Theiler’s murine encephalomyelitis virus (TMEV) establishes a persistent infection in the central nervous system (CNS) leading to an inflammatory demyelinating disease of the CNS in which the histology and clinical course is similar to multiple sclerosis (MS). Disease pathogenesis is primarily due to T-cell–mediated destruction of myelin, which has been attributed to cytopathic effects of the virus, but immune-mediated destruction of myelin mediated via both virus-specific and myelin-specific T cells appear to play the major role. To determine if bone marrow transplantation would be an effective therapy for a virus-initiated autoimmune disease and to better separate viral cytopathic effects from immune-mediated demyelination, we ablated the immune system of TMEV-infected animals with 1,100 cGy total body irradiation, and then the animal’s immunity was reconstituted by transplantation of disease-susceptible SJL/J mice with syngeneic marrow or disease-susceptible DBA/2J with marrow from disease-resistant (C57Bl/6 × DBA/2)F1 (B6D2) donors. Hematopoietic transplant performed after onset of disease resulted in 42% mortality in SJL/J syngeneic transplants, 47% mortality in diseased DBA2 recipients restored with marrow from naive B6D2 donors, and 12% in diseased DBA2 recipients receiving marrow from B6D2 donors previously infected with TMEV. Delayed type hypersensitivity (DTH) to both virion and myelin proteins was decreased in surviving mice that underwent transplantation; however, CNS viral titers were significantly elevated compared with nontransplanted controls. We conclude that a functional immune system with appropriate T-cell responses are important in prevention of lethal cytopathic CNS effects from TMEV. Relevant to the clinical use of bone marrow transplantation, attempts to ablate the immune system in viral-mediated immune diseases or virus-initiated autoimmune disease may have acute and lethal consequences. Our results raise concern about the attempted use of autologous hematopoietic transplantation in patients with MS, an autoimmune disease with a suspected virus etiology, particularly if the graft is aggressively depleted of lymphocytes.
THEILER’S MURINE encephalomyelitis virus (TMEV) is a naturally occurring enteric murine picornavirus.1 Infection of susceptible mouse strains leads to a biphasic disease that is first manifest as an acute gray matter inflammation followed by chronic, immune-mediated white matter demyelination of the central nervous system (CNS) that serves as a model for multiple sclerosis (MS).2 Virus persists within the CNS throughout a susceptible host’s life.3 Target cells for early infection are neural, glial, and endothelial cells, whereas cells harboring virus during chronic demyelination are predominately CNS macrophages and, to a lesser extent, oligodendrocytes.4,5 The neurologic consequences of TMEV arise from early acute viral cytopathic effects on gray matter neurons that evolve into chronic immune T-cell–mediated demyelination of CNS white matter.6,7 The immune system’s role in preventing acute disease is supported by the effective clearance of TMEV from the CNS of disease-resistant murine strains and abrogation of this resistance by either immunosuppressive total body irradiation (TBI) or infection of athymic mice that would otherwise be disease-resistant.8-10 The immune system’s role in causing chronic disease is documented by prevention of demyelination in disease-susceptible strains of mice after treatment with immunosuppressive agents such as cyclophosphamide, antithymocyte globulin, irradiation, or anti-major histocompatibility complex (MHC) class II or anti-CD4 monoclonal antibody therapy.7 11-14
After infection of SJL mice with the BeAn 8386 strain of TMEV, demyelination is initiated by CD4+ T cells specific for virus epitopes that arise within 7 to 10 days postinfection and target CNS-persistent virus leading to macrophage-mediated bystander destruction of myelin.7,15,16 Approximately 4 weeks after onset of clinical disease, T-cell responses to myelin epitopes arise in an ordered temporal progression17 consistent with a role for both virus- and myelin-specific responses in the chronic phase of disease. The latter appearance of myelin-specific responses and the lack of cross-reactivity between TMEV and myelin epitopes indicate that CNS autoimmunity arises by epitope spreading and is not due to shared virus and myelin epitopes.17-19
Hematopoietic stem cell transplantation has been proposed as a therapy for immune-mediated disorders such as multiple sclerosis.20,21 Because inflammatory cells such as autoreactive lymphocytes and activated macrophages arise from the hematopoietic progenitor stem cell compartment, the rationale is to ablate the immune system, followed by immune reconstitution with hematopoietic stem cells from an unaffected animal in the hopes of ablating autoreactive lymphocytes and re-establishing tolerance to self-epitopes. The efficacy of this is supported by experiments demonstrating that either syngeneic or allogeneic hematopoietic stem cell transplantation from an unaffected animal is capable of preventing, ameliorating, and/or curing experimental autoimmune encephalomyelitis (EAE), another autoimmune animal model of MS.22-26 Recent short-term outcome studies on limited numbers of patients with MS suggest that immune ablation and hematopoietic stem cell reconstitution with autologous hematopoietic stem cells may prevent progression of and in some cases improve the pathogenesis of MS.22 23
Because the histology and clinical course of TMEV are similar to human MS and because epidemiological studies suggest MS may be initiated and/or exacerbated by virus infections,27 we evaluated the outcome of hematopoietic stem cell transplantation in TMEV-induced demyelinating disease. The results demonstrate that stem cell transplantation of mice with ongoing TMEV-induced demyelinating disease results in a high incidence of mortality concomitant with a significant elevation of CNS virus titers. Thus, attempts to ablate the immune system in viral-mediated immune diseases or virus-initiated autoimmune diseases associated with persistent infection may have acute and lethal consequences.
MATERIALS AND METHODS
Animals.
Six-week-old female SJL mice were obtained from Harlan Laboratories (Madison, WI). DBA/2J and B6D2 F1 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were maintained on standard mouse chow and water ad libitum in a containment animal facility. Neomycin sulfate (0.7 mmol/L), tetracycline (0.1 mmol/L), and trimethoprim/sulfamethoxazole (0.4 mmol/L) were added in the drinking water for 2 weeks after bone marrow transplantation (BMT) to prevent infections.
Induction of TMEV-induced demyelinating disease.
BeAn 8386 virus was plaque purified and amplified in BHK-21 cells. A working stock was prepared by passage in BHK-21 cells. Female mice were anesthetized with methoxyflurane and intracerebrally inoculated in the right cerebral hemisphere with approximately 2.9 × 106 PFU of BeAn virus in 30 μL. All animals were examined several times per week for the first 4 weeks and at least once weekly thereafter. Sham-infected control animals received 30 μL Dulbecco’s modified Eagle’s medium (DMEM).
Treatment.
By definition, day 0 was the day of intracerebral inoculation. At 2 time points after infection (day between days 70 and 90), mice were divided into control and treatment groups. Control mice received no further therapy. The treatment groups underwent myeloablation and marrow rescue from same-sex animals using 107 nucleated bone marrow cells from either (1) syngeneic disease-susceptible naive mice, (2) allogeneic naive but disease-resistant B6D2 mice, or (3) allogeneic healthy disease-resistant B6D2 mice previously inoculated with TMEV. Myeloablation consisted of TBI at a dose of 1,100 cGy in 2 fractions of 550 cGy administered 6 hours apart 1 day before marrow infusion.
Clinical evaluation.
TMEV was scored clinically according to neurologic deficit using the following numerical scores: 0, asymptomatic; 1, mild waddling gait; 2, more severe waddling gait, righting reflexes normal to mildly impaired, able to right itself in under 3 seconds; 3, spastic paralysis, righting reflexes severely impaired, unable to right itself in under 3 seconds; 4, dehydration; 5, total hind limb paralysis, dehydration, malnutrition; and 6, death. All clinical scoring was performed by the same observer.
Histology.
Ten to 12 1-μm–thick, Epon-embedded sections stained with toluidine blue were examined from each spinal cord and scored as follows: +/−, mild inflammation without demyelination; +, inflammation with focal demyelination; ++, inflammation with multiple foci of demyelination; and +++, marked inflammation with bilateral, converging areas of demyelination. Glial scarring was characterized according to the majority of sections involved as follows: absent, mild (focal glial bands in the margin of the cord); moderate (gliosis extending to at least 50% of the thickness of the anterior and/or lateral columns); or severe (glial scarring covering the entire thickness of the anterior column). Mice selected for histologic examination had clinical scores that represented the average for their group. The neuropathologist performing histologic scoring was blinded to animal treatment and clinical score.
Delayed type hypersensitivity (DTH).
Virion peptide-specific (VP1 233-250, VP2 70-86, and VP3 24-37) and myelin epitope-specific (PLP 139-151) DTH responses were quantitated using a 24-hour in vivo ear swelling assay. Three mice from each group were challenged with 5 μg of the indicated VP or 10 μg of proteolipid protein (PLP) peptide in 0.01 mL saline. Twenty-four hours after challenge, the increase in ear swelling was quantitated with an engineer’s micrometer (Mitutoyo Model 7326; Schlesinger Tools, Brooklyn, NY). Results were corrected for prechallenge ear thickness.
CNS cytokine analysis.
Analysis of cytokine mRNA levels in the CNS was performed by homogenizing phosphate-buffered saline (PBS) perfused spinal cord from 3 mice in each group in guanidium isothiocyanate and isolating total RNA by CsCl gradient. First-strand cDNA synthesis was performed using 2 μL (0.5 μg/μL) of RNA in 10.5 μL of diethyl pyrocarbonate (DEPC)-treated water, 1 μL of oligo-(dT) primer, and 6.5 μL of a master mix (4 μL of 5× reaction buffer, 1 μL of dNTP mix [10 mmol/L each], 0.5 μL RNAse inhibitor, and 1.0 μL of Moloney murine leukemia virus [M-MLV] reverse transcriptase). Polymerase chain reaction (PCR) primers for interferon-γ (IFN-γ), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) encompass 234, 324, and 240 bp of wild-type cDNA, respectively. First-strand cDNA was amplified 35 cycles (Perkin Elmer Thermocycler; Perkin Elmer, Norwalk, CT).
Virus plaque assays.
Standard plaque assays (previously described28) were performed for quantification of virus titers in the spinal cord, brain, and spleen. Organs from 3 mice per group were homogenized with a Virtis tissue homogenizer (Virtisher-Gardiner, New York, NY) into a 10% solution and the homogenate was layered over BHK-21 cells. After 96 hours of incubation, live cells were stained using a 0.015% neutral red. Plates were incubated for 4 hours and plaques were enumerated.
Statistical analyses.
Student’s t-tests were used to determine the statistical significance of clinical scores, DTH, and virus titers between experimental groups.
RESULTS
Syngeneic transplantation of TMEV-susceptible SJL mice.
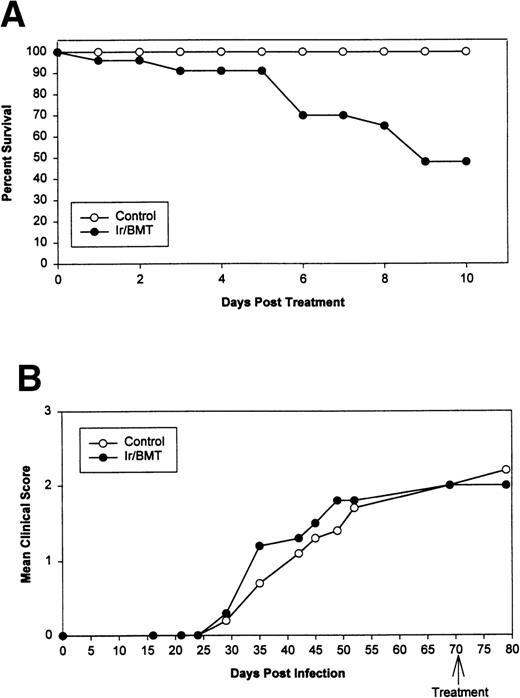
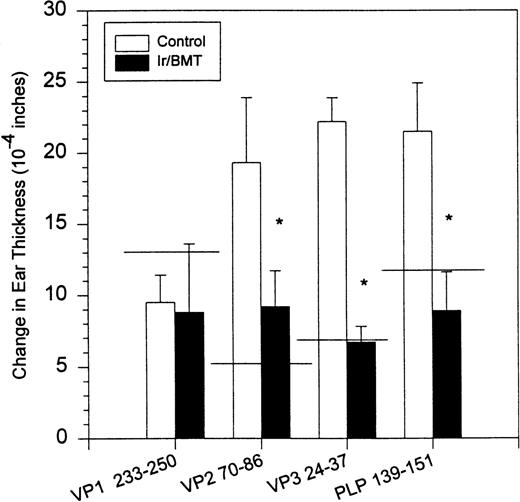
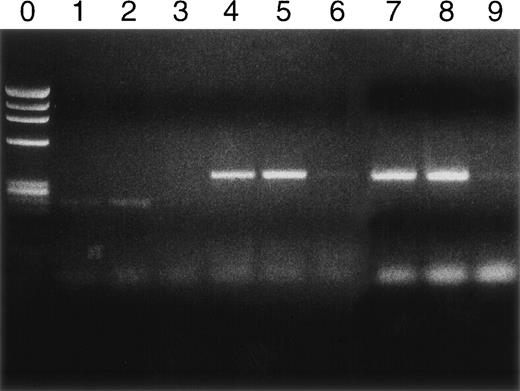
Syngeneic hematopoietic stem cell transplantation was performed on 2 separate groups of SJL mice with established TMEV-induced demyelinating disease at days 70 and 92 postinfection. Thirteen of 31 (42%) of these mice transplanted 70 days postinfection died within 2 weeks posttransplantation (Fig 1A). Interestingly, the mortality rate for SJL mice with TMEV-induced demyelinating disease was significantly greater than we have noted in similar transplants of SJL mice with established relapsing EAE, in which a mortality rate of less than 6% was observed in well over 100 mice transplanted at varying times during ongoing disease.26 There was no significant difference in median neurologic deficit in surviving transplanted animals compared with nontransplanted animals (P = .3858) as neurologic deterioration continued in both the transplanted and control groups (Fig 1B). Despite the progression of clinical disease, the myeloablation was apparently successful, because DTH responses to both virus epitopes (VP2 70-86 and VP3 24-37) and to the immunodominant epitope on proteolipid protein (PLP 139-151) were abrogated in transplanted mice assayed approximately 30 to 40 days postreconstitution (Fig 2). Evaluation of the CNS for Th1-associated (IFN-γ and TNF-α) or Th2-associated (IL-10) cytokine mRNA levels using a semiquantitative PCR analysis showed no significant differences between transplanted and nontransplanted mice assayed approximately 40 days postreconstitution (Fig 3).
Syngeneic BMT using naive SJL donor marrow for SJL/J disease-susceptible TMEV-infected recipients. (A) Survival curve. (B) Mean clinical score. Ir = 1,100 cGy TBI.
Syngeneic BMT using naive SJL donor marrow for SJL/J disease-susceptible TMEV-infected recipients. (A) Survival curve. (B) Mean clinical score. Ir = 1,100 cGy TBI.
DTH responses to viral-specific (VP1 233-250, VP2 70-86, and VP3 24-37) and myelin-specific (PLP 139-151) epitopes in SJL/J mice inoculated with TMEV. The horizontal line indicates average response in 3 normal noninfected animals. DTH assays were performed on day 132. PLP, proteolipid protein; VP, viral peptide.
DTH responses to viral-specific (VP1 233-250, VP2 70-86, and VP3 24-37) and myelin-specific (PLP 139-151) epitopes in SJL/J mice inoculated with TMEV. The horizontal line indicates average response in 3 normal noninfected animals. DTH assays were performed on day 132. PLP, proteolipid protein; VP, viral peptide.
Cytokine RNA levels by PCR. Rows 1, 2, and 3 are IFN-γ; rows 4, 5, and 6 are TNF-; and rows 7, 8, and 9 are IL-10. Naive controls never inoculated with TMEV are rows 3, 6, and 9. TMEV diseased but untransplanted animals are rows 1, 4, and 7. Mice with TMEV treated by transplantation are rows 2, 5, and 8. Assays were performed on day 128.
Cytokine RNA levels by PCR. Rows 1, 2, and 3 are IFN-γ; rows 4, 5, and 6 are TNF-; and rows 7, 8, and 9 are IL-10. Naive controls never inoculated with TMEV are rows 3, 6, and 9. TMEV diseased but untransplanted animals are rows 1, 4, and 7. Mice with TMEV treated by transplantation are rows 2, 5, and 8. Assays were performed on day 128.
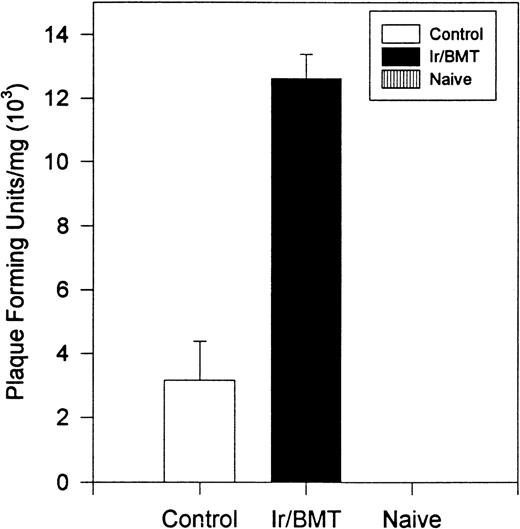
Interestingly, transplanted animals exhibited significantly higher titers of infectious TMEV within the spinal cord than did untreated controls (Fig 4). In transplanted mice, approximately 4-fold more infectious virus was present in the spinal cords than in the nontransplanted mice (12.6 × 103PFU/mg v 3.1 × 103 PFU/mg, P = .004). No viral plaques were found in the brain or spleen of either TMEV-infected group or in any tissues in the uninfected controls. Histologic examination of the spinal cords showed an acute inflammatory infiltrate with less glial scarring in transplanted mice versus a prominent chronic demyelination and gliosis with minimal residual infiltration in nontransplanted mice (Fig5).
Viral titer within the spinal cord of animals infected with TMEV. Naive mice were never inoculated with TMEV. Control mice were inoculated with TMEV but had no further treatment. Ir/BMT, treatment with TBI and BMT from uninfected healthy syngeneic animals. Assays were performed on day 128. These results are representative of 2 assays.
Viral titer within the spinal cord of animals infected with TMEV. Naive mice were never inoculated with TMEV. Control mice were inoculated with TMEV but had no further treatment. Ir/BMT, treatment with TBI and BMT from uninfected healthy syngeneic animals. Assays were performed on day 128. These results are representative of 2 assays.
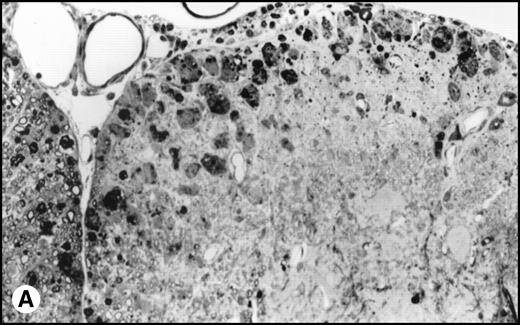
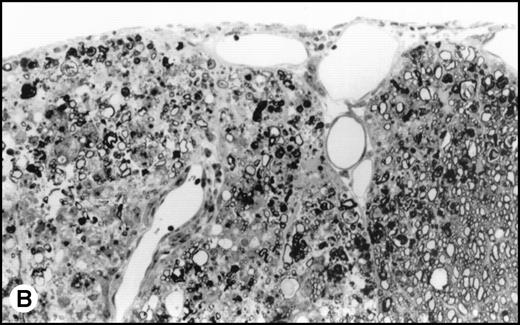
(A) Histology of SJL/J spinal cords from mice infected with TMEV. Section of spinal cord of an SJL/J mouse, 130 days after infection with TMEV. The right anterior column is completely demyelinated and numerous large lipid-laden macrophages are present close to the central sulcus. The gray uniform background reflects conspicuous gliosis in the demyelinated area (1-μm–thick, Epon-embedded section, stained with toluidine blue; original magnification × 220). (B) Section from spinal cord of an SJL/J mouse 130 days after infection with TMEV and after treatment with radiation and BMT. Inflammatory cells are still around the large venule in the parenchyma of the left anterior column, indicating active disease, but many axons are still surrounded by myelin sheaths and gliosis is less prominent (1-μm–thick, Epon-embedded section, stained with toluidine blue; original magnification × 220).
(A) Histology of SJL/J spinal cords from mice infected with TMEV. Section of spinal cord of an SJL/J mouse, 130 days after infection with TMEV. The right anterior column is completely demyelinated and numerous large lipid-laden macrophages are present close to the central sulcus. The gray uniform background reflects conspicuous gliosis in the demyelinated area (1-μm–thick, Epon-embedded section, stained with toluidine blue; original magnification × 220). (B) Section from spinal cord of an SJL/J mouse 130 days after infection with TMEV and after treatment with radiation and BMT. Inflammatory cells are still around the large venule in the parenchyma of the left anterior column, indicating active disease, but many axons are still surrounded by myelin sheaths and gliosis is less prominent (1-μm–thick, Epon-embedded section, stained with toluidine blue; original magnification × 220).
Allogeneic transplantation using TMEV-resistant donors.
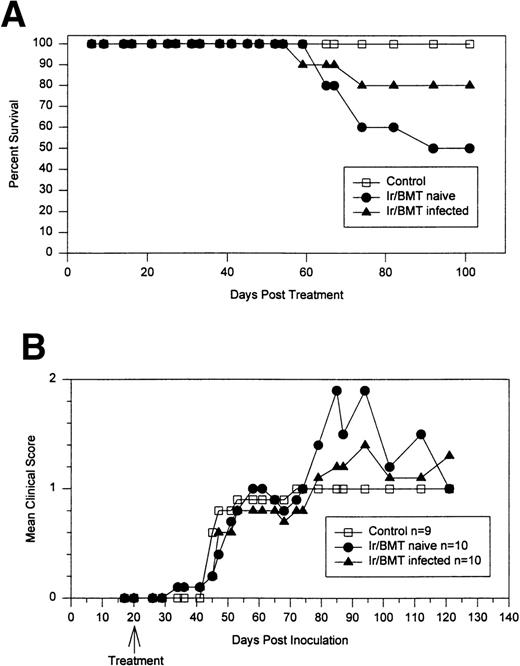
To determine if the efficacy of the transplantation may be increased by using marrow from disease-resistant donors, disease-susceptible DBA/2J mice were treated with TBI and infused with marrow from either naive or TMEV-infected disease-resistant B6D2 mice. At day 20 postinfection, before the onset of clinical disease, susceptible DBA/2J mice were treated with TBI and infused with marrow from either naive or TMEV infected disease-resistant B6D2 mice (Fig6). Control mice developed chronic progressive disease with no mortality (0/9). There was 50% mortality (5/10) in DBA/2J mice transplanted with bone marrow from naive disease-resistant B6D2 mice and 20% mortality (2/10) in DBA/2J mice receiving allogeneic marrow from B6D2 donors that had previously been infected with TMEV. Mortality was secondary to progressive neurologic disability, with most animals dying between 60 and 90 days posttransplant. In a second experiment (data not shown) performed after disease onset (day 94 postinfection), DBA/2 mice transplanted with allogeneic marrow from naive disease-resistant B6D2 donors had a 43% mortality (3/7), whereas only 1 of 14 (7%) mice transplanted with marrow from a TMEV-infected B6D2 donor died. Therefore, the combined mortality from 2 different experiments was 8 of 17 (47%) for recipients of naive B6D2 marrow and 3 of 24 (12%; P = .029) for recipients of marrow from TMEV-infected, but healthy, B6D2 donors.
Allogeneic BMT using naive or TMEV-infected B6D2 disease-resistant donor marrow for DBA/2J disease-susceptible recipients inoculated with TMEV. (A) Percentage of animals surviving. (B) Neurologic score of surviving mice.
Allogeneic BMT using naive or TMEV-infected B6D2 disease-resistant donor marrow for DBA/2J disease-susceptible recipients inoculated with TMEV. (A) Percentage of animals surviving. (B) Neurologic score of surviving mice.
DISCUSSION
Resistance and susceptibility to demyelination after TMEV infection are both immune-mediated processes. Susceptibility/resistance to TMEV-induced demyelinating disease is controlled by multiple loci, including Tmevd-1 on chromosome 6 near the genes encoding the β chain of the T-cell receptor29; Tmevd-2 on chromosome 3 near the Car-2 locus30; and the MHC class I H-2D region on chromosome 17.31-34 Disease susceptibility correlates with development of strong virus-specific Th1 responses, as exemplified by expression of DTH reactivity31,35,36 and predominant production of Th1-derived cytokines37 and IgG2a-predominant antivirus antibody responses.38 Disease-susceptible mice also display a lifelong persistent CNS virus infection.3 In contrast, disease resistance is associated with clearance of virus from the CNS within 2 to 3 weeks postinfection36,39 and development of only low levels of virus-specific DTH.36Recent evidence indicates that virus clearance is largely mediated by an abundant MHC class I-restricted CD8+ CTL response arising within the first 10 days postinfection.40 Studies have shown that depletion of CD8+ T cells can confer susceptibility to some otherwise resistant inbred strains.41
In disease-susceptible strains, TMEV-induced demyelinating disease is a 2-stage process. Infection with the DA strain of TMEV leads to early inflammatory infiltration within the CNS gray matter that leads to a limited amount of neuronal necrosis that is consistent with lytic infection of neurons in cell culture.42 The gray matter inflammation is cleared within 2 weeks postinfection and is replaced by mononuclear inflammation and demyelination of white matter. Use of the BeAn strain of TMEV that was employed in these studies obviates much of the gray matter pathology. Relatively weak CTL responses in susceptible strains40 result in the inability to clear virus from the CNS and lead to establishment of persistent infection.3White matter damage is initiated by virus-specific CD4+ T cells that lead to macrophage-mediated bystander destruction of myelin.7,15,16 Myelin-specific T-cell responses do not play a role in disease initiation as responses to immunodominant epitopes on MBP and PLP are not detected before disease onset,17,18 and peripheral tolerance to myelin components before virus infection fails to affect the course of demyelination.16 However, chronic disease is associated with the development of T-cell responses to multiple myelin epitopes that arise via epitope spreading.17 Taken together with the fact that TMEV-specific T-cell responses persist throughout the disease course in susceptible SJL mice,35 the data are consistent with a role for both anti–virus-specific and anti–myelin-specific T-cell responses in chronic disease.
Because MS27 and perhaps other T-cell–mediated autoimmune diseases may be initiated as a secondary consequence to a virus infection, we have attempted to use hematopoietic stem cell transplantation as a tool to separate the overlapping roles of direct virus cytopathology, virus-specific immunity, and myelin epitope-specific autoimmunity in TMEV-induced demyelinating disease. The results clearly show that transplantation of syngeneic marrow from naive TMEV-susceptible donors to diseased SJL/J recipients resulted in 40% mortality within 10 to 15 days after transplantation (Fig 1). Although the deaths may have been due to radiation induced CNS injury, the mortality rate was significantly higher than that of SJL mice undergoing BMT for the treatment of relapsing EAE, a purely autoimmune inflammatory demyelinating disease, in which we and others have historically observed an approximate 6.0% mortality rate.22-26
Surviving mice displayed significantly diminished immune DTH responses to both virus and myelin epitopes, indicating the effectiveness of the myeloablative therapy (Fig 2). The acute neurologic deterioration correlated with an increased CNS viral load (Fig 4). Histologic evaluation (Fig 5) showed a predominance of acute gray matter inflammation in transplanted mice compared with predominant white matter demyelination in untreated animals. The slightly exacerbated clinical disease course, increased CNS virus levels, and pattern of histology are consistent with an exacerbation of direct viral cytopathology, not immune-mediated CNS damage. This finding is similar to that previously reported by Lipton and Dal Canto,11 who showed that high-dose cyclophosphamide or antithymocyte serum administered shortly after virus infection prevented TMEV-induced immune-mediated demyelination, but resulted in a mortality rate of 77% to 88%. Thus, severe immunosuppression of TMEV-infected animals is capable of causing fatal neurologic consequences that were also likely due to uncontrolled virus growth.
To reconstitute viral immunity more rapidly, we attempted allogeneic transplantation of marrow from naive disease-resistant B6D2 mice into diseased DBA/2J mice. This resulted in an equally high early mortality of 50%, although death was delayed to 60 to 70 days posttransplant. Therefore, we attempted allogeneic transplantation using disease-resistant but previously inoculated B6D2 donors rather than naive B6D2 donors. This resulted in a mortality rate (20%) that was substantially lower than that of mice receiving bone marrow from naive allogeneic or syngeneic donors. Lower mortality may have been due to adoptive transfer of viral-specific cytotoxic lymphocytes infused with the donor marrow.43 Because mouse bone marrow contains relatively few lymphocytes, adoptive transfer of both splenocytes and marrow from previously infected disease-resistant mice may prevent or further decrease exacerbation of viral cytopathic effects after transplantation.
BMT is currently being investigated in clinical trials as therapy for human autoimmune diseases. Whereas the experimental results in autoimmune disorders such as EAE indicate amelioration or cure after transplantation, the current results in TMEV-induced demyelinating disease suggest that this therapy may be dangerous in virus-associated autoimmune diseases. Transplantation did improve the autoimmune component of TMEV by decreasing immune responses to viral and PLP peptides. However, nonspecific attempts to suppress this virus-initiated autoimmune process also suppress viral immunity and can apparently result in lethal consequences.
Further advances in transplant of viral-associated autoimmune diseases should recognize the importance of controlling viral cytotoxicity after transplantation with either peritransplant antiviral drugs and/or virus-specific adoptive immunotherapy at the time of graft infusion. This is particularly true in MS, in which an infectious etiology is strongly suspected based on a variety of observations, including epidemiological data,44-49 abnormal humoral and/or cellular responses to viruses,50-55 isolation of virus or presence of viral proteins within CNS plaques,56-58 and animal models of virus-initiated, inflammatory CNS demyelinating diseases that mimic MS clinically and histologically.1,2 The rationale for treating MS by hematopoietic stem cell transplantation is based on the unproven assumption that MS is an autoimmune disease targeting myelin proteins and that the procedure will ablate activated autoreactive T cells and also lead to the re-establishment of self-tolerance to myelin epitopes. This theory is supported by cure or amelioration of disease after transplantation of autoimmune diseases such as EAE,22-26 collagen-induced arthritis,59and adjuvant arthritis,60 which are induced by active immunization with self-proteins/peptides in adjuvant. However, the current data clearly show that stem cell transplantation of an autoimmune-like disease initiated by infection with TMEV, a natural mouse pathogen, although dampening autoimmune responses, also inhibits antiviral responses, resulting in virus reactivation and significant mortality.
It should be pointed out that TMEV-induced demyelinating disease may be an exception, because the virus establishes a lifelong persistent CNS infection. Hematopoietic stem cell transplantation may be perfectly appropriate for treating virus-induced autoimmune diseases in which the initial virus infection has been cleared, thus alleviating the danger of virus reactivation. Current results from transplantation of MS patients have not shown undue mortality,1,2 6 perhaps suggesting that the disease etiology is diverse, with only a subset being virally mediated, or that a potential virus infection may have already been cleared or is relatively noncytopathic. However, given the limited number of patients transplanted to date, the potential for virus reactivation is a real concern and may depend on the level of immunosuppression of the recipient and/or the degree of lymphocyte depletion of the graft.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Richard K. Burt, MD, Department of Medicine, Northwestern University Medical School, 250 E Superior St, Room 1456, Wesley Pavilion, Chicago, IL 60611.