A 44-year-old woman with a 12-year history of Sjögren’s syndrome (SS) developed a low-grade mucosa-associated lymphoid tissue (MALT) lymphoma in the parotid gland. Two years later, she presented with generalized lymphadenopathy and hepatosplenomegaly and a follicular lymphoma was diagnosed. To investigate the relationship of the two histologically distinct lymphomas, we re-examined their histology and immunophenotype and studied the lymphomatous tissue from the parotid, cervical lymph node, and spleen using molecular genetic methods. Histologic and immunophenotypic studies confirmed the previous diagnoses and also identified a previously unnoticed focus of follicular lymphoma in the second parotid gland biopsy. Polymerase chain reaction (PCR) amplification of the rearranged Ig heavy-chain gene showed the same sized dominant product in the MALT lymphoma and the follicular lymphoma. Similarly, PCR analysis of the t(14:18) translocation yielded an identical sized band from both MALT and follicular lymphoma. Cloning and sequencing of the Ig PCR products showed an identical CDR3 sequence from each lesion, indicating a common clonal lineage. The follicular lymphoma of the parotid gland lymph node and the follicular lymphoma of the spleen showed an identical mutation signature to that of the salivary gland MALT lymphoma. We propose that follicular lymphoma in the parotid gland lymph node may have resulted from colonization of lymphoid follicles by MALT lymphoma cells, following which the tumor cells were induced to express a follicular lymphoma phenotype, due to Bcl-2 overexpression caused by t(14;18), leading to a change in clinical behavior resulting in rapid widespread dissemination of disease. These observations suggest that the distinct phenotypes of low-grade B-cell lymphomas may be the consequence of interplay between genetic and local microenvironmental factors.
DIFFERENT LYMPHOID malignancies are characterized by distinct cytomorphological, genetic, and clinical features, which together form the basis of modern lymphoma classifications. In this respect, genetic characteristics have adopted increasing importance. An example is the t(14;18) translocation, which results in overexpression of the bcl-2 gene and occurs in 90% of follicular lymphomas, but is only rarely present in other low-grade B-cell lymphomas.1 t(14;18) is believed to occur during immunoglobulin (Ig) gene rearrangement in pre-B cells and to be the first genetic abnormality leading to the development of follicular lymphoma.2,3 Although t(14;18) alone is unable to cause malignant transformation,4-7 the translocation appears to be a crucial genetic element in predisposing the affected cell to develop into follicular lymphoma in humans.1
B-cell lymphoma of mucosa-associated lymphoid tissue (MALT)-type is an extranodal low-grade B-cell lymphoma that arises from lymphoid tissue acquired after chronic inflammation, which usually has an autoimmune basis. MALT lymphoma of the salivary glands typically occurs in patients with chronic sialadenitis (MESA), which is often a consequence of the autoimmune disorder Sjögren’s syndrome (SS). Patients with SS have a 44 times greater risk of developing non-Hodgkin’s lymphoma compared with normal individuals,8 and most of these are MALT lymphomas.8-10 The lymphomas occurring in individual SS patients frequently involve several sites, but always belong to a single histological subtype.9 10 We report a SS patient with both MALT and follicular lymphoma, both of which were derived from the same precursor cell carrying t(14;18).
MATERIALS AND METHODS
Patient.
The patient was a 44-year-old woman who presented with a 12-year history of dry mouth, symmetrical bilateral arthritis of both hands, and swelling of the right parotid gland. Parotid biopsy showed the histological features of SS (MALT lymphoma on review). One year later, a repeat parotid gland biopsy showed the histological features of MALT lymphoma. Eighteen months after the first biopsy, the patient presented with enlargement of cervical lymph nodes and biopsy confirmed MALT lymphoma involvement. Two years after the first biopsy, the patient developed generalized lymphadenopathy with hepatosplenomegaly. Laparotomy with mesenteric lymph node biopsy, liver biopsy, and splenectomy was performed together with an inguinal lymph node biopsy. Histologic examination of each of these tissues showed the classic features of follicular lymphoma.
Histology and immunohistochemistry.
Routine hematoxylin and eosin (H&E)-stained sections were prepared from each of the biopsies and their histology was reviewed. Paraffin sections were immunostained with CD20, CD3, CD21, CD10, anti-IgM, anti-IgD, anti-κ Ig light chain, anti-λ Ig light chain, and anti–bcl-2 protein using the streptavidin-biotin method preceded by heat retrieval of antigen using a domestic pressure cooker.11 All antibodies were purchased from Dako (Bucks, UK) with the exception of CD10, which was purchased from Novacastra (Newcastle upon Tyne, UK).
DNA extraction and microdissection.
Genomic DNA was extracted from whole sections of both frozen and fixed tissue samples of parotid gland, cervical and parotid lymph nodes, and spleen as described previously.12 DNA samples were also prepared from representative tumor cell populations of both MALT and follicular lymphoma microdissected from CD10-stained sections of both parotid gland (CD10−) and spleen (CD10+) using the method of Pan et al.13 Normal liver cells were microdissected and the DNA extracted was used as germline control.
Polymerase chain reaction (PCR) of the rearranged Ig heavy-chain gene and t(14;18) breakpoint.
The rearranged IgH gene was amplified from framework (Fr) 1, Fr2, and Fr3 to the joining (J) region with consensus primers using previous published PCR protocols.12 PCR products were analyzed on 6% (Fr1-JH, Fr2-JH) or 10% (Fr3-JH) polyacrylamide gels.
The t(14;18) was amplified using primers directed to the major breakpoint region (MBR) of the bcl2 gene and to the JHregion14 and products were analyzed on 7.5% polyacrylamide gels.
All PCR reactions were performed using a hot start procedure15 and appropriate positive and negative controls were included in each experiment. All samples were analyzed in duplicate. To avoid any potential cross-contamination, analysis of different tumor lesions was performed at different times.
Cloning and sequencing of PCR products.
The Fr2-JH PCR products were purified on Sephacryl S-400 MicroSpin columns (Pharmacia, St Albans, UK), then ligated to the pGEM-T vector and transformed into JM109 competent cells according to the manufacturer’s protocol (Promega, Southampton, UK). The transformed cells were selected on LB-ampicillin agar plates containing X-gal and isopropyl-1-thio-β-D-galactoside (IPTG). White colonies were screened using PCR with vector primers (Sp6 and T7). The PCR products showing the expected insert size were sequenced using an ABI sequencer with dye terminators (Perkin Elmer, Warrington, UK). At least 6 PCR clones from each sample were sequenced at both directions.
The Fr1-JH and t(14;18) breakpoint PCR products were directly sequenced using an ABI sequencer with dye terminators (Perkin Elmer).
Sequence analysis.
The VH and JH germline segments used by the tumor IgH gene were identified by sequence comparison to the V base, which is a comprehensive database of human Ig germline gene sequences compiled from the published sequences, using online DNAPLOT (MRC Centre for Protein Engineering,http://www.mcr-cpe.cam.ac.uk/imt-doc/vbase-home-page.html). Mutations in the VH region were identified by comparing the tumor sequence with the closest published germlines, whereas mutations in the CDR3 region were recorded according to the most closely related PCR clone.
The probability of the observed replacement or silent mutations in the CDRs or Fr regions by chance was calculated using a binomial mutation model as described elsewhere.16
Bcl2 and JH sequences in the t(14;18) breakpoint were identified by comparison to the published t(14;18) breakpoint and JH sequences using Wisconsin GCG software (provided by the Human Genome Mapping Project, Cambridge, UK) and the GenBank Database.
RESULTS
Histology and immunohistochemistry.
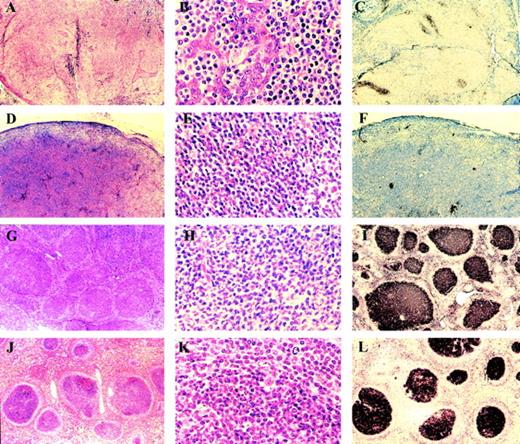
Both parotid gland biopsies showed the histological features of low-grade B-cell lymphoma of MALT-type (Fig1A and B). The parotid gland was diffusely infiltrated by sheets of centrocyte-like (CC-L) cells, which infiltrated parotid duct epithelium to form characteristic lymphoepithelial lesions. There were occasional preserved reactive B-cell follicles. The CC-L cells were CD20+, CD5−, CD10−, IgM+, IgD− and showed κ Ig light-chain restriction. Residual CD21+ follicular dendritic cell (FDC) meshworks were present sometimes associated with small aggregates of CD10+ follicle center cells (Fig 1C). The cervical lymph node biopsy showed effacement of its architecture by a diffuse infiltrate of CC-L cells with the identical immunophenotype to those comprising the parotid MALT-type lymphoma. As in the parotid, there was immunohistochemical evidence of follicle center remnants (Fig 1D through F).
(A through C) Parotid gland MALT lymphoma. Low magnification (A) shows diffuse infiltrate with numerous lymphoepithelial lesions. High magnification (B) shows centrocyte-like cells invading ductal epithelium. The lymphoma is CD10−(C) in contrast to residual reactive follicle centers. (D through F) Cervical lymph node. Low magnification (D) shows effacement of lymph node architecture by a diffuse infiltrate of centrocyte-like cells seen at high magnification (E). The tumor cells are CD10− (F), but small clusters of CD10+ residual follicle center cells are present. (G through I) Inguinal lymph node showing the features of follicular lymphoma at low magnification (G) and high magnification (H). The tumor is CD10+ (I). (J through L) Spleen showing involvement by follicular lymphoma at low magnification (J), high magnification (K), and CD10 expression (L).
(A through C) Parotid gland MALT lymphoma. Low magnification (A) shows diffuse infiltrate with numerous lymphoepithelial lesions. High magnification (B) shows centrocyte-like cells invading ductal epithelium. The lymphoma is CD10−(C) in contrast to residual reactive follicle centers. (D through F) Cervical lymph node. Low magnification (D) shows effacement of lymph node architecture by a diffuse infiltrate of centrocyte-like cells seen at high magnification (E). The tumor cells are CD10− (F), but small clusters of CD10+ residual follicle center cells are present. (G through I) Inguinal lymph node showing the features of follicular lymphoma at low magnification (G) and high magnification (H). The tumor is CD10+ (I). (J through L) Spleen showing involvement by follicular lymphoma at low magnification (J), high magnification (K), and CD10 expression (L).
The inguinal, mesenteric lymph nodes, and spleen showed the histological features of follicular lymphoma. There was effacement of normal lymphoid architecture by an infiltrate comprising closely packed follicles characterized by a large follicle center surrounded by a poorly formed mantle. Similar follicles were observed in the hepatic portal triads. The follicle centers were comprised of centrocytes and scattered centroblasts which were CD20+, CD5−, CD10+, bcl-2 protein+, IgM+, IgD− and showed κ light-chain restriction (Fig 1G through L).
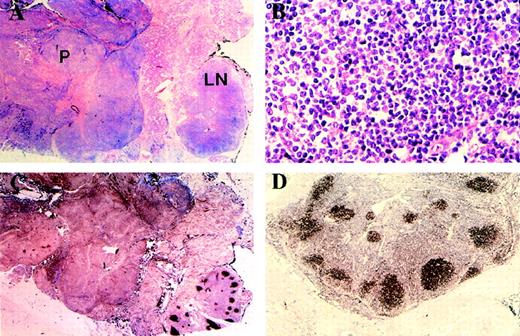
Review of the second parotid gland biopsy showed a small intraparotid lymph node at one pole with retention of normal lymph node architecture. However, on closer inspection, the B-cell follicles were devoid of mantle zones and lacked tingible body macrophages. Moreover, immunohistochemical staining showed that they shared the immunophenotype of the follicular lymphoma as described above (CD10+, bcl-2 protein+, IgM+, IgD−, κ Ig light chain+, λ− [Fig 2A through D]).
Low magnification (A) shows parotid infiltrated by MALT lymphoma (P) and intraparotid lymph node (LN) with preservation of normal architecture. High magnification of intraparotid lymph node follicle center (B) shows centrocytes with occasional centroblasts and absence of tingible body macrophages. Immunostaining with CD10 (C) shows absence of staining in MALT lymphoma. The lymph node follicle centers are positive and emphasize the normal nodal architecture. Expression of bcl-2 protein by the lymph node follicle centers (D) is indicative of the neoplastic nature of the follicle center cells.
Low magnification (A) shows parotid infiltrated by MALT lymphoma (P) and intraparotid lymph node (LN) with preservation of normal architecture. High magnification of intraparotid lymph node follicle center (B) shows centrocytes with occasional centroblasts and absence of tingible body macrophages. Immunostaining with CD10 (C) shows absence of staining in MALT lymphoma. The lymph node follicle centers are positive and emphasize the normal nodal architecture. Expression of bcl-2 protein by the lymph node follicle centers (D) is indicative of the neoplastic nature of the follicle center cells.
Analysis of the rearranged Ig gene.
PCR analysis of the rearranged Ig gene was first performed on DNA samples prepared from whole sections of parotid gland, cervical lymph nodes, and spleen biopsies. All samples yielded an identical sized band on polyacrylamide gels with both Fr2-JH and Fr3-JHprimers. The Fr2-JH PCR products were cloned and sequenced. A dominant clone was found in each of the above samples and was identical in the CDR3 sequence in each lesion (Fig3), indicating a common clonal origin. To verify that Fr2-JHPCR truly reflected the MALT lymphoma examined rather than a biased amplification of a minute disseminated follicular lymphoma cell population due to somatic mutation of the MALT lymphoma clone or vice versa, we performed Fr1-JH PCR, which is less affected by somatic mutation, from high molecular DNA isolated from frozen samples of parotid gland and spleen biopsies. Direct sequencing Fr1-JH PCR yielded identical results to those obtained from Fr2-JH PCR. Furthermore, Fr3-JH PCR analysis of representative tumor cell populations microdissected from both MALT (CD10− CCL cells of the parotid gland biopsy) and follicular lymphoma (neoplastic follicle of the spleen) showed the same sized monoclonal band.
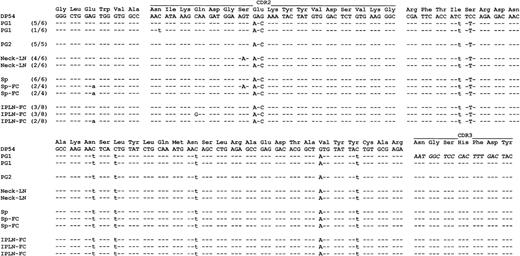
Comparison of IgH sequence among different lesions. PG1, first parotid gland biopsy; PG2, second parotid gland biopsy; Neck-LN, neck lymph node; Sp, spleen; Sp-FC, spleen CD10+ follicle center cells; IPLN-FC, CD10+ follicle center cells of the intraparotid lymph node in the second parotid biopsy. The number in brackets shows the number of common clones of the number of total tumor clones sequenced. Identity to the germline sequences is shown by dashes, replacement mutations by uppercase, and silent mutations by lowercase letters.
Comparison of IgH sequence among different lesions. PG1, first parotid gland biopsy; PG2, second parotid gland biopsy; Neck-LN, neck lymph node; Sp, spleen; Sp-FC, spleen CD10+ follicle center cells; IPLN-FC, CD10+ follicle center cells of the intraparotid lymph node in the second parotid biopsy. The number in brackets shows the number of common clones of the number of total tumor clones sequenced. Identity to the germline sequences is shown by dashes, replacement mutations by uppercase, and silent mutations by lowercase letters.
The VH sequence of the tumor-derived Ig gene was aligned with the closest germline VH segment obtained from a sequence similarity search of the GenBank Database and mutations were identified. Comparison of the Ig gene sequences of the different lymphoma lesions showed the same sequence shared by MALT lymphoma of the parotid gland, cervical lymph node, and surprisingly by the follicular lymphoma of the spleen (Fig 3). The common lymphoma clone showed a significantly low ratio of replacement/silent (R/S) mutation in the Fr region (P < .02) and intraclonal sequence variations were observed.
The microdissected neoplastic follicle centers in the intraparotid lymph node of the second parotid gland biopsy and spleen were similarly studied using the above strategy. The CD10+ cells of the follicles were found to be clonally identical to the MALT lymphoma (Fig3).
Analysis of the t(14;18) breakpoint.
PCR analysis of the t(14;18) breakpoint in DNA samples prepared from the whole section of the lymphoma of the parotid gland, cervical lymph node, and spleen yielded an identical sized band on polyacrylamide gels. To ascertain that the finding of t(14;18) translocation in MALT lymphoma was not the result of dissemination of the follicular lymphoma cells, representative CD10− tumor cells from the MALT lymphoma were microdissected, analyzed, and identical products were seen. Sequencing of these PCR products showed identical breakpoint sequences in each lesion (data not shown).
PCR analysis of DNA extracted from microdissected normal liver cells showed no evidence of the translocation, which confirmed that t(14;18) in the lymphoma was acquired rather than a germline characteristic.
DISCUSSION
The sequence of events in the case reported here appears to be as follows. The initial parotid gland enlargement, 12 years after the onset of SS, was due to the development of MALT lymphoma, which was confirmed in the second parotid biopsy 1 year later and in the cervical lymph node after a further 6 months. At this time, follicular lymphoma was already present in germinal centers of an otherwise normal appearing intraparotid lymph node and soon manifested as typical stage IV disease with involvement of peripheral lymph nodes, spleen, and liver.
MALT lymphoma and follicular lymphoma are clinicopathologically distinct tumors that are recognized in the revised European American lymphoma classification as different “diseases.”17The former is an extranodal lymphoma that tends to remain localized (stage IE or IIE) for a prolonged period, which is in keeping with its remarkable clinical indolence.18 The normal cell counterpart of MALT lymphoma is thought to be the marginal zone B cell, which accounts for its cytological features, CD10 negative immunophenotype, and typical growth pattern as a diffuse infiltrate initially around reactive B-cell follicles. MALT lymphoma is not yet defined by a molecular genetic abnormality, although recently it has been suggested that t(11;18) may be characteristic.19,20Rare instances of t(14;18) in MALT lymphoma have been reported.21,22 By contrast, follicular lymphoma is a nodal tumor that only infrequently arises in extranodal sites and is usually widely disseminated (stage IV) at the time of diagnosis. The normal cell counterpart is the follicle center cell (centroblast and centrocyte), and the tumor typically grows as a collection of neoplastic follicles comprising CD10+ centroblasts and centrocytes with a minor interfollicular diffuse component in some cases.23 In over 90% of cases, t(14;18) is present.1
Analysis of the rearranged Ig gene and the t(14;18) breakpoint showed that the MALT and follicular lymphomas in this patient had identical CDR3 and breakpoint sequences, indicating a common clonal origin from the same precursor cell, which harbored the t(14;18) translocation. How can we explain the presence in this patient of these two clinicopathologically and immunophenotypically different lymphomas that, on molecular grounds, are clonally identical?
One explanation is that a “premalignant” cell bearing the t(14;18) may have undergone separate transforming events at two distinct sites resulting in the two phenotypic lymphomas observed. Alternatively, the explanation may lie, in part, in the phenomenon known as follicular colonization.24,25 In this process MALT lymphoma cells selectively colonize reactive (ie, non-neoplastic) follicle centers where they may undergo a degree of transformation. This may occur either in the original extranodal site or in adjacent lymph nodes. Follicular colonization is typically a focal phenomenon and the cells in the colonized follicles retain their MALT lymphoma cytomorphology and immunophenotype, being CD10− and, interestingly, often losing their bcl-2 expression.26Because individual follicles in the intraparotid lymph node showed the immunophenotypic and genotypic features of follicular lymphoma, the possibility must be considered that the presence of t(14;18) in the MALT lymphoma clone influenced the cells that colonized the follicle centers in the intraparotid lymph node to acquire the cytomorphology, immunophenotype, and biological characteristics of follicular lymphoma.The t(14;18) translocation rarely occurs in MALT lymphoma, but is the genetic hallmark of follicular lymphoma.1,22 The finding that the same cell with the t(14;18) translocation evolved into both MALT and follicular lymphoma is unprecedented. It has been shown that the t(14;18) translocation is a crucial element in the genesis of follicular lymphoma, but not in other lymphomas, despite occurrence of the translocation at pre-B cells, several stages earlier in B-cell development than the normal cell counterpart of this tumor. This is best explained by the effect of deregulated bcl-2 expression on cell growth or survival, which is maximized in the germinal center microenvironment in which normal germinal center B cells undergo active cell proliferation or apoptosis and do not express Bcl-2 before leaving the germinal center.27 The same mechanisms may apply to transformed clones, as shown in the present case. Thus, the genesis of particular subtypes of B-cell lymphoma may depend on the interplay between genetic [t(14;18)] and environmental (the follicle center) factors.
ACKNOWLEDGMENT
We thank Donata Penso for technical help on part of the sequencing work.
Supported by the Leukaemia Research Fund and Associazione Italiana per la Ricerca sul Cancro.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Peter G. Isaacson, MD, Department of Histopathology, University College London Medical School, Rockefeller Building, University St, London WC1E 6JJ, UK; e-mail:p.isaacson@ucl.ac.uk.