Inherited mutations in the erythropoietin receptor (EPOR) causing premature termination of the receptor cytoplasmic region are associated with dominant familial erythrocytosis (FE), a benign clinical condition characterized by hypersensitivity of erythroid progenitor cells to EPO and low serum EPO (S-EPO) levels. We describe a Swedish family with dominant FE in which erythrocytosis segregates with a new truncation in the negative control domain of the EPOR. We show that cells engineered to concomitantly express the wild-type (WT) EPOR and mutant EPORs associated with FE (FE EPORs) are hypersensitive to EPO-stimulated proliferation and activation of Jak2 and Stat5. These results demonstrate that FE is caused by hyperresponsiveness of receptor-mediated signaling pathways and that this is dominant with respect to WT EPOR signaling.
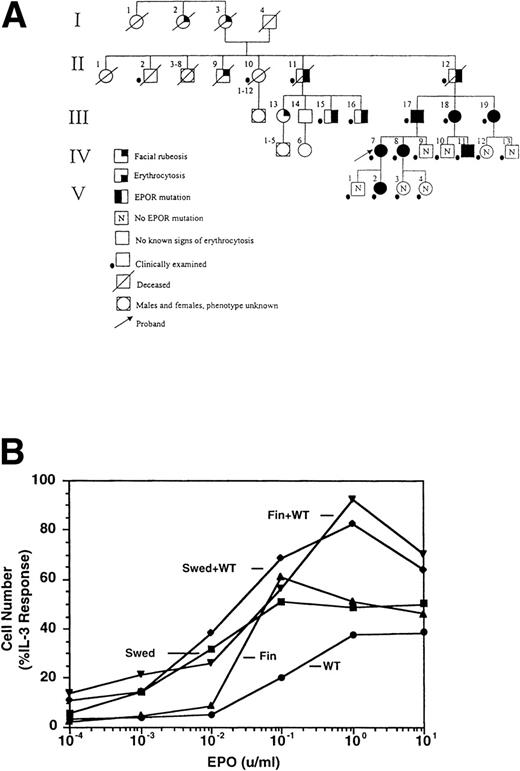
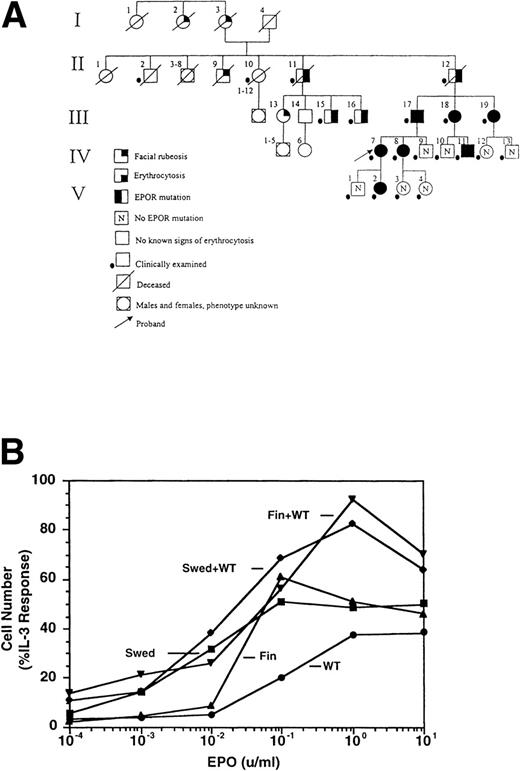
THE SWEDISH erythropoietin receptor (EPOR) allele contains a direct tandem duplication of nucleotides 5968 to 5975. This results in a frameshift beyond residue 405, introducing 25 amino acids not related to the EPOR, and a premature stop codon, deleting 79 residues at the C-terminus of the receptor (data not shown). Affected family members (Fig 1A, Table 1) were plethoric and often had additional symptoms, including hypertension, headaches, dizziness, nosebleeds, and exertional dyspnea, which were most pronounced in the males. These symptoms were alleviated by phlebotomies, and phlebotomy treatment has been recommended.
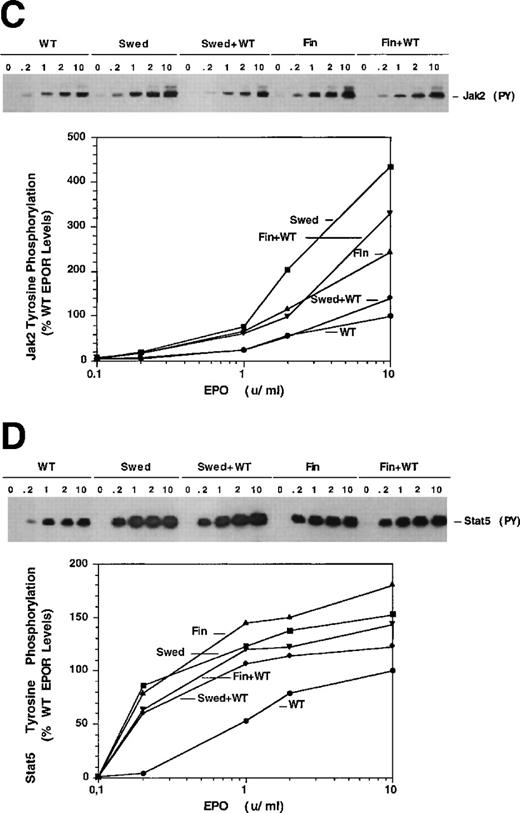
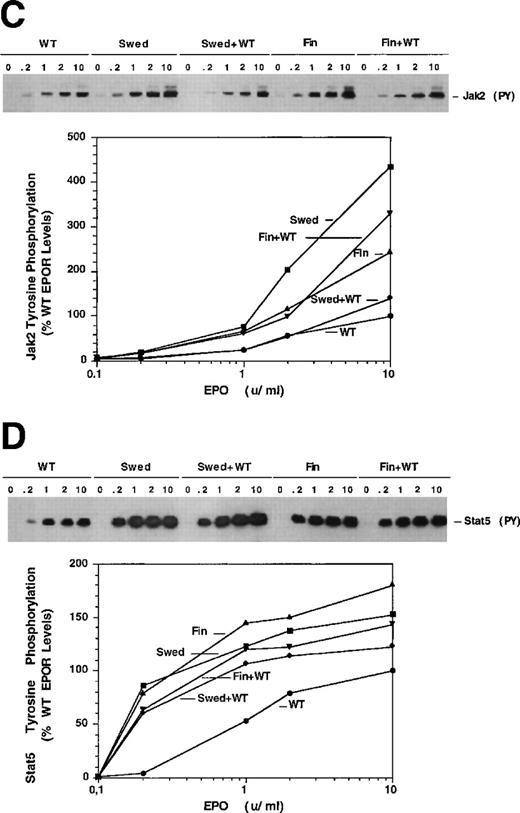
Dominant erythrocytosis and EPO hypersensitivity. (A) Pedigree of the Swedish family. (B) EPO-dependent proliferation in 32D cell lines. Cells expressing WT (•), mFin (▴), mSwed (▪), mFin+WT (▾), or mSwed+WT (⧫) were assayed for growth in EPO-containing medium. (C and D) EPO-dependent Jak2 and Stat5 activation in 32D cell lines. Cells were stimulated with EPO and Jak2 and Stat5 activation determined by immunoprecipitation and antiphosphotyrosine immunoblot assays. Methods: The inheritance pattern of familial erythrocytosis among members of a 5-generation family from southern Sweden was determined by clinical observation. In some cases the presence of the mutant EPOR allele was assayed by direct genomic sequencing or an allele-specific hybridization test. 32D cell lines were established as described.12 Cells were grown in media containing serial dilutions of EPO (from 10−4 to 10 U/mL), or in media containing interleukin-3 (IL-3). Viable cells were counted after 3 days. The number of cells in EPO cultures is expressed as a percentage of the cells present in IL-3 cultures (% IL-3 response). For Jak2 and Stat5 activity assays, 32D cells were stimulated for 10 minutes at 37°C with 0.2, 1.0, 2.0, or 10.0 U/mL EPO, or were left unstimulated, as indicated. Proteins were immunoprecipitated from detergent cell extracts with an antibody specific for Jak2 or Stat5, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose. The filters were probed with antiphosphotyrosine antibody 4G10 and developed with chemiluminescent reagents.9 12 A computer-generated image of the region of the gel containing Jak2 (≈130 kD) or Stat5 (≈90 kD) is shown. Chemiluminescent signals were quantified on a lumi-imager and are expressed as a percentage of the level of Jak2 or Stat5 phosphorylation elicited by EPO stimulation of the WT receptor at 10 U/mL EPO (arbitrarily set to 100%). The levels of protein phosphorylation were normalized to the levels of immunoprecipitated Jak2 or Stat5 for each sample.
Dominant erythrocytosis and EPO hypersensitivity. (A) Pedigree of the Swedish family. (B) EPO-dependent proliferation in 32D cell lines. Cells expressing WT (•), mFin (▴), mSwed (▪), mFin+WT (▾), or mSwed+WT (⧫) were assayed for growth in EPO-containing medium. (C and D) EPO-dependent Jak2 and Stat5 activation in 32D cell lines. Cells were stimulated with EPO and Jak2 and Stat5 activation determined by immunoprecipitation and antiphosphotyrosine immunoblot assays. Methods: The inheritance pattern of familial erythrocytosis among members of a 5-generation family from southern Sweden was determined by clinical observation. In some cases the presence of the mutant EPOR allele was assayed by direct genomic sequencing or an allele-specific hybridization test. 32D cell lines were established as described.12 Cells were grown in media containing serial dilutions of EPO (from 10−4 to 10 U/mL), or in media containing interleukin-3 (IL-3). Viable cells were counted after 3 days. The number of cells in EPO cultures is expressed as a percentage of the cells present in IL-3 cultures (% IL-3 response). For Jak2 and Stat5 activity assays, 32D cells were stimulated for 10 minutes at 37°C with 0.2, 1.0, 2.0, or 10.0 U/mL EPO, or were left unstimulated, as indicated. Proteins were immunoprecipitated from detergent cell extracts with an antibody specific for Jak2 or Stat5, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose. The filters were probed with antiphosphotyrosine antibody 4G10 and developed with chemiluminescent reagents.9 12 A computer-generated image of the region of the gel containing Jak2 (≈130 kD) or Stat5 (≈90 kD) is shown. Chemiluminescent signals were quantified on a lumi-imager and are expressed as a percentage of the level of Jak2 or Stat5 phosphorylation elicited by EPO stimulation of the WT receptor at 10 U/mL EPO (arbitrarily set to 100%). The levels of protein phosphorylation were normalized to the levels of immunoprecipitated Jak2 or Stat5 for each sample.
To investigate the molecular mechanisms of erythrocytosis, the Swedish mutation and a previously described mutation from a Finnish family (6002G → A)1 2 were engineered in the murine EPOR (mSwed and mFin, respectively). Clonal 32D cell lines expressing these EPOR isoforms were established. To approximate EPOR expression patterns predicted for heterozygous individuals, clonal cell lines coexpressing wild-type (WT) and mutant EPORs were also established (data not shown). The mFin and mSwed EPORs rendered hypersensitive proliferative responses to EPO and dominantly enhanced the proliferative response of the WT EPOR (Fig 1B).
EPO hypersensitivity could be caused by differential receptor metabolism or altered signal transduction. Cell-surface receptor expression did not vary more than 2-fold, as judged by flow cytometry analysis, and the intracellular processing of newly synthesized WT, mFin, or mSwed was similar (data not shown). These results suggest that differential receptor metabolism is unlikely to be the sole cause of the observed EPO-hypersensitivity.
To examine EPOR signaling, we assayed EPO-dependent activation of Jak2 and Stat5 by immunoprecipitation and immunoblot analysis.3-5 Cells expressing the mutant receptors alone or coexpressing mutant and WT EPORs had elevated levels of activated Jak2 and Stat5 at all EPO concentrations tested, relative to cells expressing the WT receptor alone (Fig 1C and D). Stat5 also demonstrated hypersensitivity to EPO, with half-maximal activation at ≈0.2 U/mL EPO in cells expressing mutant EPORs compared to 1.0 U/mL EPO in WT cells. Similar levels of Jak2 or Stat5 were present in all samples (data not shown).
Heterozygous individuals with FE are predicted to express both WT and mutant EPORs,1,6-8 although this has not yet been documented. Our study provides the first analysis of cells engineered to coexpress WT and FE EPOR isoforms. The results show that FE alleles confer dominant and hypersensitive EPO-dependent proliferation and activation of Jak2 and Stat5. The hypersensitive response may reflect mechanistic differences in Jak2 activation or inactivation by FE EPORs, compared with WT EPOR. For example, the mutant receptors may abrogate one or more negative-regulatory signals, such as SHP-1 or CIS,9-11 that normally suppress Jak2 activity, while still stimulating positive regulatory signals. Collectively, our results indicate that dominant FE is caused by enhanced receptor signaling because expression of a mutant allele in the heterozygous state is sufficient to unbalance WT signal transduction.
ACKNOWLEDGMENT
We thank Adriana Acurio, Hong Lu, and Melanie Wickert for excellent technical assistance; Karen Ramirez for flow cytometry analysis; Dr Joan Egrie (Amgen, Thousand Oaks, CA) for the generous gift of recombinant EPO; and Dr Harvey F. Lodish for initiating this collaboration and for advice during the course of these studies.
Supported by grants from the National Cancer Institute, National Institutes of Health (CA-77447) and the Texas Higher Education Coordinating Board Advanced Research Program (15-120) (to S.S.W.), and by grants from the National Cancer Institute, National Institutes of Health (CA-67941 and CA-16058) (to A.d.l.C.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephanie S. Watowich, PhD, MD Anderson Cancer Center, Box 178, 1515 Holcombe Blvd, Houston, TX 77030.