Disparity for HLA in unrelated donor bone marrow transplantation (BMT) increases the risk of graft rejection and graft-versus-host disease (GVHD) and may compromise transplant outcome. We have compared the outcome of matched and mismatched transplants from unrelated donors in 137 children with acute lymphoblastic leukemia (ALL). Their disease status was complete remission (CR)-1, 24 patients; CR-2, 88 patients; CR-3, 18 patients; CR-4, 2 patients; and relapse, 5 patients. CAMPATH monoclonal antibodies were used for T-cell depletion and cyclosporin A was given to 134 children together with short-course methotrexate in 43, mainly when there was HLA disparity. Fifty-two donor/recipient pairs were HLA-mismatched, 41 at HLA-A and -B and 11 at HLA-DR and -DQ loci. Overall graft failure was increased in recipients of marrow mismatched at either HLA-A, -B, -DR, or -DQ (15.7% v 4.8%; P = .057) mainly because there was a higher proportion of children with primary graft failure (11.8% v 1.2%; P = .012). The presence of an HLA-C locus mismatch did not independently increase the likelihood of graft failure. There was no significant difference in the incidence of acute GVHD ≥ grade 2 between the matched and mismatched groups (P = .849). For patients in CR-2, the risk of relapse post-BMT was significantly lower if leukemic relapse occurred off-treatment (P = .005). The Kaplan-Meier overall and leukemia-free survival (LFS) estimates for recipients of matched and mismatched BMT, respectively, at 36 months were 49% versus 42% (P = .380) and 45% versus 40% (P = .654). Although HLA mismatching results in an increased occurrence of primary graft failure with T-cell–depleted allografts, it allows more donors to be identified rapidly for children with ALL without compromising overall transplant outcome.
CHEMOTHERAPY FOR CHILDREN with acute lymphoblastic leukemia (ALL) has dramatically improved outcome over the past 20 years so that, on nationally directed protocols, cure rates of 70% to 80% can be expected.1,2 The main cause of treatment failure is disease relapse and when this occurs on, or shortly after, stopping treatment, subsequent cures are unlikely to be achieved with further chemotherapy.3,4 Allogeneic bone marrow transplantation (BMT) is most frequently performed in children with relapsed ALL, and several reports have indicated that survival is improved when compared with chemotherapy alone.5-7 A proportion of children with ALL in first complete remission (CR-1) have features that suggest they are at increased risk of relapse.8 These include infant ALL associated with 11q23/MLL rearrangement or Philadelphia-positive ALL, both in children9,10 and adults,10 and studies have suggested that allogeneic BMT can improve long-term disease-free survival in these patients. The majority, however, lack a matched sibling donor (MSD) and the use of unrelated donor marrow has been explored.10-13 Data from prospective randomized studies are lacking and the role of unrelated donor BMT remains controversial. Moreover, in children with high-risk ALL, it is often not possible to identify rapidly a molecularly HLA-A, -B, -DR, and -DQ matched donor. Available reports to date using both matched and mismatched unrelated donor marrow show that leukemia-free survival (LFS) in the range 23% to 40% can be achieved.14-16
The outcome of unrelated donor BMT for leukemia depends on the transplant protocol used. The use of T-replete bone marrow has been associated with a higher incidence of severe acute graft-versus-host disease (GVHD).11,17 With both T-depleted and T-replete marrow, an increase in graft failure has been documented when compared with MSD BMT.11-13 Mismatching for HLA-A, -B, and -C has been associated with a higher risk of graft failure.18-20 The incidence and severity of acute GVHD is also higher in most reported series where HLA-A, -B, -C, or -D incompatibility is present,17,21-26 although in 1 recent report of patients with chronic myeloid leukemia, the presence of a single HLA-A or -B mismatch did not increase the risk.27Other factors such as the type of posttransplant immunosuppression and marrow cell dose may also have a significant effect on outcome.28 We have previously reported our results for unrelated donor BMT in a group of 52 children with ALL in second complete remission.29 In this report, we extend our observations to 137 consecutive first transplants in children less than 18 years of age in CR-1, 2, 3, 4, or relapse receiving bone marrow either matched (85) or mismatched (52) for HLA-A, -B, -DR, and -DQ antigens from unrelated donors. Our results show that while primary graft failure is higher in recipients of mismatched grafts, overall survival (OS) is similar.
MATERIALS AND METHODS
Patients.
A total of 147 children with ALL had unrelated donor transplants between October 1988 and July 1997. Informed consent was obtained in each case. Most received similar conditioning therapy and grafts depleted of T cells using CAMPATH antibodies. Ten patients who received either T-cell–replete marrow (1), CD34+-selected grafts (4), or were recipients of second transplants (5) were excluded from analysis. Patients were aged 1 to 17 years (median, 8 years). The male:female ratio was 87:50. Disease status was as follows: CR-1, 24 cases; CR-2, 88 cases; CR-3, 18 cases; CR-4, 2 cases; and relapse, 5 cases (Table 1). Ninety-four patients relapsed in the bone marrow either alone or additionally in the central nervous system (CNS) (21 patients) or testes (11 patients) or both (3 patients). Fourteen and 4 children had isolated CNS and testicular relapses, respectively, and 1 relapsed in both sites. Children were only transplanted for isolated testicular relapse if this occurred on, or within, 3 months of stopping chemotherapy. Patients were transplanted in first complete remission when 1 of the following factors was present: t(4;11) in infancy (n = 4), t(9;22) (n = 7), failure to remit using standard therapy (n = 6), high Oxford Hazard Score (n = 4),30 or biphenotypic leukemia (n = 1). In 2 further patients, high presenting leukocyte counts (> 70 × 109/L and 250 × 109/L) were associated with trisomy 8 and trisomy 2 and 16, respectively.30 For patients in CR-1, the median time from diagnosis to transplant was 214 days (range, 63 to 598). The median time from diagnosis to relapse for children in CR-2 was 824 days (range, 152 to 2,011) and from relapse to BMT, 166 days (range, 60 to 651). These intervals did not differ between matched and mismatched patients (Table 1). The median follow-up of survivors is 38 months (range, 6 to 104).
Baseline Characteristics of Patients
| . | Matched (n = 85) . | Mismatched (n = 52) . | P Value . |
|---|---|---|---|
| Age in years, median (range) | 9 (1-17) | 7 (1-15) | .069 |
| Sex of recipient M:F | 57:28 | 30:22 | .356 |
| Sex of donor M:F | 47:37* | 24:28 | .350 |
| Disease status | |||
| CR1 | 15 (17.6%) | 9 (17.3%) | .778† |
| CR2 | 53 (62.4%) | 35 (67.3%) | |
| CR3 | 13 (15.3%) | 5 (9.6%) | |
| CR4 | 2 (2.4%) | 0 (0%) | |
| Not in remission | 2 (2.4%) | 3 (5.8%) | |
| CR1 median days diagnosis to BMT (range) | 201 (63-598) | 191 (138-316) | |
| CR2 median days diagnosis to relapse (range) | 852 (152-2,011) | 823 (198-1,883) | .951 |
| CR2 median days relapse to BMT (range) | 158 (60-651) | 169 (84-368) | |
| CMV status recipient/donor | |||
| Neg/neg | 52 (61.2%) | 24 (46.2%) | .124 |
| Any other | 33 (38.8%) | 28 (53.8%) | |
| TNC harvested (×108/kg), median (range) | 4.0 (1.9-15.0) | 5.6 (2.8-14.8) | .006 |
| No methotrexate | 79 (92.9%) | 15 (28.9%) | <.001 |
| Methotrexate | 6 (7.1%) | 37 (71.1%) |
| . | Matched (n = 85) . | Mismatched (n = 52) . | P Value . |
|---|---|---|---|
| Age in years, median (range) | 9 (1-17) | 7 (1-15) | .069 |
| Sex of recipient M:F | 57:28 | 30:22 | .356 |
| Sex of donor M:F | 47:37* | 24:28 | .350 |
| Disease status | |||
| CR1 | 15 (17.6%) | 9 (17.3%) | .778† |
| CR2 | 53 (62.4%) | 35 (67.3%) | |
| CR3 | 13 (15.3%) | 5 (9.6%) | |
| CR4 | 2 (2.4%) | 0 (0%) | |
| Not in remission | 2 (2.4%) | 3 (5.8%) | |
| CR1 median days diagnosis to BMT (range) | 201 (63-598) | 191 (138-316) | |
| CR2 median days diagnosis to relapse (range) | 852 (152-2,011) | 823 (198-1,883) | .951 |
| CR2 median days relapse to BMT (range) | 158 (60-651) | 169 (84-368) | |
| CMV status recipient/donor | |||
| Neg/neg | 52 (61.2%) | 24 (46.2%) | .124 |
| Any other | 33 (38.8%) | 28 (53.8%) | |
| TNC harvested (×108/kg), median (range) | 4.0 (1.9-15.0) | 5.6 (2.8-14.8) | .006 |
| No methotrexate | 79 (92.9%) | 15 (28.9%) | <.001 |
| Methotrexate | 6 (7.1%) | 37 (71.1%) |
1 donor gender unknown.
CR3, CR4 and not in remission groups combined for comparison.
Donor selection.
This was from the British Bone Marrow Registry in 68 cases and the Anthony Nolan Bone Marrow Trust in 69 cases. Seventy-one donors were male and 65 were female. In 1 case, information on donor gender was not available. If HLA-A, -B, -DR, and -DQ matched donors could not be identified, we aimed to select those matched at HLA-DR and -DQ wherever possible. The majority of mismatches in this series is, therefore, at HLA-A and -B. HLA typing was the major selection criterion, then preference was given to younger, male donors. Cytomegalovirus (CMV)-seronegative patients preferentially received marrow from CMV-seronegative donors (Table 1).
Histocompatibility studies.
We initially used serologic testing using 2-stage National Institutes of Health (NIH) complement-dependent microlymphocytotoxicity for HLA-A and -B loci and restriction fragment length polymorphism (RFLP) for HLA-DR and -DQ as previously described.31 Subsequently, typing using polymerase chain reaction (PCR) and sequence-specific primers (SSP) was used and where samples were available, retrospective HLA-C typing by PCR-SSP was performed.31 32 Overall, for HLA-A and -B, 116 donor/recipient pairs were typed by serology and 21 using PCR-SSP; 82 donor/recipient pairs were HLA-C typed retrospectively using PCR-SSP. For class II antigens, 40 donor/recipient pairs were typed using RFLP and 97 using PCR-SSP. The definition of HLA-A and -B was similar using serological and molecular techniques, as molecular typing was usually performed at low/medium resolution to determine specificity at the antigen rather than the allele level. Similarly, HLA-C, -DR, and -DQ typing was performed at a comparable resolution. For statistical analysis of HLA typing methodology, patients transplanted before October 22, 1993 when serology and RFLP typing were used, were compared with the remainder who were predominantly typed by SSP.
Conditioning regimens.
All children received CAMPATH-1G monoclonal antibody (5 to 20 mg/d depending on body weight) on day −9 to −5 and cyclophosphamide 60 mg/kg on days −6, −5 with hydration and mesna (160% of the cyclophosphamide dose). Total body irradiation (TBI) of 1,440 cGy was given in 8 fractions on day −3 to 0 at 20 cGy/min, except in 9 children less than 4 years old who received single fraction TBI (1,000 cGy; 5 to 8 cGy/min) instead on day −1 and 3 children less than 3 years old who were conditioned with cyclophosphamide and busulphan; the dose of busulphan being 4 to 5 mg/kg/d for 4 days as described previously.29
Engraftment.
Patients were considered evaluable for engraftment if they survived for at least 28 days posttransplant. Neutrophil engraftment was defined as absolute neutrophil count (ANC) ≥0.5 × 109/L on 3 consecutive measurements. Primary graft failure was defined as failure to reach ANC ≥0.5 × 109/L at any time and secondary graft failure sustained loss of neutrophils to <0.1 × 109/L after initial engraftment had occurred.
GVHD prophylaxis and grading.
Bone marrow was harvested and processed as previously described.29 The total nucleated counts (TNC) harvested were calculated for each recipient (Table 1). Donor marrow was depleted of T cells ex vivo using CAMPATH-1M (116 of 137) and CAMPATH-1G (18 of 137) monoclonal antibodies together with third-party group AB serum as a source of complement as previously described.33 In 18 transplants, CAMPATH-1G was used instead of CAMPATH-1M due to a concern (subsequently unsubstantiated) regarding in vitro efficiency of T-cell lysis. CAMPATH-1M was then reintroduced and has been used since. Three patients received a T-cell addback of 0.1 to 0.5 × 106 cells/kg on day 0 as part of a study investigating the impact of T-cell addition on graft rejection and GVHD34 and in 3 transplants, recipients were given CAMPATH-1G monoclonal antibody (5 to 20 mg/d) from day −5 to day +4 to achieve in vivo T-cell depletion.
Cyclosporin A (CSA) was given to all patients except 3, transplanted early in the program when it was not routinely given, who received no posttransplant immunosuppression. In the remainder, it was given from day −1 to between 3 to 6 months post bone marrow transplant, and then tapered over 3 months in the absence of GVHD. Levels were measured twice weekly and dosage modified to maintain therapeutic levels. We gave 3 doses of intravenous methotrexate 15 mg/m2 on day 1 and 10 mg/m2 on days 3 and 6 to 37 patients because of HLA mismatch. In 6 patients who received matched grafts, methotrexate was given at the physician’s discretion. Acute and chronic GVHD were graded using standard criteria.35 36 Patients were considered evaluable for chronic GVHD if they engrafted and survived for 100 days.
Supportive care and follow-up.
This was as previously described.29 Briefly, all patients were transplanted in high-efficiency particle air filtration rooms. CMV-seronegative blood products were given to all patients until April 1996 after which CMV-seronegative red blood cells and leukodepleted platelet concentrates were used. This change in policy has not resulted in any CMV infection or disease in CMV-seronegative donor/recipient pairs (unpublished data, 1999). For patients at high risk of CMV infection, prophylaxis with intravenous immunoglobulin (IVIg) 200 mg/kg every 3 weeks from day −1 to day +90 and IV aciclovir 500 mg/m2 every 8 hours was given. Surveillance cultures of buffy coat and throat swabs were performed weekly and bronchoalveolar lavage (BAL) at 1, 2, and 3 months. More recently, PCR screening of blood samples twice weekly has replaced BAL.37 CMV reactivation was treated with ganciclovir 5 mg/kg twice a day for 14 days, then daily for 14 more days, and IVIg was administered if there was evidence of pneumonitis. Ciprofloxacin, itraconazole, and aciclovir were given prophylactically to all children; cotrimoxazole was given from day +28 to 6 months and then lifelong penicillin V was started. Granulocyte colony-stimulating-factor (G-CSF) was started at day +10 until the ANC was >1 × 109/L for 3 days. Bone marrow biopsies were performed at 1, 2, 3, 6, 9, and 12 months posttransplant and every 6 months thereafter or when indicated.
Statistics.
Primary outcome measures of engraftment and GVHD were analyzed using continuity corrected χ2 tests or, where frequencies were small, 2-tailed Fisher’s Exact tests. Between subgroup comparisons of patient ages, T-cell counts, and TNC subgroups were made using Mann-Whitney U-tests.
A limited series of multivariable analyses were performed as appropriate and with consideration given to the number of events per variable.38 Logistic regression was used for binary outcome (graft failure and acute GVHD). For time to engraftment, survival, and event-free survival, univariable analyses were performed by constructing Kaplan-Meier curves for subsets of patients and comparing them using logrank tests. Multivariable analysis was performed using the Cox Proportional Hazards model.
RESULTS
HLA typing.
HLA-C typing was performed retrospectively in 82 donor/recipient pairs where sufficient archival material was available. Transplants are referred to as matched where there was HLA-A, -B, -DR, and -DQ identity. The additional impact of HLA-C mismatching in both matched and mismatched groups was then evaluated. Eighty-five donor/recipient pairs were matched and 52 mismatched. There were 17 mismatched at HLA-A, 16 at -B, 7 at -A+B, 5 at -DR, 5 at -DQ, 1 at +DR+DQ, and 1 at -B+DQ. Sixteen (30.2%) of 53 matched donor/recipient pairs were mismatched at HLA-C compared with 22 (75.9%) of 29 in the mismatched group. Of the 17 HLA-A mismatches 12 of 17 were at the specificity level as defined by World Health Organization (WHO) nomenclature39; 9 of 12 were serologically defined and 3 of 12 were defined by PCR-SSP. Five of 12 were mismatches between antigens, which were within the same cross-reactive epitope groups. Of the 16 HLA-B mismatched pairs, there were 18 mismatches (2 pairs were mismatched for both HLA-B specificities). Only 2 of these mismatches were within the same cross-reactive epitope groups. Of 7 pairs that were HLA mismatched for both HLA-A and -B, 4 of 7 were within cross-reactive epitope groups, and 3 mismatches were serologically unrelated. None of the HLA-A and -B antigens were typed to the allele level. All HLA-C mismatches were defined by PCR-SSP and were allelic. Of 12 HLA-DR and/or -DQ mismatched pairs, 1 was an HLA-DR mismatch defined by RFLP and 5 were allelic mismatches of DRB1. Seven of 12 mismatches were of HLA-DQ and were defined by RFLP.
Engraftment.
One hundred thirty-five patients who survived for a minimum of 28 days posttransplant were considered evaluable for engraftment. One hundred twenty-three children engrafted including 80 of 84 (95.2%) in the matched group and 43 of 51 (84.3%) in the mismatched group; Table 2. We considered the proportion of all patients with primary graft failure and then secondary graft failure, omitting patients with primary graft failure from the denominator. There was a higher proportion of children with primary graft failure in the mismatched group (P = .012). The proportion of patients with any graft failure was also higher in the mismatched group, largely reflecting the increase in primary graft failure (P = .057; see above). There was no difference in the occurrence of secondary graft failure (P = 1.000).
Univariable Analysis of Engraftment
| . | Engraftment n = 123 . | Primary Graft Failure n = 7 . | Secondary Graft Failure n = 5 . | P Values for Types of Graft Failure . | ||
|---|---|---|---|---|---|---|
| Primary . | Secondary . | All . | ||||
| Matched | 80 (95.2%) | 1 (1.2%) | 3 (3.6%) | .012 | 1.000 | .057 |
| Mismatched | 43 (84.3%) | 6 (11.8%) | 2 (3.9%) | |||
| HLA-C | ||||||
| Matched | 40 (95.2%) | 0 (0.0%) | 2 (4.8%) | .047 | 1.000 | .141 |
| Mismatched | 32 (84.2%) | 4 (10.5%) | 2 (5.3%) | |||
| Matched | ||||||
| HLA-C matched | 34 (94.4%) | 0 (0.0%) | 2 (5.6%) | .308 | 1.000 | 1.000 |
| HLA-C mismatched | 15 (93.8%) | 1 (6.3%) | 0 (0.0%) | |||
| HLA-C not tested | 31 (96.9%) | 0 (0.0%) | 1 (3.1%) | |||
| Mismatched | ||||||
| HLA-C matched | 6 (100%) | 0 (0.0%) | 0 (0.0%) | 1.000 | 1.000 | .553 |
| HLA-C mismatched | 17 (77.3%) | 3 (13.6%) | 2 (9.1%) | |||
| HLA-C not tested | 20 (87.0%) | 3 (13.0%) | 0 (0.0%) | |||
| No methotrexate | 86 (92.5%) | 2 (2.2%) | 5 (5.4%) | .030 | .320 | .515 |
| Methotrexate | 37 (88.1%) | 5 (11.9%) | 0 (0.0%) | |||
| Matched | ||||||
| No methotrexate | 74 (94.9%) | 1 (1.3%) | 3 (3.8%) | 1.000 | 1.000 | 1.000 |
| Methotrexate | 6 (100%) | 0 (0.0%) | 0 (0.0%) | |||
| Mismatched | ||||||
| No methotrexate | 12 (80.0%) | 1 (6.7%) | 2 (13.3%) | .657 | .092 | .679 |
| Methotrexate | 31 (86.1%) | 5 (13.9%) | 0 (0.0%) | |||
| Disease status | ||||||
| CR1 | 21 (87.5%) | 3 (12.5%) | 0 (0.0%) | .341* | 1.000* | .429* |
| CR2 | 82 (93.2%) | 2 (2.3%) | 4 (4.5%) | |||
| CR3/CR4/relapse | 20 (87.0%) | 2 (8.7%) | 1 (4.3%) | |||
| CMV status | ||||||
| Neg/neg | 68 (89.5%) | 6 (7.9%) | 2 (2.6%) | .136 | .658 | .550 |
| Any other | 55 (93.2%) | 1 (1.7%) | 3 (5.1%) | |||
| Donor gender | ||||||
| Male | 62 (88.6%) | 5 (7.1%) | 3 (4.3%) | .444 | 1.000 | .371 |
| Female | 60 (93.8%) | 2 (3.1%) | 2 (3.1%) | |||
| HLA methodology | ||||||
| Pre-SSP | 36 (90.0%) | 3 (7.5%) | 1 (2.5%) | .422 | 1.000 | .749 |
| SSP | 87 (91.6%) | 4 (4.2%) | 4 (4.2%) | |||
| Patient age | ||||||
| Median | 8 | 7 | 6 | .473 | .777 | .456 |
| Range | (1-17) | (1-15) | (4-15) | |||
| TNC × 108/kg | ||||||
| Recipient weight | 5.01 | 7.54 | 4.01 | .069 | .258 | .541 |
| Median range | (1.90-15.0) | (3.57-10.8) | (2.05-7.40) | |||
| . | Engraftment n = 123 . | Primary Graft Failure n = 7 . | Secondary Graft Failure n = 5 . | P Values for Types of Graft Failure . | ||
|---|---|---|---|---|---|---|
| Primary . | Secondary . | All . | ||||
| Matched | 80 (95.2%) | 1 (1.2%) | 3 (3.6%) | .012 | 1.000 | .057 |
| Mismatched | 43 (84.3%) | 6 (11.8%) | 2 (3.9%) | |||
| HLA-C | ||||||
| Matched | 40 (95.2%) | 0 (0.0%) | 2 (4.8%) | .047 | 1.000 | .141 |
| Mismatched | 32 (84.2%) | 4 (10.5%) | 2 (5.3%) | |||
| Matched | ||||||
| HLA-C matched | 34 (94.4%) | 0 (0.0%) | 2 (5.6%) | .308 | 1.000 | 1.000 |
| HLA-C mismatched | 15 (93.8%) | 1 (6.3%) | 0 (0.0%) | |||
| HLA-C not tested | 31 (96.9%) | 0 (0.0%) | 1 (3.1%) | |||
| Mismatched | ||||||
| HLA-C matched | 6 (100%) | 0 (0.0%) | 0 (0.0%) | 1.000 | 1.000 | .553 |
| HLA-C mismatched | 17 (77.3%) | 3 (13.6%) | 2 (9.1%) | |||
| HLA-C not tested | 20 (87.0%) | 3 (13.0%) | 0 (0.0%) | |||
| No methotrexate | 86 (92.5%) | 2 (2.2%) | 5 (5.4%) | .030 | .320 | .515 |
| Methotrexate | 37 (88.1%) | 5 (11.9%) | 0 (0.0%) | |||
| Matched | ||||||
| No methotrexate | 74 (94.9%) | 1 (1.3%) | 3 (3.8%) | 1.000 | 1.000 | 1.000 |
| Methotrexate | 6 (100%) | 0 (0.0%) | 0 (0.0%) | |||
| Mismatched | ||||||
| No methotrexate | 12 (80.0%) | 1 (6.7%) | 2 (13.3%) | .657 | .092 | .679 |
| Methotrexate | 31 (86.1%) | 5 (13.9%) | 0 (0.0%) | |||
| Disease status | ||||||
| CR1 | 21 (87.5%) | 3 (12.5%) | 0 (0.0%) | .341* | 1.000* | .429* |
| CR2 | 82 (93.2%) | 2 (2.3%) | 4 (4.5%) | |||
| CR3/CR4/relapse | 20 (87.0%) | 2 (8.7%) | 1 (4.3%) | |||
| CMV status | ||||||
| Neg/neg | 68 (89.5%) | 6 (7.9%) | 2 (2.6%) | .136 | .658 | .550 |
| Any other | 55 (93.2%) | 1 (1.7%) | 3 (5.1%) | |||
| Donor gender | ||||||
| Male | 62 (88.6%) | 5 (7.1%) | 3 (4.3%) | .444 | 1.000 | .371 |
| Female | 60 (93.8%) | 2 (3.1%) | 2 (3.1%) | |||
| HLA methodology | ||||||
| Pre-SSP | 36 (90.0%) | 3 (7.5%) | 1 (2.5%) | .422 | 1.000 | .749 |
| SSP | 87 (91.6%) | 4 (4.2%) | 4 (4.2%) | |||
| Patient age | ||||||
| Median | 8 | 7 | 6 | .473 | .777 | .456 |
| Range | (1-17) | (1-15) | (4-15) | |||
| TNC × 108/kg | ||||||
| Recipient weight | 5.01 | 7.54 | 4.01 | .069 | .258 | .541 |
| Median range | (1.90-15.0) | (3.57-10.8) | (2.05-7.40) | |||
Combining CR1/CR2.
Graft failure occurred in 0 of 17 patients with an HLA-A mismatch alone, in 4 of 16 children mismatched for HLA-B, 1 of 6 mismatched for HLA-A plus -B, 1 of 1 mismatched for HLA-B and -DQ, and 2 of 11 mismatched for HLA-DR or -DQ. Using Bonferronis’ adjustment of Fisher’s exact test, there was a borderline association between mismatch for HLA-B and any graft failure (P = .063), but not for HLA-A or -DR or -DQ when each was compared separately with the matched group. The difference was more significant when primary graft failure alone was considered (P = .039) (data not shown).
We examined the effect of HLA-C matching where data were available (80 of 137 cases). In cases matched at -C, 95.2% had durable engraftment; there was no primary graft failure, and 4.8% had secondary graft failure. In those mismatched at -C, the respective proportions were 84.2%, 10.5%, and 5.3%. There was a significantly increased chance of primary graft failure in recipients of an HLA-C mismatched graft (P = .047; Table 2). No differences were seen for secondary (P = 1.000) and any (P = .141) graft failure. We also subdivided both the -A, -B, -DR, and -DQ matched and mismatched groups of children into those who were either matched, mismatched, or not tested for HLA-C. There was no significant effect of HLA-C matching with either group (Table 2), but numbers of patients were small. Multivariable analysis was not possible. Use of methotrexate was associated with an incidence of primary (P = .030), but not secondary (P = .320) or any (P = .515) graft failure. However, methotrexate was given mostly to recipients of mismatched grafts (Table 1). A logistic regression analyis was possible with overall graft failure and showed that mismatching was significantly associated with any graft failure (adjusted odds ratio, 5.2; 95% confidence interval, 1.1 to 24.0; P = .036), whereas methotrexate administration was not significantly associated (P = .471). We also analyzed whether disease status, donor/recipient CMV status, donor gender, HLA methodology, patient age, TNC dose, and postgraft immunosuppression influenced engraftment. Patients with primary graft failure had a higher TNC in univariable analysis (P = .069), but any effect of cell dose was not significant when adjusted for the HLA matching in a logistic regression analysis (P = .290).
Of 4 children in the matched group who rejected their grafts, 3 were rescued with autologous marrow, while 1 relapsed shortly after day 28. In the mismatched group, 7 children with graft rejection had autologous marrow rescue and 1 relapsed shortly after day 28. Only 1 of these 12 children survives; of the remainder, 8 died either due to relapse after first BMT and 3 due to treatment-related toxicity or relapse after a second unrelated donor BMT.
The median time to engraftment was 15 days. We constructed Kaplan-Meier curves to compare the kinetics of engraftment between recipients of matched versus mismatched grafts and in children who received methotrexate versus those who did not. There was no difference in time to engraftment between recipients of matched and mismatched grafts (P = .461), but there was a suggestion of slower engraftment for patients who received methotrexate in both groups. Overall, patients who received methotrexate engrafted more slowly, median, 16 days (95% confidence interval, 15 to 18 days) versus 15 days (95% confidence interval, 15 to 16 days) (P = .037; Fig 1). Seventy-five percent of patients who received methotrexate engrafted by day 22 (95% confidence interval, 18 to 27 days) compared with day 18 for those who did not receive methotrexate (95% confidence interval, 17 to 20 days). These variables were also considered in a multivariable Cox Proportional Hazard model. This confirmed that methotrexate significantly delayed engraftment (P = .046), whereas HLA mismatching did not (P = .425).
Kaplan-Meier plots of the rate of engraftment in patients receiving methotrexate (MTX+) versus those who did not (MTX−). The difference is significant (P = .037).
Kaplan-Meier plots of the rate of engraftment in patients receiving methotrexate (MTX+) versus those who did not (MTX−). The difference is significant (P = .037).
GVHD.
One hundred twenty-three children were evaluable for acute GVHD; 80 in the matched and 43 in the mismatched group. No significant effect of HLA-A, -B, -DR, and -DQ or HLA-C matching was seen (Table 3). A multivariable analysis using HLA-A, -B, -DR, and -DQ matching and HLA-C matching as variables was performed and neither were significant (data not shown). Use of methotrexate did not affect the probability of grade 2-4 acute GVHD (Table 3) and when matching and methotrexate administration were analyzed together in a logistic regression, neither were significant (data not shown). The incidence of acute GVHD was not significantly influenced by remission status, donor/recipient CMV status, donor gender, or HLA-typing methodology (Table 3). TNC and patient age did not differ between children with grade 0-1 versus those with grade 2-4 acute GVHD (TNC 5.01 [1.90 to 14.99] v 4.97 [2.64 to 14.86], respectively, P = .928; patient age 8 [1 to 17]v 8.5 [1 to 16] years, respectively, P = .747; figures given as median [range]). A series of logistic regression analyses were performed to look at the effect of HLA matching with adjustments for the other variables listed in Table 3, and none were found to be significant. The incidence of grade 3-4 acute GVHD for the matched and mismatched groups was 2.5% versus 7.0%, respectively (P = .342).
Univariable Analysis of Acute GVHD
| . | Grade 2-4 . | . | P Value . |
|---|---|---|---|
| Matched | 16/80 | (20.0%) | .849 |
| Mismatched | 10/43 | (23.3%) | |
| HLA-C | |||
| Matched | 7/40 | (17.5%) | .627 |
| Mismatched | 8/32 | (25.0%) | |
| Matched | |||
| HLA-C matched | 7/34 | (20.6%) | .717 |
| HLA-C mismatched | 4/15 | (26.7%) | |
| HLA-C not tested | 5/31 | (16.1%) | |
| Mismatched | |||
| HLA-C matched | 0/6 | (0.0%) | .539 |
| HLA-C mismatched | 4/17 | (23.5%) | |
| HLA-C not tested | 6/20 | (30.0%) | |
| No methotrexate | 20/86 | (23.3%) | .525 |
| Methotrexate | 6/37 | (16.2%) | |
| Matched | |||
| No methotrexate | 16/74 | (21.6%) | .340 |
| Methotrexate | 0/6 | (0.0%) | |
| Mismatched | |||
| No methotrexate | 4/12 | (33.3%) | .427 |
| Methotrexate | 6/31 | (19.4%) | |
| Disease status | |||
| CR1 | 6/21 | (28.6%) | .529 |
| CR2 | 15/82 | (18.3%) | |
| CR3/CR4/relapse | 5/20 | (25.0%) | |
| CMV status | |||
| Neg/neg | 13/68 | (19.1%) | .698 |
| Any other | 13/55 | (23.4%) | |
| Donor gender3-150 | |||
| Male | 13/62 | (21.0%) | .925 |
| Female | 13/60 | (21.7%) | |
| HLA methodology | |||
| Pre-SSP | 7/36 | (19.4%) | .958 |
| SSP | 19/87 | (21.8%) |
| . | Grade 2-4 . | . | P Value . |
|---|---|---|---|
| Matched | 16/80 | (20.0%) | .849 |
| Mismatched | 10/43 | (23.3%) | |
| HLA-C | |||
| Matched | 7/40 | (17.5%) | .627 |
| Mismatched | 8/32 | (25.0%) | |
| Matched | |||
| HLA-C matched | 7/34 | (20.6%) | .717 |
| HLA-C mismatched | 4/15 | (26.7%) | |
| HLA-C not tested | 5/31 | (16.1%) | |
| Mismatched | |||
| HLA-C matched | 0/6 | (0.0%) | .539 |
| HLA-C mismatched | 4/17 | (23.5%) | |
| HLA-C not tested | 6/20 | (30.0%) | |
| No methotrexate | 20/86 | (23.3%) | .525 |
| Methotrexate | 6/37 | (16.2%) | |
| Matched | |||
| No methotrexate | 16/74 | (21.6%) | .340 |
| Methotrexate | 0/6 | (0.0%) | |
| Mismatched | |||
| No methotrexate | 4/12 | (33.3%) | .427 |
| Methotrexate | 6/31 | (19.4%) | |
| Disease status | |||
| CR1 | 6/21 | (28.6%) | .529 |
| CR2 | 15/82 | (18.3%) | |
| CR3/CR4/relapse | 5/20 | (25.0%) | |
| CMV status | |||
| Neg/neg | 13/68 | (19.1%) | .698 |
| Any other | 13/55 | (23.4%) | |
| Donor gender3-150 | |||
| Male | 13/62 | (21.0%) | .925 |
| Female | 13/60 | (21.7%) | |
| HLA methodology | |||
| Pre-SSP | 7/36 | (19.4%) | .958 |
| SSP | 19/87 | (21.8%) |
1 donor gender unknown.
Thirteen of 113 (12%) evaluable patients developed chronic GVHD. In 6 (5%), it was extensive (3 survive) and in 7, it was of limited extent (3 survive). Of the 6 survivors with chronic GVHD, all are in full-time employment or education. An additional patient developed fatal acute transfusion-associated GVHD after day 100.
LFS.
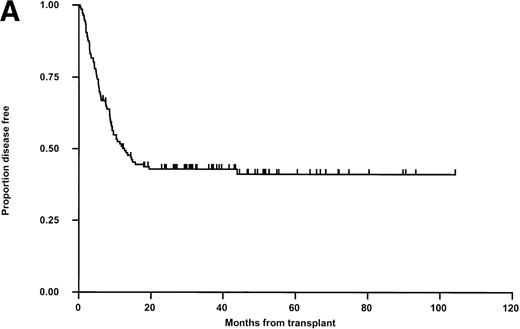
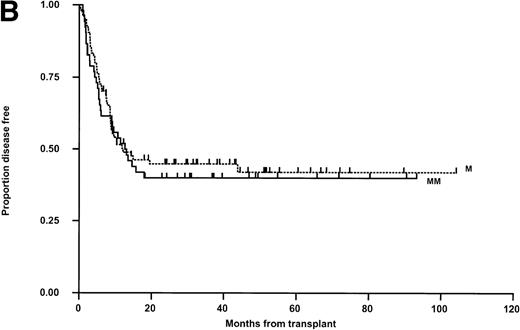
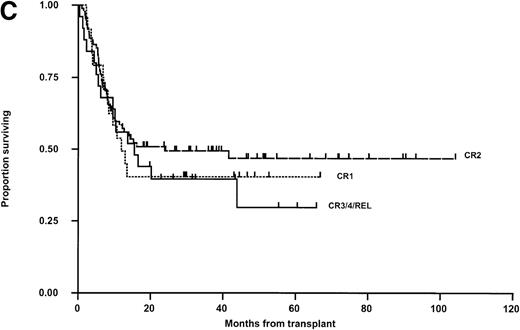
LFS for the whole group is shown in Fig 2A. Relapse was the main cause of death (50 of 74 patients; 67.6%, Table 4). Thirty patients in the matched group (35.3%) and 20 in the mismatched group (38.5%) relapsed posttransplant (Fig 2B). In 11 cases, this occurred after primary (6) or secondary (5) nonengraftment (vide supra). In univariable Kaplan-Meier analysis, LFS was not significantly influenced by matching for HLA-A, -B, -DR, -DQ (Fig 2B) or HLA-C (Table 5). These results were confirmed when both variables were analyzed together in a Cox Proportional Hazards model. Administration of methotrexate did not influence LFS in either the matched or mismatched groups. Methotrexate and HLA-matching were tested in a multivariable Cox Regression model and neither were significantly associated. None of the other parameters previously described were significantly associated with LFS (Table 5). There was a nonsignificant trend towards poorer outcome for recipients of grafts from male donors and patients transplanted in CR3/4/relapse (Fig 2C). A multivariable analysis using just matching, disease status, CMV (any positive), donor sex, and age failed to show significant determinants (P > .100 for all), however there was a suggestion that LFS was slightly better with grafts from female donors (Hazard rate ratio 0.67; 95% confidence interval, 0.42 to 1.06; P = .085). Further, relapse was not correlated with the interval from diagnosis to BMT in CR-1 patients and from diagnosis to relapse, or relapse to BMT in CR-2 patients (data not shown). Sixty-two percent of CR-2 patients in the matched group relapsed on therapy compared with 61% in the mismatched group.
Kaplan-Meier plots of LFS for (A) the whole group, (B) matched (M) versus mismatched (MM) transplants, and (C) LFS according to remission status. No statistically significant differences were seen. Tick marks indicate censoring.
Kaplan-Meier plots of LFS for (A) the whole group, (B) matched (M) versus mismatched (MM) transplants, and (C) LFS according to remission status. No statistically significant differences were seen. Tick marks indicate censoring.
Cause of Death
| . | Matched Group (n = 85) . | Mismatched Group (n = 52) . | Total (n = 137) . |
|---|---|---|---|
| Relapse | 30 (35.3%) | 20 (38.5%) | 50 (36.5%) |
| Viral | 4 (4.7%) | 5 (9.6%) | 9 (6.6%) |
| aGVHD/sepsis | 2 (2.4%) | 2 (3.8%) | 4 (2.9%) |
| Fungal | 1 (1.2%) | 1 (1.9%) | 2 (1.5%) |
| TTP | 3 (3.5%) | 1 (1.9%) | 4 (2.9%) |
| Other | 3 (3.5%) | 2 (3.8%) | 5 (3.6%) |
| Total | 43 (50.5%) | 31 (59.6%) | 74 (54%) |
| . | Matched Group (n = 85) . | Mismatched Group (n = 52) . | Total (n = 137) . |
|---|---|---|---|
| Relapse | 30 (35.3%) | 20 (38.5%) | 50 (36.5%) |
| Viral | 4 (4.7%) | 5 (9.6%) | 9 (6.6%) |
| aGVHD/sepsis | 2 (2.4%) | 2 (3.8%) | 4 (2.9%) |
| Fungal | 1 (1.2%) | 1 (1.9%) | 2 (1.5%) |
| TTP | 3 (3.5%) | 1 (1.9%) | 4 (2.9%) |
| Other | 3 (3.5%) | 2 (3.8%) | 5 (3.6%) |
| Total | 43 (50.5%) | 31 (59.6%) | 74 (54%) |
Univariable Analysis of OS and LFS
| . | No. . | Kaplan- Meier 36-Month OS % (SE %) . | P Value . | Kaplan- Meier 36-Month LFS % (SE %) . | P Value . |
|---|---|---|---|---|---|
| Matched | 855-150 | 48.7 (5.6) | .380 | 44.8 (5.6) | .654 |
| Mismatched | 52 | 41.7 (6.9) | 39.9 (6.8) | ||
| HLA-C | |||||
| Matched | 44 | 48.6 (8.1) | .700 | 45.0 (7.8) | .858 |
| Mismatched | 385-150 | 44.6 (8.1) | 43.2 (8.1) | ||
| Not tested | 55 | 45.5 (6.7) | 41.8 (6.7) | ||
| No methotrexate | 945-150 | 47.3 (5.3) | .652 | 43.7 (5.3) | .933 |
| Methotrexate | 43 | 43.4 (7.7) | 41.3 (7.6) | ||
| Matched | |||||
| No methotrexate | 795-150 | 47.2 (5.8) | .438 | 43.0 (5.8) | .346 |
| Methotrexate | 6 | 66.7 (19.3) | 66.7 (19.3) | ||
| Mismatched | |||||
| No methotrexate | 15 | 46.7 (12.9) | .712 | 46.7 (12.9) | .699 |
| Methotrexate | 37 | 39.4 (8.2) | 37.1 (8.0) | ||
| Disease status | |||||
| CR1 | 24 | 40.4 (10.2) | .622 | 41.3 (10.1) | .596 |
| CR2 | 885-150 | 49.4 (5.5) | 45.5 (5.5) | ||
| CR3/CR4/relapse | 25 | 39.6 (9.9) | 36.0 (9.6) | ||
| CMV status | |||||
| Neg/neg | 76 | 46.3 (5.8) | .968 | 43.9 (5.8) | .935 |
| Any other | 615-150 | 44.7 (6.7) | 41.3 (6.6) | ||
| Donor gender | |||||
| Male | 715-150 | 41.5 (6.1) | .171 | 38.4 (6.0) | .137 |
| Female | 65 | 51.4 (6.3) | 48.4 (6.3) | ||
| HLA methodology | |||||
| Pre-SSP | 40 | 47.5 (7.9) | .776 | 47.5 (7.9) | .655 |
| SSP | 975-150 | 45.3 (5.2) | 40.7 (5.2) | ||
| Patient age | |||||
| <8 yr | 62 | 39.9 (6.3) | .231 | 40.1 (6.3) | .469 |
| >8 yr | 755-150 | 51.1 (6.0) | 45.3 (6.0) | ||
| TNC | |||||
| <Median | 695-150 | 46.3 (6.2) | .885 | 43.2 (6.1) | .994 |
| >Median | 68 | 45.4 (6.2) | 42.6 (6.2) |
| . | No. . | Kaplan- Meier 36-Month OS % (SE %) . | P Value . | Kaplan- Meier 36-Month LFS % (SE %) . | P Value . |
|---|---|---|---|---|---|
| Matched | 855-150 | 48.7 (5.6) | .380 | 44.8 (5.6) | .654 |
| Mismatched | 52 | 41.7 (6.9) | 39.9 (6.8) | ||
| HLA-C | |||||
| Matched | 44 | 48.6 (8.1) | .700 | 45.0 (7.8) | .858 |
| Mismatched | 385-150 | 44.6 (8.1) | 43.2 (8.1) | ||
| Not tested | 55 | 45.5 (6.7) | 41.8 (6.7) | ||
| No methotrexate | 945-150 | 47.3 (5.3) | .652 | 43.7 (5.3) | .933 |
| Methotrexate | 43 | 43.4 (7.7) | 41.3 (7.6) | ||
| Matched | |||||
| No methotrexate | 795-150 | 47.2 (5.8) | .438 | 43.0 (5.8) | .346 |
| Methotrexate | 6 | 66.7 (19.3) | 66.7 (19.3) | ||
| Mismatched | |||||
| No methotrexate | 15 | 46.7 (12.9) | .712 | 46.7 (12.9) | .699 |
| Methotrexate | 37 | 39.4 (8.2) | 37.1 (8.0) | ||
| Disease status | |||||
| CR1 | 24 | 40.4 (10.2) | .622 | 41.3 (10.1) | .596 |
| CR2 | 885-150 | 49.4 (5.5) | 45.5 (5.5) | ||
| CR3/CR4/relapse | 25 | 39.6 (9.9) | 36.0 (9.6) | ||
| CMV status | |||||
| Neg/neg | 76 | 46.3 (5.8) | .968 | 43.9 (5.8) | .935 |
| Any other | 615-150 | 44.7 (6.7) | 41.3 (6.6) | ||
| Donor gender | |||||
| Male | 715-150 | 41.5 (6.1) | .171 | 38.4 (6.0) | .137 |
| Female | 65 | 51.4 (6.3) | 48.4 (6.3) | ||
| HLA methodology | |||||
| Pre-SSP | 40 | 47.5 (7.9) | .776 | 47.5 (7.9) | .655 |
| SSP | 975-150 | 45.3 (5.2) | 40.7 (5.2) | ||
| Patient age | |||||
| <8 yr | 62 | 39.9 (6.3) | .231 | 40.1 (6.3) | .469 |
| >8 yr | 755-150 | 51.1 (6.0) | 45.3 (6.0) | ||
| TNC | |||||
| <Median | 695-150 | 46.3 (6.2) | .885 | 43.2 (6.1) | .994 |
| >Median | 68 | 45.4 (6.2) | 42.6 (6.2) |
1 patient excluded for LFS, relapse date uncertain.
We also conducted an investigation of the factors influencing LFS in just the group of 88 patients transplanted in CR-2. An initial univariable analysis showed that only duration of CR-1 (≤730, and >730 days) and donor gender were significant (P = .005 and .041, respectively, Fig3). Other factors analyzed, including HLA-matching, CMV status, patient age, or TNC did not reach statistical significance. Matching, CR-1 duration, and donor gender were entered into a Cox Proportional Hazards regression model. Length of CR-1 was the only variable that significantly affected LFS (P = .007, Table 6). Again recipients of a graft from a female donor had a survival advantage, but this did not reach statistical significance (P = .067; Table 6).
Kaplan-Meier plots of LFS in CR-2 patients who relapsed on (≤730 days) or off (>730 days) therapy. The difference is significant (P = .005). Tick marks indicate censoring.
Kaplan-Meier plots of LFS in CR-2 patients who relapsed on (≤730 days) or off (>730 days) therapy. The difference is significant (P = .005). Tick marks indicate censoring.
Multivariable Analysis of LFS for Children in CR-2
| Variable . | Hazard Rate Ratio . | (95% Confidence Interval) . | P Value . |
|---|---|---|---|
| Matched | 1 | (baseline) | .811 |
| Mismatched | 1.08 | (0.58-2.01) | |
| CR-1 | |||
| ≤730 d | 1 | (baseline) | .007 |
| >730 | 0.44 | (0.24-0.80) | |
| Male donor | 1.0 | baseline | .067 |
| Female donor | 0.57 | (0.31-1.05) |
| Variable . | Hazard Rate Ratio . | (95% Confidence Interval) . | P Value . |
|---|---|---|---|
| Matched | 1 | (baseline) | .811 |
| Mismatched | 1.08 | (0.58-2.01) | |
| CR-1 | |||
| ≤730 d | 1 | (baseline) | .007 |
| >730 | 0.44 | (0.24-0.80) | |
| Male donor | 1.0 | baseline | .067 |
| Female donor | 0.57 | (0.31-1.05) |
In the matched group, 1 patient had a second unrelated donor transplant 29 months after the first following chemotherapy to induce a further remission and died of transplant-related toxicity. Two patients in the mismatched group also had second transplants at 6 and 17 months post first BMT after further chemotherapy. Neither was successful, with death from transplant toxicity in 1 and a further relapse in the other. One child who relapsed after a mismatched graft received chemotherapy followed by donor lymphocyte infusions, but did not enter a further remission.
Overall outcome and cause of death.
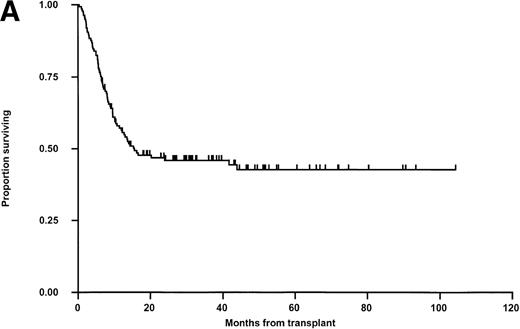
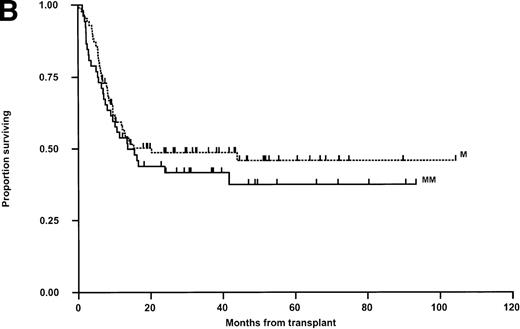
Relapse was the principal cause of treatment failure (50 patients; 36.5%) followed by viral infections in 9 children (6.6%) and GVHD with associated sepsis in 4 (2.9%) (Table 4). Overall survival was analyzed in exactly the same manner as LFS with the same parameters tested in both univariable and multivariable analysis. The results are shown in Fig 4. In univariable analysis, OS was not influenced by any parameter except that, as for LFS, there was a nonsignificant trend toward a poorer overall outcome in older children and in recipients of grafts from male donors. Multivariable analysis using just matching, disease status, CMV (any positive), donor sex, and age again failed to show significant determinants, although there was a trend towards better outcome for children receiving transplants from female donors (Hazard rate ratio, 0.66; 95% confidence interval, 0.41 to 1.06, P = .087) and a slight trend toward poorer survival with increased age (P = .080).
Kaplan-Meier plots of OS for (A) the whole group, (B) matched (M) versus mismatched (MM) transplants, and (C) OS according to remission status. No statistically significant differences were seen. Tick marks indicate censoring.
Kaplan-Meier plots of OS for (A) the whole group, (B) matched (M) versus mismatched (MM) transplants, and (C) OS according to remission status. No statistically significant differences were seen. Tick marks indicate censoring.
At the time of analysis, 60 patients were alive and more than 6 months post-BMT. Forty-seven patients were at school full-time, 6 were at university full-time, and 3 are in full-time employment. Of the remaining 4 patients, 2 had relapsed disease, 1 was having a second BMT, and 1 was preschool age.
DISCUSSION
The principal finding of this study was an increased incidence of primary graft failure in children receiving HLA mismatched grafts (11.8% v 1.2%; P = .012, Table 2). There was no difference in the incidence of secondary graft failure, and the only parameter in our patients that predicted for a significant increase in graft failure was HLA mismatch. Most mismatches were at HLA-A and -B loci. Other reports have shown that graft failure is more common in recipients of unrelated donor bone marrow mismatched at HLA-A, -B, or -C loci.19-21 Our data suggest a trend toward an increased incidence of any graft failure in recipients of HLA-B mismatched marrow when compared with recipients of matched grafts (P = .063). This seems to result mainly from more primary graft failure (P= .039). While an HLA-C mismatch did increase the chance of graft failure, the presence of additional HLA-C mismatches in the HLA-A, -B, -DR, and -DQ mismatched children did not independently predict for this outcome as far as we are able to ascertain. Because most children who received mismatched marrow were given methotrexate in addition to cyclosporin as postgraft immunosuppression, we analyzed these variables together. The number of children with sustained engraftment was still lower in mismatched donor/recipient pairs irrespective of methotrexate administration. Methotrexate did result, however, in slower overall engraftment.
Three other centers have reported series of 25 or more unrelated donor marrow allografts for children with ALL.14-16 In these, sustained engraftment was observed in 93%, 100%, and 100% compared with 91% in our patients. Graft failure is a serious complication of unrelated donor BMT; only 1 of 7 patients who had cryopreserved back-up bone marrow infused is currently alive, and 3 further patients died after a second unrelated donor allogeneic BMT from either transplant toxicities or relapse. It is noteworthy that 2 patients (1 in the matched and 1 in the mismatched group) had, by definition, primary graft failure (ie, neutrophils <0.5 × 109/L at day 28), but relapsed before day 35. It is possible that either rapid leukemic relapse inhibited engraftment or that failure to engraft donor cells permitted the reemergence of leukemic cells. Analysis of engraftment excluding these patients would not alter our findings. It is now recognized that long-term survivors of unrelated donor allografts for acute leukemia who are in sustained hematologic remission may revert to predominantly recipient type hemopoiesis without noticeable changes in the peripheral blood count.40It is possible that a proportion of our patients are not full donor chimeras even though they are in continued hematologic remission. Further molecular analysis, not routinely available for our patients, would be required to confirm this and compare the incidence in recipients of matched and mismatched grafts.
We observed a low incidence of acute GVHD grades 2-4 in recipients of both matched and mismatched grafts (20.0% v 23.3%, respectively). The incidence of grade 3-4 acute GVHD was 2.5% versus 7.0%. These incidences do not significantly differ between the 2 groups. Similarly, there was a low incidence of chronic GVHD resulting in 7 deaths overall (5.1%). The low incidence of acute and chronic GVHD in recipients of mismatched grafts did not result from the use of posttransplant methotrexate in these patients and probably reflects, in part, the efficacy of T-cell depletion with CAMPATH antibodies. These were used as conditioning therapy in all and for marrow manipulation in 134 patients. Although immunofluorescent measurements indicate approximately 3 logs of ex vivo T-cell depletion, more than 99% residual cells are still coated with antibody (unpublished observations, 1987) and continued T-cell destruction is likely in vivo. In 2 previous reports of T-cell–depleted unrelated donor BMT in children with acute leukemia, the incidence of acute GVHD, grade 2-4, was 22% and 33%.16,41 In 1 report where 45 of 50 patients received T-cell–replete grafts, the incidence of acute GVHD was 49% and 67% for matched versus mismatched grafts, respectively,15 and in a further report 89%.14
The actuarial LFS for the 137 children in this series is 43% at 36 months posttransplant. These results compare favorably with other reports of unrelated BMT in childhood ALL where LFS was 23% at 3 years in 43 patients (although 28 of 43 patients were in third remission or relapse),14 30% at 2 years in 35 patients,15and 40% at 4 years in 25 patients.16 These differences may, in part, reflect our T-cell–depletion strategy using CAMPATH-1 antibodies (vide supra). It has been reported that in patients with ALL who receive CAMPATH-1 purged grafts, the risk of relapse is similar to that in recipients of T-cell–replete marrow.42 One surprising finding in this study was that recipients of marrow from female donors had a marginally better survival. An increase in acute GVHD is reported after bone marrow transplantation from parous female donors. It is possible that our results are explained by effective T-cell depletion and a low consequent rate of acute GVHD in both the matched and mismatched groups.
We analyzed whether patients in the mismatched group were at equivalent risk of relapse to those recipients of matched grafts by comparing the intervals from diagnosis to transplant for patients transplanted in CR-1 and from diagnosis to relapse and relapse to transplant in children transplanted in CR-2. We found no differences; moreover, the proportion of patients relapsing on treatment in the matched and mismatched group was 62% versus 61%, respectively. When patients in CR-2 were considered separately, we found that children relapsing on-therapy (CR-1 duration ≤730 days) had a significantly higher probability of posttransplant relapse. This correlates well with our previous observation that minimal residual disease (MRD) is still detectable at commencement of BMT in most patients who relapse on therapy, but only in a small minority of those who relapse after completion of first line treatment.43
In summary, we believe that our results using T-cell–depleted transplants are sufficiently encouraging to justify further studies that investigate the role of unrelated BMT in the management of childhood ALL. We have shown that selecting mismatched donors increases the incidence of primary graft failure, but does not significantly affect overall outcome. The ability to use mismatched donors clearly increases the number of children who can have unrelated donor BMT and may reduce the time from relapse to transplant. The other striking finding of our report is the low incidence of acute and chronic GVHD and the excellent functional status of long-term survivors. Further studies are needed to better define which groups of children with ALL benefit most from unrelated donor BMT (compared with alternative treatments) and which poor-outcome groups require different strategies such as intensified conditioning or posttransplant immune modulation.
ACKNOWLEDGMENT
We thank the nursing and medical staff of the transplant unit for patient care. Drs Mike Potter and Nick Goulden contributed greatly to the care of patients during this study. We are grateful to Bob Thorne and Heather Hawkins for help with data collection and to Bridget Hunt for preparing the manuscript.
E.C. was supported by a program research grant from the National Blood Authority.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Derwood H. Pamphilon, MD, Consultant Haematologist, Royal Hospital for Sick Children, St Michael’s Hill, Bristol BS2 8BJ, UK; e-mail:derwood.pamphilon@nbs.nhs.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal