To the Editor:
There is growing evidence that environmental and genetic risk factors often interact to induce clinically manifest venous thromboembolism (VTE). The role of gene-gene interactions, although much rarer, is supported by cosegregation of genetic defects observed in patients with familial thrombophilia. In this context, factor V (FV) Leiden and the prothrombin G20210A gene (FII) mutation are of particular interest because of their high prevalence in the normal population; about 5% and 2%, respectively, in whites.1,2 The FII mutation has been reported in 10% of FV Leiden carriers with VTE,3while FV Leiden is present in 30% to 40% of symptomatic carriers of the FII mutation.2,4 Homozygosity for these mutations is less common, with a prevalence of 0.02% for FV Leiden and 0.014% for the FII mutation.1 2 We present here a case of double-homozygosity for these defects.
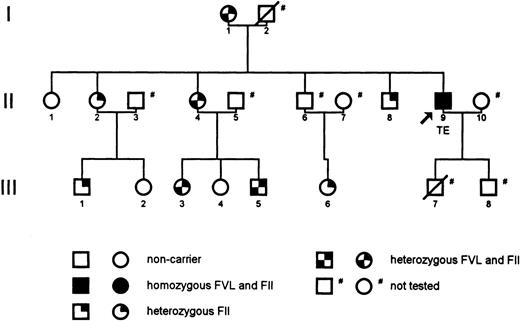
A 34-year-old man presented with a first episode of idiopathic deep-vein thrombosis. He had never been exposed to environmental risk factors for thrombosis. Treatment consisted of subcutaneous low-molecular-weight heparin for 1 week followed by acenocoumarol for 3 months. At 18 months follow-up, he had experienced no recurrence. Laboratory studies for thrombophilic disorders showed double-homozygosity for FV Leiden and the FII mutation, detected by polymerase chain reaction. None of his 15 relatives had a history of VTE. Four were double-heterozygous carriers, 4 were single carriers of the FII mutation, and 3 were noncarriers (Fig1). Four relatives, 2 of whom had died, were not tested but they were obligate carriers of at least 1 (N = 1) or both (N = 3) mutations. The patient’s father, who had a history of smoking and hypercholesterolemia, died of myocardial infarction at 57 years of age and a son died from cribdeath. His mother experienced a myocardial infarction when she was 61 years old; she had a history of hypertension and diabetes mellitus. Fetal loss had occurred only in 1 noncarrier. Table 1 summarizes characteristics of this family.
Pedigree of the reported family. The arrow indicates the propositus, who is a double-homozygous carrier of factor V Leiden (FVL) and the prothrombin G20210A gene mutation (FII). Individuals with a slash through the symbol are deceased. TE, venous thromboembolism.
Pedigree of the reported family. The arrow indicates the propositus, who is a double-homozygous carrier of factor V Leiden (FVL) and the prothrombin G20210A gene mutation (FII). Individuals with a slash through the symbol are deceased. TE, venous thromboembolism.
Double-homozygosity for FV Leiden and the FII mutation is extremely rare. Theoretically, it is expected in 3 per 100 million whites. The thrombotic risk of this combined abnormality is unknown. Homozygosity for FV Leiden increases the risk of VTE approximately 80-fold compared with noncarriers.1 The risk in homozygous carriers of the FII mutation has not yet been estimated; only case reports have been published.
Remarkably, only our double-homozygous patient has developed venous thrombosis, while none of his single- or double-heterozygous carrier relatives have experienced VTE thus far, despite their exposure to several environmental risk factors (Table 1). The compound carriership in this family is apparently not associated with a high risk of VTE. This finding agrees with a recent observation that the thrombotic risk in FII mutants is hardly influenced by the simultaneous presence of FV Leiden.4 Others, however, reported a high risk of recurrence in double-heterozygous mutants.3 Concomitant protective genetic factors in families with a hardly increased risk of VTE may explain this discrepancy.
Both mutations have also been associated with myocardial infarction.5 Their contribution to this event in 2 double-heterozygous carriers, who already exhibited several established risk factors, remains speculative. None of the carriers showed fetal loss, another possible expression of thrombophilia.6
Elevated plasma levels of prothrombin in carriers of the FII mutation have been correlated with the thrombotic risk.2 In our double-homozygous patient, prothrombin activity was shown to be clearly higher than in noncarriers, while heterozygous carriers had values that fell in between.
This family illustrates that expression of the supposed high risk of thrombosis, due to gene-gene interaction and exposure to environmental risk factors, is not a matter of course in compound carriers of FV Leiden and the FII mutation. Our findings emphasize the need for further studies to assess the implications of these common mutations in compound carriers.