by Fujita et al,10which showed 80% endothelialization of PET grafts preclotted and seeded with bone marrow blood at 1 week, but some unwanted osteocytes and microcalcification were found in the grafts implanted for 4 weeks. Based on the reports cited above, we developed our experimental design concept of CD34+ cell seeding, hoping to achieve enhancement of graft endothelialization while avoiding these undesirable side effects.
The recent availability of the necessary monoclonal antibodies allowed us to initiate studies on the in vivo functions of canine CD34+ cells. Monoclonal antibody 1H6, 1 of the 10 monoclonal antibodies that recognize CD34,14 has been used extensively to isolate canine hematopoietic progenitors for stem cell transplantation studies (McSweeney PA, unpublished data). Flow cytometry indicated that a high level of enrichment of CD34+ cells was achieved and that the viability of the separated CD34+ cells was nearly 100%, as assessed by trypan blue exclusion. These cells, seeded in and on the grafts in our experimental model, had the potential to differentiate once the proper biologic environment was provided after implantation. Zauli et al22 showed that the number of progenitor cells, including CD34+ cells, was increased in cultures stimulated by a combination of cytokines. Rafii et al9 have further emphasized the necessity of particular growth factors for the differentiation of CD34+ cells to endothelial cells. Studies have demonstrated that platelet-derived growth factor, vascular endothelial growth factor, and basic fibroblast growth factor can be released from platelets and other blood cells.22-24 We have found that the fibrin coagulum in the PET graft interstices and in the space between the graft wall and the silicone sheet wrap entraps many erythrocytes, leukocytes, and platelets, potentially providing a rich source of growth factors to facilitate the maturation of CD34+ cells into endothelium.
In our experience, the CD34 selection procedure typically depletes non-CD34+ cells by an order of 3 to 4 logs (Bruno B, McSweeney PM, unpublished data). The CD34+ cells are enriched from a starting percentage of approximately 2% in unmodified bone marrow14 to > 75% after enrichment. The enrichment of CD34+ cells correlated in our study with an enhancement of flow surface endothelialization and mural microvessel formation, strongly suggesting that the CD34+ cells were responsible for this activity. Although a smaller population of non-CD34+ cells could be contributing to these processes, this appears less likely. Analysis of the sample of separated CD34+ cells showed that contaminating cells were predominantly monocytes or small lymphocytes that nonspecifically bind to the microbeads. The latter population cannot be accurately defined in dogs because of the lack of useful B-cell and natural killer-cell markers, but we postulate that these are B cells because they express CD45 and because in human studies B cells are less readily depleted from CD34 cells than other lymphocyte populations. On the other hand, unlike the bone marrow-seeded grafts,10 we could not find any osteocytes or microcalcification in the 4-week seeded grafts. This indicated that the growth of the osteocyte and its precursors was either somewhat inhibited or excluded by the separation procedures.
Our composite graft model was designed so that the only sources of endothelium were either fallout from the circulating blood or differentiation of the CD34+ cells trapped in the interstices of the PET graft and in the space between the PET and outer silicone rubber sheet wrap after seeding. There have been several reports of endothelial cell outgrowth from cultured blood.7,9,11 Lin et al25 recently determined that CD34+ circulating endothelial precursor cells have greater proliferative potential than the mature endothelial cells that also circulate in the blood. Our study appears to support this finding, because the study grafts seeded with CD34+ cells had an average of 90% endothelial coverage. In contrast, the control grafts exposed to peripheral blood only, which has been shown to have a mature endothelial population and a lesser number of precursor cells,25had endothelial coverage of 26%. However, longer-term studies are needed to demonstrate further the advantages from CD34+cell seeding, including long-term patency.
Histologic evidence showed that there were more microvessels (many containing erythrocytes in the lumen) in the seeded grafts than in the control grafts. They were located in the neointima, in the graft wall, and in the space between the graft wall and the external silicone rubber wrap (Figure 3). Clowes et al26define the role of microvessels in the mechanisms of graft healing, and their findings suggest that microvessels may be closely associated with, and provide the endothelial cellular source for, graft surface endothelialization. Because our model prevented communication with existing microvessels in the perigraft tissue, the development of these new microvessels may be associated with the process of differentiation of CD34+ cells into endothelial cells.9 25 The presence of the microvessels also suggests that they can develop in the absence of existing vessels.
When the experimental techniques become feasible, labeling the isolated CD34+ cells with a long-lasting marker (which carries through steps of cell differentiation and division and can be seen in the mature endothelial cells) will provide definitive evidence that the enhancement of endothelialization originates and benefits from transplanted CD34+ cells.
Our observation may have clinical relevance if the CD34 origin of endothelial cells can be further substantiated by our ongoing studies. As the process of CD34+ cell separation becomes quicker and less expensive, it may be possible to perform bone marrow aspiration and separation during the surgical procedure. The CD34+cells could then be used to seed small-caliber bypass grafts. This may be more effective and less time consuming and traumatic than harvesting arm veins or using autologous endothelial cells, and the unwanted side effects of bone marrow seeding, such as osteocytes and microcalcification in the graft wall, could presumably be avoided.
This study demonstrated in the dog that CD34+ cells seeded into grafts can enhance vascular graft endothelialization and accompanying microvessel formation. It supports our concept of a bone marrow origin for these phenomena, and it suggests practical therapeutic applications from these basic biologic insights.
Acknowledgments
The authors thank C. R. Bard, Inc, and W. L. Gore and Associates, Inc, for their donation of graft material. They also thank Dorothy Mungin, histology technician, Warren Berry, medical photographer, Mary Ann Sedgwick Harvey, medical editor, and Mary-Ann Nelson, medical illustrator.
Supported in part by National Institutes of Health grants HL03701, DK42716, and CA15704.
Reprints:Moses Hong-De Wu, Department of Experimental Surgery, The Hope Heart Institute, 528 18th Avenue, Seattle, WA 98122.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.