Allogeneic bone marrow transplantation (BMT) is the only curative therapy for chronic myelogenous leukemia (CML), though several studies indicate that prolonged survival can result from interferon- (IFN-) treatment. IFN- is now often used as initial therapy for CML, before donor availability is known. Because identifying potential donors can take several weeks to months, it is important to know whether IFN- adversely affects outcome of a subsequent BMT. If it does, initiation of IFN- therapy might be delayed until donor availability is determined and avoided in patients for whom BMT is planned. We studied 873 patients who received HLA-identical sibling BMT for chronic-phase CML in 153 centers participating in the International Bone Marrow Transplant Registry. The object was to compare outcome in the 664 who received only hydroxyurea before BMT with outcome in the 209 who received IFN- with or without hydroxyurea. The median duration of IFN- therapy was 2 months (range, 1 to 39 months). Cox proportional hazards analysis was used to compare engraftment, graft-versus-host disease (GVHD), nonrelapse mortality, relapse, survival, and leukemia-free survival after adjustment for other prognostic variables. We found a higher risk of nonengraftment among patients given IFN- than among those given hydroxyurea alone (2% versus 0.2%; P = 0.01). Patients who received IFN- had a lower risk of relapse (relative risk, 0.17; 95% confidence interval, 0.04-0.70). Probabilities of GVHD, nonrelapse mortality, survival, and leukemia-free survival were similar in the two treatment groups. These results suggest that a short course of IFN- does not adversely affect survival after a subsequent HLA-identical sibling BMT for chronic-phase CML.
Two common treatments for chronic myelogenous leukemia (CML) are allogeneic bone marrow transplantation (BMT) and interferon alpha (IFN-α). Transplants from HLA-identical sibling donors produce long-term disease-free survival, and presumably cure, in about 60% of patients who undergo BMT during the first chronic phase.1-6 However, BMT cannot be performed immediately after diagnosis because of the time required for donor identification, referral to a transplant center, and pretransplantation patient evaluation. Early nonrelapse mortality is between 10% and 30%, but most patients who survive the early complications have few or no long-term sequelae and good quality of life.
In contrast to BMT, IFN-α therapy can be started shortly after diagnosis and, either alone or in combination with hydroxyurea, it produces hematologic remissions in most patients with newly diagnosed CML. It produces major cytogenetic remissions in only 20% to 50%.7-13 These patients have prolonged survival, although the Philadelphia (Ph) chromosome eventually returns or increases in most of them. Mortality related to IFN-α treatment is near zero, though side effects are common. Some investigators recommend IFN-α as initial therapy, reserving BMT for patients in whom cytogenetic remission is not achieved.14-16 However, this strategy has not been tested and other studies indicate that BMT within the first year of diagnosis is the treatment of choice in patients with a suitable donor.17-19
It is unknown whether prior treatment with IFN-α affects the outcome of a subsequent BMT, as has been shown for busulfan, which increases nonrelapse mortality.20 IFN-α exerts immune-modulatory effects that might affect the outcome of allogeneic BMT. These include up-regulation of class I and class II major histocompatibility antigen expression, enhancing the cytotoxicity of T lymphocytes and natural killer cells, and regulating the secretion of other inflammatory and anti-inflammatory cytokines.21 These effects could alter graft failure, hematopoietic recovery, incidence or severity of acute or chronic graft-versus-host disease (GVHD), and CML relapse. Previous studies analyzing effects of prior IFN-α therapy on BMT outcome had contradictory results. These single-institution series included relatively few patients or were not limited to HLA-identical sibling BMTs.22-24
IFN-α is increasingly used as initial therapy for CML, before donor availability is known. Because identifying potential donors can take several weeks to months, it is important to know whether IFN-α adversely affects outcome of a subsequent BMT. If it does, initiation of IFN-α therapy might be delayed until donor availability is determined and avoided if a BMT is planned. We addressed this issue by using the large observational database of the International Bone Marrow Transplant Registry (IBMTR) to compare outcomes in HLA-identical sibling BMT recipients whose pretransplantation therapy included IFN-α treatment with outcomes in those whose pretransplantation therapy did not include this agent.
Patients and methods
Patients
The eligibility criteria were age > 10 years, a non-T-cell–depleted HLA-identical sibling BMT for Ph-positive CML in the first chronic phase between 1987 and 1994, posttransplantation GVHD prophylaxis using cyclosporine with or without other drugs, and use of hydroxyurea alone, IFN-α alone, or both agents for CML treatment before pretransplantation conditioning. Patients known to have received IFN-α for < 4 weeks were excluded. Because only 2 patients who received IFN-α underwent BMT within 3 months of diagnosis and none who had BMT > 4 years after diagnosis received IFN-α, we restricted the comparisons to patients who had BMT between 3 and 48 months after diagnosis to minimize any confounding effect of time to treatment. Among the 51 patients who had BMT < 3 months after diagnosis (2 who received IFN-α and 49 who received hydroxyurea), there was 1 relapse; the 3-year probability of survival was 71% (95% CI, 53%-83%). A total of 873 patients who underwent BMT 3 to 48 months after diagnosis were studied after the BMT; 664 of them had received only hydroxyurea before BMT and 209 had received IFN-α with or without hydroxyurea before the procedure.
Endpoints
The primary outcome studied was leukemia-free survival, defined as survival in continuous complete remission. Events were relapse or death from any cause. Patients alive in continuous complete remission were censored at last follow-up. Relapse of CML was defined using clinical and hematologic criteria; serial cytogenetic or molecular tests were not available for all patients. Patients were considered to have had a relapse at the time of hematologic recurrence or, in the absence of hematologic recurrence, at the time of initiation of therapy for relapse. Other outcomes studied were neutrophil recovery (time to an absolute neutrophil count > 0.5 × 109/L), platelet recovery (time to a platelet count > 20 × 109/L without transfusion), graft failure (failure to achieve a neutrophil count > 0.5 × 109/L or a subsequent decline to levels < 0.5 × 109/L), grade II-IV and grade III-IV acute GVHD,25 chronic GVHD,26 nonrelapse mortality (death while in continuous complete remission; patients were censored at relapse or last follow-up), relapse (patients were censored at death while in continuous complete remission or last follow-up), and survival (patients were censored at last follow-up).
Statistical methods
Initially, the 873 patients were grouped into 4 categories for analysis according to IFN-α therapy and interval from diagnosis to BMT because of previous studies indicating an association between disease duration and BMT outcome. Group 1 included 463 patients who were treated with hydroxyurea alone and underwent BMT 3 to 12 months after diagnosis; group 2 included 201 patients who were treated with hydroxyurea alone and had BMT > 12 to 48 months after diagnosis; group 3 included 113 patients who received IFN-α with or without hydroxyurea and had BMT 3 to 12 months after diagnosis; and group 4 included 96 patients who were given IFN-α with or without hydroxyurea and had BMT > 12 to 48 months after diagnosis. The 4 groups were compared for differences in relevant patient, disease, and nonrelapse characteristics by using χ2 and Fisher exact tests. Univariate analyses of BMT outcomes used the cumulative incidence method for estimating nonrelapse mortality and relapse and the Kaplan-Meier estimator for other outcomes, analyzed with the log-rank test. Multivariate analyses used Cox proportional hazards regression with forward stepwise variable selection to identify prognostic factors other than pretransplantation treatment and disease duration that influenced outcome.27 All variables were tested for the assumption of proportional hazards by using a time-dependent covariate approach. A random-effects score test found no evidence of center effects.28 Because both univariate and multivariate analyses showed no difference between patients who had BMT 3 to 12 months after diagnosis and those who had BMT > 12 months after diagnosis, final models included only 2 treatment groups: IFN-α therapy (with or with hydroxyurea) and hydroxyurea therapy alone. Only the outcomes in those 2 groups are presented. Other factors considered in the model-building procedure are listed in Table1.
Results
Patient and treatment characteristics
Patient, disease, and treatment characteristics in the 4 initial study groups are summarized in Table 1. For all patients in the study, median age was 36 years (range, 10-62 years). Median time from diagnosis to BMT was 9 months (range, 3-46 months). Fifty-three percent of patients received total body irradiation for pretransplantation conditioning; 77% received cyclosporine and methotrexate as GVHD prophylaxis. Median follow-up was 2.8 years. There were several significant differences in pretreatment and treatment characteristics in the 4 groups. Most notably, patients who received IFN-α tended to be older, were more likely to have had marrow fibrosis before BMT, had lower white blood cell counts before BMT, had undergone BMT more recently, and were less likely to have received total body irradiation than those given hydroxyurea alone.
IFN- therapy
In the IFN-α–therapy group, details regarding dose, duration, and response were not available for all patients. Available data are summarized in Table 2. Patients received IFN-α for a median of 2 months (range, 1-39 months) at a median starting dose of 3 × 106 U/d (range, 1-10 units). Sixty-seven percent of patients received IFN-α for < 6 months. Eighty-five percent of patients had at least a hematologic remission while taking IFN-α. Twenty-three percent had a documented cytogenetic response. The main reason for discontinuing IFN-α was to proceed to BMT; only 13% of patients had BMT after a lack of response to IFN-α or progression of disease after an initial response to IFN-α.
In multivariate analyses, IFN-α dose, schedule, and response (Table2) were assessed for their effect on posttransplantation outcome. Forty-seven patients without detailed information on IFN-α therapy were first compared with patients for whom such data were available. Outcomes for the 2 groups were not significantly different. Among the 162 patients for whom data were available, neither dose, schedule, response, nor duration of IFN-α treatment was associated with BMT outcomes. The 47 patients who received IFN-α for longer than 6 months had rates of relapse, nonrelapse mortality, and leukemia-free survival that were similar to those of patients who received the agent for shorter periods.
Engraftment
Among patients who survived at least 21 days after BMT, 4 patients who received IFN-α and 1 patient who received only hydroxyurea failed to engraft (2% versus 0.2%; P = .01). Among patients who had engraftment, 1 patient who received IFN-α and 2 patients who received hydroxyurea alone subsequently lost their graft (0.5% versus 0.3%; P = .56). Median time to neutrophil recovery was 20 days (range, 9-54 days) with IFN-α and 20 days (range, 3-51 days) with hydroxyurea alone (P = .81). Median times to platelet recovery were 21 days (range, 9-104 days) and 21 days (range, 6-181 days), respectively (P = .95).
Acute and chronic GVHD
The 100-day probability of grade II-IV acute GVHD among the 209 patients who received IFN-α was 38% (95% confidence interval [CI], 31%-44%), whereas the probability in the 664 who received only hydroxyurea was 34% (95% CI, 30%-37%) (P = .48). The 100-day probabilities of grade III-IV acute GVHD in the two groups were 15% (95% CI, 11%-21%) and 14% (95% CI, 11%-17%), respectively (P = .66). The 3-year probabilities of chronic GVHD were 58% (95% CI, 49%-66%) and 56% (95% CI, 51%-60%), respectively (P = .91).
Nonrelapse mortality
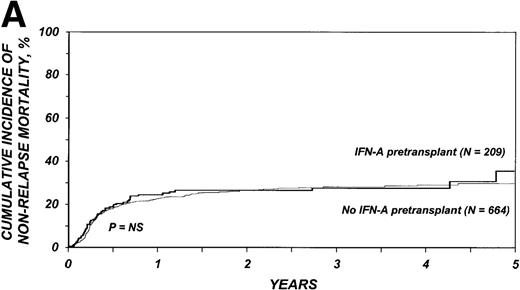
The 3-year cumulative incidence of nonrelapse mortality was 27% (95% CI, 21%-34%) among the 209 patients who received IFN-α and 28% (95% CI, 24%-32%) among the 664 patients who received only hydroxyurea (P = .75) (Figure1A). In multivariate analyses, the relative risk of nonrelapse mortality in patients given IFN-α compared with those who received only hydroxyurea was 1.21 (95% CI, 0.89-1.65;P = .22; Table 3).
Outcomes in patients with chronic myelogenous leukemia (CML).
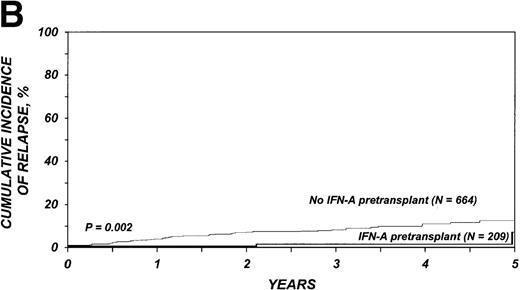
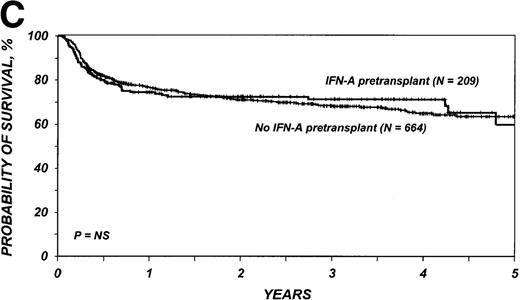
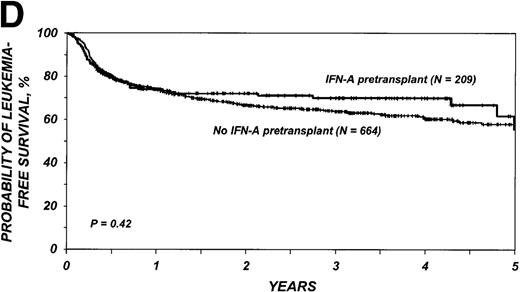
Cumulative incidence of nonrelapse mortality (A) and relapse (B) and actuarial probability of survival (C) and leukemia-free survival (D) after HLA-identical sibling bone marrow transplantation for CML in first chronic phase, according to whether pretransplantation treatment was with interferon-α with or without hydroxyurea or hydroxyurea alone. P values were calculated with the log-rank test.
Outcomes in patients with chronic myelogenous leukemia (CML).
Cumulative incidence of nonrelapse mortality (A) and relapse (B) and actuarial probability of survival (C) and leukemia-free survival (D) after HLA-identical sibling bone marrow transplantation for CML in first chronic phase, according to whether pretransplantation treatment was with interferon-α with or without hydroxyurea or hydroxyurea alone. P values were calculated with the log-rank test.
Relapse
The 3-year cumulative incidence of relapse was 1% (95% CI, < 1%-5%) among the 209 who received IFN-α compared with 8% (95% CI, 6%-10%) among the 664 patients who received only hydroxyurea (P = .002) (Figure 1B). In multivariate analysis, the relative risk of relapse in patients given IFN-α compared with those given only hydroxyurea was 0.17 (95% CI, 0.04-0.70; P = .01, Table 3).
Survival and leukemia-free survival
The 3-year probability of survival was 71% (95% CI, 64%-77%) among the 209 patients who received IFN-α and 68% (95% CI, 64%-72%) among the 664 patients who received only hydroxyurea (P = .98) (Figure 1C). Corresponding probabilities of leukemia-free survival in the two groups were 70% (95% CI, 63%-76%) and 64% (95% CI, 60%-68%) (P = .42) (Figure 1D). In multivariate analysis, the relative risk of treatment failure (inverse of leukemia-free survival) with IFN-α compared with hydroxyurea was 0.99 (95% CI, 0.73-1.33; P = .92) (Table 3).
Discussion
This analysis of 873 patients who received HLA-identical sibling BMT for chronic-phase CML indicates that there was no significant difference in leukemia-free survival between those who received a short course of IFN-α as part of their pretransplantation therapy and those who received only hydroxyurea. Earlier reports of the effects of prior IFN-α therapy on BMT outcome gave conflicting results. A study at M. D. Anderson Cancer Center did not find any deleterious effects,22 but numbers of patients were small, limiting the power to detect significant effects. A study using data from the UK Medical Research Trial compared IFN-α with hydroxyurea and revealed no differences between patients in the 2 arms who eventually received allogeneic BMT.11 Similar findings were reported in a randomized trial of the German CML Study Group that compared interferon and hydroxyurea: outcomes in patients who subsequently underwent BMT were similar regardless of initial treatment.29 In contrast, another German trial reported poorer BMT outcome in patients given IFN-α therapy for longer than 12 months, though this effect resulted primarily from increased graft failure and nonrelapse mortality in the 12 patients who received HLA-mismatched allografts or allografts from unrelated donors.23,24 The last 3 studies11,23,24,29 included transplants from unrelated and related donors and many patients who received IFN-α for long periods. Another study of 181 persons who received IFN-α before transplants from unrelated donors suggested that 6 months of IFN-α treatment increased the incidence and mortality of GVHD.30
The current analysis differs from these previous studies in the numbers of patients studied and in its focus on persons who received HLA-identical sibling BMT, most of whom were given IFN-α for < 6 months. Our data indicate that current practice patterns in many centers include short exposure to IFN-α before BMT, presumably during the wait to identify a donor and performance of the pretransplantation evaluation. Thus, analyzing the effects of short-term IFN-α exposure on BMT outcome is clinically relevant because identification of a deleterious effect would suggest that this agent be avoided until eligibility for BMT is determined. In our large group of patients, we found no adverse effect of IFN-α on acute or chronic GVHD, nonrelapse mortality, survival, or leukemia-free survival. Results in 47 patients given IFN-α for longer than 6 months were similar to those given shorter courses of IFN-α, and multivariate analyses failed to reveal an effect of IFN-α dose or duration of therapy; however, only 17 patients received IFN-α for more than a year.
It is possible that there are differences in the effect of IFN-α on the outcome of HLA-identical sibling BMT compared with unrelated-donor or HLA-mismatched BMT. These might relate to histocompatibility differences, differences in patient selection for HLA-identical sibling BMT and unrelated-donor BMT, or differences in duration of IFN-α therapy before BMT. The last point is particularly important, since the conclusions of this study can be applied only to patients who receive a relatively short course of IFN-α. In the studies reported by Beelen and colleagues,23 24 adverse effects of IFN-α exposure on BMT outcome were most pronounced in patients who received IFN-α for longer than 12 months.
In our study, there were more graft failures in patients who received IFN-α before BMT. The magnitude of this increase was small (< 2%), though statistically significant. This finding may reflect changes in the marrow microenvironment because marrow fibrosis was more common at the time of BMT in patients treated with IFN-α. The IFN-α group also had a significantly lower relapse risk, a new finding that needs confirmation, especially because policies for monitoring, diagnosing, and treating CML relapse were not uniform in this observational study. This is particularly important in CML, in which criteria for treating relapse may vary from an increase in molecular markers to cytogenetic relapse to frank hematologic relapse.
In contrast to other studies, including an analysis from the IBMTR,20 this study did not find a correlation between the interval from diagnosis to BMT and BMT outcome. This may have been because people who underwent BMT < 3 months after diagnosis were excluded, though their survival did not appear markedly superior to that of patients who had BMT later. Additionally, in recent years, there has been a shift toward earlier BMT. We found that 65% of BMTs were done within the first year after diagnosis and about 95% within 2 years. This contrasts to the previous IBMTR study reporting an adverse effect of time to BMT, in which >15% of patients underwent BMT ≥ 2 years after diagnosis. Moreover, an upper restriction of 48 months between diagnosis and BMT was placed on patients included in this study, whereas no upper restriction was made in the previous study. The exclusion of very early and very late BMTs and compression of time to BMT make it more difficult to detect an effect of disease duration.
This study did not address the question of whether BMT or IFN-α is the best definitive therapy for CML. Neither did it evaluate the strategy of administering a trial of IFN-α to identify patients likely to do well without BMT (those with complete cytogenetic response) and then proceeding to BMT in the patients who are not identified. Such a strategy would require a long course of IFN-α therapy (at least 9-12 months) before it could deem patients nonresponsive to the treatment. Both these questions would best be addressed by randomized trials, though it is unlikely that these will be done given the number of patients required. In the absence of such trials, comparative studies of large transplant and nontransplant databases and decisionanalysis techniques that consider results with transplant and nontransplant therapy may be used to address this question.17-19 Such studies generally indicate that BMT in the first year after diagnosis produces the best long-term survival in young patients.
The current study indicates that the use of IFN-α for CML therapy in patients planning to have an HLA-identical sibling BMT, though associated with a small increase in graft failure, does not adversely affect leukemia-free survival and may decrease the risk of posttransplantation relapse.
Supported by Public Health Service Grants P01-CA-40053 and U24-CA-76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute of the US Department of Health and Human Services; and grants from Schering-Plough Oncology and Alpha Therapeutic Corporation; Amgen, Inc; Anonymous; Baxter Fenwal; Berlex Laboratories; BioWhitakker, Inc; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol-Myers Squibb Company; Cell Therapeutics, Inc; Centeon; Center for Advanced Studies in Leukemia; Chimeric Therapies; Chiron Therapeutics; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Genentech, Inc; Human Genome Sciences; Immunex Corporation; Kettering Family Foundation; Kirin Brewery Company; Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation; Herbert H. Kohl Charities, Inc; Nada and Herbert P. Mahler Charities; Milstein Family Foundation; Milwaukee Foundation/Elsa Schoeneich Research Fund; NeXstar Pharmaceuticals; Samuel Roberts Noble Foundation; Novartis Pharmaceuticals; Orphan Medical; Ortho Biotech, Inc; John Oster Family Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer, Inc; RGK Foundation; Roche Laboratories; Rockwell Automation Allen-Bradley Company; SangStat Medical Corporation; Schering AG; Searle; SEQUUS Pharmaceuticals; SmithKline Beecham Pharmaceutical; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Foundation; SyStemix; United Resource Networks; and Wyeth-Ayerst Laboratories.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Reprints:Mary M. Horowitz, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.