Abstract
Therapy of B-cell chronic lymphocytic leukemia (CLL) has been limited by both the nonselectivity of therapeutic agents toward normal residual immune cells and inherent drug resistance. Identification of agents that spare normal immune effector cells, thus facilitating addition of immune-based therapies, and that modulate factors associated with drug resistance in CLL might represent a major therapeutic advance. Depsipeptide (FR901228) is a novel agent entering clinical trials that has selective in vitro activity against resistant leukemia cell lines. To assess its in vitro activity in CLL, we exposed peripheral mononuclear cells from CLL patients (n = 10) to varying concentrations of this agent. Viability of the CLL cells was reduced by 50% (LC50) at 4 hours, 24 hours, and 4 days at depsipeptide concentrations of 0.038, 0.024, and 0.015 μmol/L, respectively. Depsipeptide had marked selective cytotoxicity when compared with normal blood mononuclear cells, in which the LC50 was 3.44 μmol/L at 4 hours (P = .03), 0.965 μmol/L at 24 hours (P = .01), and 0.0318 μmol/L at 96 hours (P = .04). Inhibition of bone marrow progenitor cell growth was also minimal after incubation with 0.015 μmol/L (19% inhibition of colony forming unit-granulocyte-macrophage [CFU-GM]; 17% inhibition burst forming unit-erythroid [BFU-E]) and 3.44 μmol/L (24% inhibition of CFU-GM; 57% inhibition BFU-E) of depsipeptide for 4 hours, followed by a 14-day incubation period. Expression of apoptotic proteins after depsipeptide exposure (0.015 μmol/L) included no change in bcl-2, elevation of bax, and decreased expression of p27. These data demonstrate that depsipeptide has significant selective in vitro activity against human CLL cells concurrent with favorable alterations of the bcl-2:bax protein ratio and decrease in p27 expression. Such findings strongly support the early introduction of depsipeptide into clinical trials for patients with CLL.
B-CELL CHRONIC lymphocytic leukemia (CLL) is the most common leukemia in the Western hemisphere, with approximately 10,000 new cases diagnosed each year.1 The overall prognosis relative to other forms of leukemia in the absence of therapy is good, with even the most advanced stage CLL patients having a median survival of 3 years.2 However, unlike most of the other forms of acute and chronic leukemia, substantial therapeutic progress has not been made over the past 40 years in either prolongation of survival or the introduction of curative therapy. The addition of fludarabine early in the treatment of symptomatic CLL patients has led to a higher rate of complete responses (27% v3%) and duration of progression-free survival (33 v 17 months) as compared with previously used alkylator-based therapies.3 Although attaining a complete clinical response after therapy is the initial step toward improving survival in CLL, the majority of patients either do not attain complete remission or fail to respond to fludarabine. Furthermore, all patients with CLL treated with fludarabine eventually relapse, making its role as a single agent purely palliative. Reasons for drug resistance in CLL are many and include the cellular overexpression of bcl-2, increased bcl-2:bax ratio, 17p21 deletions (p53 locus), and cytokine deregulation (interleukin-4 [IL-4] or basic fibroblast growth factor).4-12 Vrhovac et al12 recently reported that overexpression of p27 in patients with CLL is also associated with an inferior overall survival. Overexpression of p27 was inducible by IL-4 incubation (an antiapoptotic cytokine in CLL) in this study12 and another previously published report.13 Identification of a therapeutic agent that favorably alters protein expression of p27 and other key apoptosis-related proteins may represent a therapeutic target to exploit in this disease.
In addition to drug resistance, patients with CLL have compromised bone marrow function and an inherent immune deficiency as a consequence of their underlying disease.14 Both the immune dysfunction and marrow deficiency are accentuated by currently applied therapies for CLL (ie, fludarabine and chlorambucil). This acutely increases the severity of pre-existing cytopenias and predisposition toward developing secondary infections. Furthermore, cellular immune deregulation induced by fludarabine can be long-lasting, thus potentially limiting application of highly promising immune-based therapies (ie, monoclonal antibodies or gene therapy). One ideal property for a new agent entering clinical trials in CLL, therefore, would include selective cytotoxicity toward the leukemic cell with minimal effect on normal bone marrow progenitors or immune effector cells. We describe here depsipeptide, a novel bicyclic depsipeptide currently under evaluation in phase I clinical trials, that demonstrates marked in vitro selective cytotoxicity toward human CLL cells as well as favorable changes in protein expression of key apoptotic-related proteins.
MATERIALS AND METHODS
Patients, Cell Separation, and Culture Conditions
Written, informed consent was obtained to procure cells from patients with previously diagnosed CLL as defined by the modified National Cancer Institute (NCI) criteria15 and normal healthy volunteers. All of the CLL patients had been without prior therapy for a minimum of 2 months. Clinical data provided in Table 1 include modified Rai stage, previous treatment, presence of active disease, and fludarabine response status at the time of cell acquisition. Patients were considered to have active disease if they required initiation of therapy within 2 months of donating cells. Criteria for being considered fludarabine refractory included lack of partial or complete response to treatment with this agent or relapse within 6 months of last fludarabine treatment. Response was judged according to the modified NCI criteria.15 Mononuclear cells were isolated from the peripheral blood using density gradient centrifugation (Ficoll-Paque Plus; Pharmacia Biotech, Piscataway, NJ). Cells were immediately cultured (1 × 107 cells/mL) in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin-G, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine (Life Technologies, Grand Island, NY) and incubated at 37°C in a 5% CO2incubator. Depsipeptide (FR901228 or NSC649890) was obtained from the Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute.
Blood Viability Assay
Viability assays of isolated mononuclear cells from CLL patients were performed using the MTT assay. Cells (1 × 106 per well) in a volume of 100 μL were placed in a 96-well flat-bottom plate, and the test drug (100 μL per well at 2× final concentration) or 100 μL of medium alone was added to the plates. All experiments were performed in quadruplicate. Cells were incubated for fixed times (4, 24, and 96 hours). After the incubation, 50 μL of MTT (Sigma Chemical Co, St Louis, MO) at a concentration of 2 mg/mL was added to each well. Cells were incubated for 24 hours and centrifuged at 300g for 5 minutes, and 150 μL of supernatant was removed. A total of 200 μL of protamine sulfate (Sigma Chemical Co) in phosphate-buffered saline (PBS) at 25 mg/mL was added to each well. Centrifugation was repeated, followed by the replacement of 200 μL of protamine sulfate. Plates were centrifuged and 200 μL of protamine sulfate was removed. Plates were then allowed to air-dry. The precipitated MTT formazan was solubilized with 150 μL of dimethyl sulfoxide (DMSO) with constant agitation for 4 hours. After this, the optical density at 540 nm was obtained using an Anthos Reader 2001 (Anthos Labtec Inc, Frederick, MD) with a Biolise-Windows program. Cell viability was expressed as the ratio of absorption between drugged cells/control sample.
Bone Marrow Viability Assay
Viability assays on normal patient bone marrow progenitor cells were performed using the methyl cellulose colony-forming assay. Written, informed consent was obtained from normal bone marrow transplant donors. Bone marrow from the donor was prepared by Ficoll-Hypaque (Ficoll-Paque Plus; Pharmacia Biotech) density gradient centrifugation. The mononuclear interphase was removed and resuspended in Isocove’s modified Dulbecco’s medium (IMDM; GIBCO BRL Life Technologies, Grand Island, NY). The mononuclear cells were counted and resuspended at 4 × 105 cells/mL in IMDM medium with 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 U/mL penicillin-G, and 100 μg/mL streptomycin (Life Technologies). A 66 mmol/L stock preparation of depsipeptide was prepared in DMSO, aliquoted, and frozen at −80°C. Just before use, stock solution was further diluted to 3.44, 0.015, and 0.0015 mmol/L in DMSO to be used at a final concentration of 0.1% (vol/vol) to obtain the appropriate final concentration in culture. A total of 2 mL of cell suspension at 4 × 105 cells/mL was added to wells of a 24-well plate. To each drug incubation well, 2 μL of drug diluted to 1,000× the desired concentration in DMSO was added. Two microliters of DMSO was added to the media control well. Therefore, the final concentration of depsipeptide for incubation was 3.44, 0.015, and 0.0015 μmol/L. The cells were incubated at 37°C with 5% CO2 for 4 hours. After this, 1.6 × 105 total cells were removed from each drug incubation and media control well and washed 3 times. After the last wash, the cells were transferred to 3.6 mL of prealliquoted methylcellulose (Stem Cell Technologies, Vancouver, British Columbia, Canada; catalogue no. HCC-4434) in 0.4 mL of complete IMDM media with 10% FCS, L-glutamine, penicillin G, and streptomycin. A total of 1.0 mL of methylcellulose was added to each of three 35-mm petri dishes.
The effect of each drug on the in vitro growth of marrow-derived multipotent (colony-forming unit-mixed [CFU-GEMM]), erythroid (burst forming unit-erythroid [BFU-E]), and granulocyte-macrophage (colony forming unit-granulocyte-macrophage [CFU-GM]) hematopoietic progenitors was investigated after the in vitro transient exposure to drug for 4 hours. The progenitor cell growth was evaluated after 14 to 18 days of incubation at 37°C with 5% CO2 in a humidified atmosphere. The data are based on the actual colony counts from the assays (n = 3). The percentage of colony growth inhibition was calculated as follows: 100 − (mean colony growth in drug/mean colony growth in media × 100). The LC50 was determined by calculated extrapolation from the numerical data.
Apoptosis Assays
After incubation with 0.15, 0.038, or 0.015 μmol/L of depsipeptide in supplemented RPMI and 10% FBS for 4 hours, followed by incubation in media for 96 hours, apoptosis studies were performed using the following techniques.
TdT/propidium iodine (PI).
Cells (5 × 105) were added to cold 1% buffered formaldehyde (10% formadehyde [methanol free, ultrapure grade; Polysciences, Inc, Warrington, PA], prepared in Dulbecco’s PBS without CaCl2 and MgCl2 [PBS]) for 15 minutes on ice. Cells were then washed with PBS, resuspended in 70% methanol, and stored at −20°C. To complete the assay, cells were washed with PBS, resuspended in 50 μL of Cacodylate/biotin dUTP reaction buffer (0.2 mol/L potassium cacodylate, 25 mmol/L Tris-HCL [pH 6.6], 2.5 mmol/L cobalt chloride [CoCl2], 0.25 mg/mL bovine serum albumin [BSA], 10 U of TdT, and 0.5 nmol biotinylated deoxyuridine triphosphate) with a TdT negative control for each sample (all supplies from Boehringer Mannheim, Mannheim, Germany), and incubated for 60 minutes at 37°C. Cells were then washed twice with 2 mL of rinsing buffer (0.1% Triton X-100 and 5 mg/mL BSA in PBS), resuspended in 100 μL of fluorescein isothiocyanate (FITC)-Avidin solution (4× saline sodium citrate buffer, 0.1% Triton X-100, 5% nonfat dry milk, and 2.5 μg/mL of fluoresceinated avidin; Boehringer Mannheim), and incubated for 30 minutes in a light-protected area. Cells were subsequently washed twice with rinsing buffer, resuspended in 0.25 mL of PI/RNAse solution (100 μg/mL propidium iodine [Sigma Chemical Co] and 7.5 μg/mL RNAse-DNAse free [Boehringer Mannheim] in PBS), and incubated for 30 minutes. Samples were analyzed on a FACScan (Becton Dickinson, San Jose, CA) illuminated at 488 nm and measuring green fluorescence (detecting FITC levels) at 530 nm using an exponential scale. Red fluorescence (measuring PI content of the cells) was measured on a linear scale at greater than 600 nm. All samples were analyzed in duplicate or triplicate fashion.
Annexin/PI.
CLL cells were incubated with 0.15, 0.038, and 0.015 μmol/L of depsipeptide or medium for 4 hours, followed by incubation in media for 0, 24, 48, 72, and 96 hours. These CLL cells (5 × 105) were then washed with PBS and resuspended in binding buffer (10 mmol/L HEPES/NaOH, pH 7.4, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, and 1.8 mmol/L CaCl2) containing 2 μL of annexin V-FITC stock (BioWhittaker, Inc, Walkersville, MD) and 10 μL of 20 μg/mL PI (Sigma Chemical Co). After incubation for 10 minutes at room temperature in a light-protected area, the specimens were quantified by flow cytometry on a FACScan (Becton Dickinson).
Protein Extraction and Western Blot Analysis
The bcl-2, bax, p53, and p27Kip1 protein expressions were analyzed by Western blot after incubation in either medium or 2 concentrations of depsipeptide (0.15 and 0.015 μmol/L) for 4, 24, and 48 hours. Whole-cell lysates were prepared by pelleting 1.25 × 108 PBS washed mononuclear cells in a microcentrifuge, aspirating the supernatant, and adding 0.5 mL of cold lysis buffer as described previously.16 This cell suspension was incubated at 4°C for 40 minutes with constant agitation and then centrifuged for 15 minutes at 14,000 rpm at 4°C. The supernatant was recovered, alliquoted, and frozen at −80°C.
Protein was quantified in each supernatant by the BCA method (Pierce, Rockford, IL). Varied amounts (2 to 100 μg) of each sample were used for each protein studied based on varied expression of protein in human CLL cells. Once identified, a single loading concentration was used for each protein examined. Samples were diluted with lysis buffer to a volume of 25 μL and then added to 25 μL of 2× Laemmli’s Sample Buffer as previously described.16 The samples were boiled for 4 minutes. Rainbow-colored protein molecular weight markers (Amersham Life Science, Arlington Heights, IL) and samples were loaded onto 10% to 14% polyacrylamide gels and electrophoresed. The proteins were transferred to a 0.45-μm nitrocellulose membrane (Schleicher and Schueel, Keene, NH) using an electroblot apparatus (Hoefer, San Francisco, CA). The transfer buffer consisted of 0.1 mol/L Tris-HCL, pH 8.8, 0.192 mol/L glycine, and 10% methanol.
After transfer of the proteins, the nitrocellulose membranes were blocked for 1 hour in TBS-T (Tris-buffered saline-0.05% Tween; JT Baker, Philipsburg, NJ) containing 5% skim milk for 1 hour. The membranes were incubated with either 1 μg/mL of monoclonal mouse antihuman bcl-2 antibody clone 124 (Dako, Carpenteria, CA), 2 μg/mL polyclonal rabbit antihuman bax (Santa Cruz Biotechnology, Santa Cruz, CA), or 1 μg/mL polyclonal rabbit antihuman p27 (Oncogene Research Products, Cambridge, MA) diluted in TBS-T with 5% skim milk for 24 hours at 4°C or for 1 hour at room temperature with constant agitation. After washing 4 times with TBS-T, the blots were incubated with horseradish peroxidase (HRP)-conjugated antimouse IgG diluted 1:2,000 (H and L chains; Pierce) or HRP-conjugated antirabbit IgG (Caltag Laboratories, Burlingame, CA) diluted 1:2,000 with 5% skim milk in PBS for 1 hour at room temperature with constant agitation. The blots were washed 4 times with TBS-Tween and shown with chemiluminescent substrate (Pierce Super-Signal chemiluminescent; Pierce) for 1.5 minutes. Autoradiography was performed with x-ray film (Eastman Kodak, Rochester, NY). Gel loading equivalence was confirmed either by reprobing with 1 μg/mL polyclonal goat antihuman Actin (I-19; Santa Cruz Biotechnology) followed by HRP antigoat IgG diluted 1:5,000 or by exposing the nitrocellulose gel to Fast Green Stain to show the total protein banding pattern. To determine the quantitative reproducibility of measuring specific protein expression by Western blots, we loaded varying concentrations (0.1 ηg to 1 μg) of recombinant p27 protein (Santa Cruz Biotechnology) as described above. Over this range of p27 protein concentrations, we were able to demonstrate a dose-dependent change in signal with increasing concentrations. Protein bands were quantified by computer densitometry.
Statistics
Groups of data were compared using paired or nonpaired Student’st-tests (2-sided) as appropriate. Nonparametric data were analyzed using the Wilcoxan signed-rank test. JMP Statistics software (SAS Institute, Trumbull, CT) or Quatropro software (Novell Inc, Orem, UT) were used to perform these analyses.
RESULTS
Depsipeptide Produces Cytotoxicity in Human CLL Cells
Peripheral mononuclear cells from 10 consecutive patients with CLL were exposed to varying (0.0001, 0.001, 0.0033, 0.01, .033, 0.1, 1, 3.3, 10, and 33 μmol/L) concentrations of depsipeptide. Cells were incubated as follows: 4 hours and then developed; 4 hours and then washed of drug and developed after in vitro incubation in fresh media for a total of 4 days; 24 hours and then developed; 24 hours, washed of drug, and developed after in vitro incubation in fresh media for 4 days; and developed at 4 days. The viability of CLL cells at each concentration and time point are depicted in Table 2. All of the patients with CLL demonstrated in vitro response to depsipeptide. The average concentration of depsipeptide required to produce 50% cytotoxicity (LC50) after 4 hours of agent exposure followed by incubation in fresh media until 4 days was 0.038 μmol/L (range, 0.003 to 0.01 μmol/L; 95% confidence interval [CI], ±0.046 μmol/L). In contrast, the 24- and 96-hour exposure to depsipeptide had an LC50 of 0.024 μmol/L (range, 0.0003 to 0.02 μmol/L; 95% CI, ±0.032 μmol/L) and 0.015 μmol/L (range, 0.0006 to 0.06 μmol/L; 95% CI, ±0.0123 μmol/L), respectively.
Depsipeptide Is Selectively Cytotoxic Toward CLL as Compared With Normal Mononuclear Cells
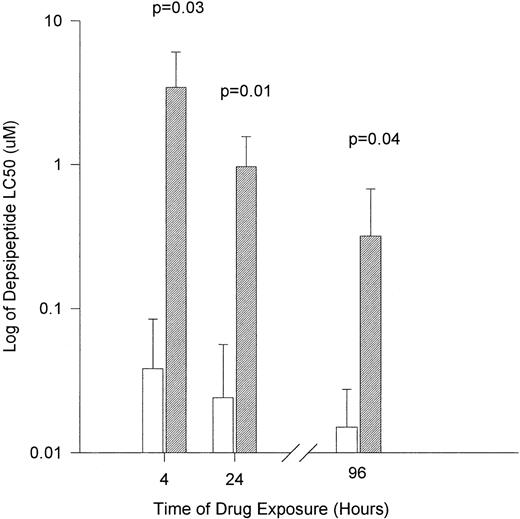
Peripheral mononuclear cells from 4 healthy volunteers were exposed to varying concentrations of depsipeptide. Cells were incubated in an identical fashion to that of the CLL cells described above. The viability of normal mononuclear cells at each concentration and time point are depicted in Table 3. The LC50 for normal mononuclear cells after 4 hours of agent exposure followed by incubation in fresh media until 4 days was 3.44 μmol/L (range, 0.5 to 6.99 μmol/L; 95% CI, ±2.61 μmol/L). In contrast, the 24- and 96-hour exposure to depsipeptide had a LC50 of 0.965 μmol/L (range, 0.5 to 1.85 μmol/L; 95% CI, ±0.589 μmol/L) and 0.3175 μmol/L (range, 0.01 to 0.77 μmol/L; 95% CI, ±0.356 μmol/L), respectively. In comparison to the CLL cells, as shown in Fig 1, depsipeptide was significantly less toxic to normal mononuclear cells at 4 hours (P = .03), 24 hours (P = .01), and 96 hours (P = .04).
The in vitro cytotoxicity achieved after exposure of CLL cells to flavopiridol for different times. (□) Human CLL cells (n = 10 patients); (▨) normal mononuclear cells (n = 4 patients). Cytotoxicity is expressed as the log of LC50 (drug concentration required to reduce viability by 50%).
The in vitro cytotoxicity achieved after exposure of CLL cells to flavopiridol for different times. (□) Human CLL cells (n = 10 patients); (▨) normal mononuclear cells (n = 4 patients). Cytotoxicity is expressed as the log of LC50 (drug concentration required to reduce viability by 50%).
Concentrations of Depsipeptide That Are Cytotoxic to CLL Cells Do Not Inhibit Growth of Normal Bone Marrow Progenitor Cells
Because patients with CLL often have significant cytopenias, identifying an agent that is selectively cytotoxic to tumor cells but not suppressive to normal hematopoiesis is most attractive. Therefore, we assessed if depsipeptide was suppressive to the growth of bone marrow colony-forming units using a short-term drug exposure that our previous in vitro drugging studies supported as optimal for clinical study. Bone marrow mononuclear isolates from 3 healthy volunteers were exposed for 4 hours to varying concentrations of depsipeptide, ranging from 0.0015 to 3.44 μmol/L. Table 4demonstrates the effect of depsipeptide at these different concentrations. It is notable that, even at the highest concentration of depsipeptide examined, greater than 50% suppression of CFU-GM was not observed. For BFU-E (IC50, 2.84 μmol/L) and CFU-GEMM (IC50, 2.37 μmol/L), similar, but slightly less pronounced selective suppression was observed. These data further support a selective cytotoxic advantage of depsipeptide against CLL cells as compared with normal marrow hematopoietic precursor cells.
Depsipeptide Induces Apoptosis in CLL Cells
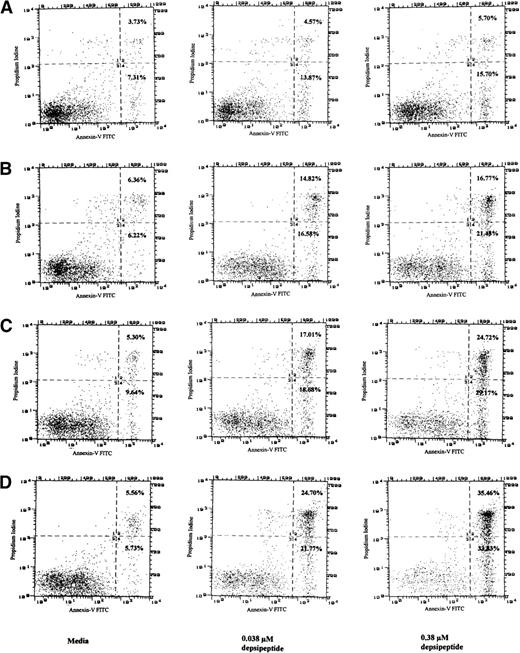
In an attempt to determine if the cytotoxicity induced by depsipeptide was due to an increase in apoptosis, mononuclear cells from CLL patients (n = 4) were incubated in medium, 0.15, 0.038, and 0.015 μmol/L of depsipeptide, and then incubated in media for 4 days. These studies demonstrated apoptosis after 4 days of incubation with both annexin-V/PI and the TdT assay (data not shown). To ascertain if the apoptosis was a time and depsipeptide concentration dependent process, CLL cells from a fludarabine-refractory patient were incubated with depsipeptide (0.038 or 0.38 μmol/L) or media for a period of 4 hours. The annexin-V/PI assay was performed immediately or after this for a proportion of the cells, whereas others were incubated in media for 24, 48, 72, and 96 hours before assessing apoptosis. No apoptosis was observed immediately after 4 hours of incubation, but Fig 2 demonstrates that depsipeptide causes time- and concentration-dependent apoptosis in CLL cells. Indeed, Fig 2demonstrates for each of the depsipeptide incubations a definitive population of cells with the altered annexin-V phospholipid observed with apoptosis, but lacking PI staining (quadrant 4). The remaining majority of cells in quadrant 2 have altered annexin-V phospholipid exposure and retention of PI, a finding seen late in apoptosis with loss of cellular membrane integrity. These data support the conclusion that depsipeptide is inducing cytotoxicity at least in part through the pathway of apoptosis.
Detection of apoptosis in CLL cells as detected by annexin-V/PI assay after exposure to media or depsipeptide (0.038 or 0.38 μmol/L) for a total of 4 hours, washing, and then incubation in media for 24 (A), 48 (B), 72 (C), and 96 hours (D). No apoptosis was noted with depsipeptide immediately after 4 hours of exposure (not shown). This demonstrates that, as the concentration of depsipeptide is increased, a time- and dose-dependent induction of apoptosis occurs. Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of PI indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 4 of (A), (B), (C), and (D) (percentages noted in far right corner) exposed to depsipeptide as compared with media supports that depsipeptide is inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.
Detection of apoptosis in CLL cells as detected by annexin-V/PI assay after exposure to media or depsipeptide (0.038 or 0.38 μmol/L) for a total of 4 hours, washing, and then incubation in media for 24 (A), 48 (B), 72 (C), and 96 hours (D). No apoptosis was noted with depsipeptide immediately after 4 hours of exposure (not shown). This demonstrates that, as the concentration of depsipeptide is increased, a time- and dose-dependent induction of apoptosis occurs. Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of PI indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 4 of (A), (B), (C), and (D) (percentages noted in far right corner) exposed to depsipeptide as compared with media supports that depsipeptide is inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.
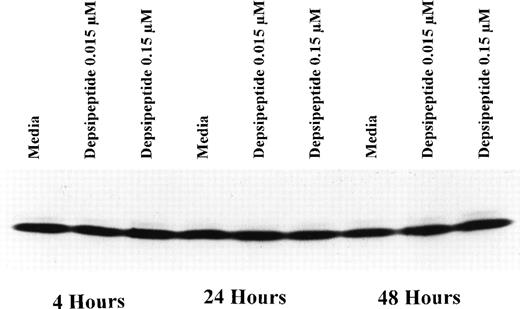
Depsipeptide Cytotoxicity Correlates With Increased Bax, But No Change in Bcl-2 Expression
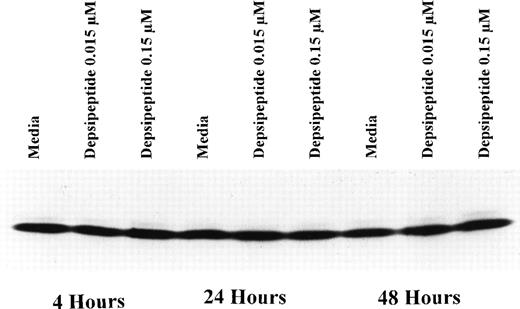
To determine if depsipeptide-induced apoptosis was occuring concurrent with favorable alteration in the antiapoptotic protein bcl-2 or alteration of the bcl-2:bax ratio, we incubated mononuclear cells from CLL patients with depsipeptide (0.015 and 0.15 μmol/L) or media with subsequent assessment of bcl-2 protein (n = 3) expression at 4, 24, and 48 hours. Figure 3 demonstrates no change in bcl-2 protein expression with depsipeptide exposure as compared with media. Contrasting with this, when CLL cells were exposed to depsipeptide, bax protein expression was increased when compared with media-matched control, as demonstrated in Fig 4. This increase in bax protein was dose- and time-dependent, occuring most prominently at the highest concentration and 2-day time point (Fig 4A). A representative Fast Green stain for bax is shown in Fig 4B, demonstrating equivalent lane protein loading.
Expression of bcl-2 protein in human CLL cells at 4, 24, and 48 hours after incubation with medium or 0.15 or 0.015 μmol/L of depsipeptide. The cells were obtained from CLL patients after informed consent was obtained, were isolated, and were cultured at 5 × 106/mL in medium or depsipeptide. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Two micrograms of protein per lane from the CLL cell lysates was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and electrophoresed. Bcl-2 protein was detected using an anti–bcl-2 monoclonal antibody (Dako). Lane equivalent loading was verified by assessment with Fast Green staining (not shown).
Expression of bcl-2 protein in human CLL cells at 4, 24, and 48 hours after incubation with medium or 0.15 or 0.015 μmol/L of depsipeptide. The cells were obtained from CLL patients after informed consent was obtained, were isolated, and were cultured at 5 × 106/mL in medium or depsipeptide. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Two micrograms of protein per lane from the CLL cell lysates was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and electrophoresed. Bcl-2 protein was detected using an anti–bcl-2 monoclonal antibody (Dako). Lane equivalent loading was verified by assessment with Fast Green staining (not shown).
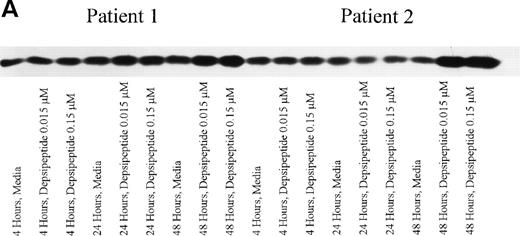
(A) Expression of bax protein in human CLL cells at 4, 24, and 48 hours after incubation with medium or 0.15 or 0.015 μmol/L of depsipeptide. The cells were obtained from CLL patients after informed consent was obtained, were isolated, and were cultured at 5 × 106/mL in medium or depsipeptide. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Two micrograms of protein per lane from the CLL cell lysates was loaded onto a 10% SDS-PAGE gel and electrophoresed. Bcl-2 protein was detected using an anti–bcl-2 monoclonal antibody (Dako). Lane equivalent loading was verified by assessment with Fast Green staining (not shown). (B) Expression of total protein on nitrocellulose membrane for gel (A) after staining with Fast Green. This demonstrates equivalent lane loading for each of the time points outlined in (A).
(A) Expression of bax protein in human CLL cells at 4, 24, and 48 hours after incubation with medium or 0.15 or 0.015 μmol/L of depsipeptide. The cells were obtained from CLL patients after informed consent was obtained, were isolated, and were cultured at 5 × 106/mL in medium or depsipeptide. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Two micrograms of protein per lane from the CLL cell lysates was loaded onto a 10% SDS-PAGE gel and electrophoresed. Bcl-2 protein was detected using an anti–bcl-2 monoclonal antibody (Dako). Lane equivalent loading was verified by assessment with Fast Green staining (not shown). (B) Expression of total protein on nitrocellulose membrane for gel (A) after staining with Fast Green. This demonstrates equivalent lane loading for each of the time points outlined in (A).
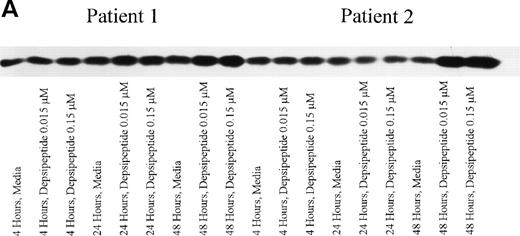
Depsipeptide Causes p27 Protein Expression to Decrease
If p27 expression is increased in patients with aggressive CLL and associated with drug resistance, a decrease in this protein might increase the apoptotic threshold of these cells. Mononuclear cells from 3 CLL patients were incubated in medium or depsipeptide (0.15 or 0.015 μmol/L). After a 4-hour exposure to this agent, both a high rate of cytotoxicity and apoptosis were noted at 4 days. Concurrent with this was the notable marked decrease in p27 protein expression at 24 and 48 hours, as shown in Fig 5. This decrease in p27 protein was both dose- and time-dependent. We have previously shown that a decrease in p27 protein expression is not observed in CLL cells undergoing apoptosis induced by flavopiridol.17 Therefore, this finding may be a drug-specific and not a nonspecific phenomenon associated with apoptosis.
Expression of p27 protein in human CLL cells at 4, 24, and 48 hours after incubation with medium or 0.15 and 0.015 μmol/L of depsipeptide. The cells were obtained from CLL patients after informed consent was obtained, were isolated, and were cultured at 5 × 106/mL in medium or depsipeptide. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Five micrograms of protein per lane from the CLL cell lysates was loaded on a 10% SDS-PAGE gel and electrophoresed. The p27 protein was detected using an anti-p27 polyclonal antibody (Oncogene). Lane equivalent loading was verified by assessment with Fast Green staining (not shown).
Expression of p27 protein in human CLL cells at 4, 24, and 48 hours after incubation with medium or 0.15 and 0.015 μmol/L of depsipeptide. The cells were obtained from CLL patients after informed consent was obtained, were isolated, and were cultured at 5 × 106/mL in medium or depsipeptide. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Five micrograms of protein per lane from the CLL cell lysates was loaded on a 10% SDS-PAGE gel and electrophoresed. The p27 protein was detected using an anti-p27 polyclonal antibody (Oncogene). Lane equivalent loading was verified by assessment with Fast Green staining (not shown).
DISCUSSION
This report represents the first preclinical evaluation of the novel antitumor bicyclic depsipeptide in CLL cells. Our data derived from human tumor samples demonstrate that depsipeptide has marked preclinical activity against CLL cells, requiring only a 4-hour exposure time to induce apoptosis. With a 4-hour exposure to depsipeptide, the cytotoxicity that is observed in CLL cells occurs at markedly lower concentrations (ie, 2 logs less) than in normal blood mononuclear cells or normal bone marrow stem cells. Depsipeptide exposure also induces a variety of changes in proapoptotic or antiapoptotic proteins, including decreasing the bcl-2:bax ratio and p27 expression. With these changes, apoptosis was noted as documented by both the TdT assay and annexin-V assay. Taken together, these findings strongly support the early introduction of depsipeptide in clinical trials involving CLL patients.
Fludarabine is widely used in the treatment of CLL and produces significant cellular immune dysfunction at concentrations that are effective for eradicating the underlying leukemia. This lack of selective cytotoxicity explains in part why opportunistic infections have been observed in advanced stage CLL patients treated with fludarabine.18-20 Furthermore, this cellular immune suppression induced by fludarabine places limitation on immune based therapies such as those with monoclonal antibodies requiring antibody-dependent cellular cytotoxicity or vaccine-based therapy. Reason for excitement about depsipeptide relates to the marked selectivity observed between CLL as compared with normal mononuclear cells. Contrasting with what we have previously described with F-ara-a,21 our data demonstrate that depsipeptide induces cytotoxicity and apoptosis at a concentration much lower than that which produces cytotoxicity in normal mononuclear cells. Additionally, depsipeptide also fails to suppress colony-forming growth after short exposure to drug concentrations that are cytotoxic to CLL cells. These data combined suggest that depsipeptide might have minimal immunosuppressive and myelosuppressive effects at drug concentrations that effectively induce apoptosis in CLL cells. This observation has similarly been noted with depsipeptide in normal human fibroblast and endothelial cell lines as compared with several solid tumor cell lines.22 Combination of depsipeptide with fludarabine or alkylator therapy might be possible without markedly accentuating toxicity. In addition, if depsipeptide is shown to have in vivo activity in CLL, its selective sparing of normal mononuclear cells might make it an ideal agent to combine with immune-based therapies.
Depsipeptide was identified by Ueda et al22,23 through screening soil samples for new antitumor activity against tumor cells overexpressing myc. Chromobacterium violaceum no. 968 was noted to produce the bicyclic depsipeptide that blocks p21 protein signal transduction. The mechanism by which depsipeptide produces cytotoxicity in CLL cells or other tumor systems is currently unknown. Initial preliminary investigations of depsipeptide in several solid tumor and 1 leukemia cell line have suggested that it induces G0-1arrest.22 Such cell cycle arrest possibly occurs through inhibition of the ras signal transduction pathway with reversion of ras-transformed tumor cells to normal morphology cells and concurrent downregulation of c-myc mRNA.24 Additionally, both in vitro and in vivo studies in both solid tumor and leukemia cell lines have demonstrated increased cytotoxicity in chemotherapy-resistant cell lines as compared with similar nonresistant cell lines. Furthermore, depsipeptide has a broad range of activity as compared with other standard chemotherapeutic agents,22,25 suggesting a novel, yet unknown mechanism of action. One recent report26suggests this agent might act via inhibition of the enzyme DNA histone deacetylase. Based on the selectivity of depsipeptide in CLL cells, study of this enzyme’s activity and relation to chemosensitivity seem to be indicated. Furthermore, our analysis of changes in apoptosis-related proteins after depsipeptide exposure demonstrates that this agent causes no change in bcl-2 protein but decreases p27 protein and increases bax protein in a dose-dependent fashion. At the present time, it is uncertain if these alterations occur in vivo in a dose-dependent fashion. Our data provide in vitro evidence to justify investigation of these parameters in the setting of a phase I trial. If such alterations are noted in vivo, treatment with depsipeptide may represent a novel therapeutic option for those CLL patients possessing high p27 or bcl-2:bax protein ratios in their leukemia cells.
In summary, our study demonstrates that depsipeptide has a marked therapeutic index in CLL cells as compared with normal mononuclear cells or bone marrow progenitor cells. Furthermore, depsipeptide appears to favorably alter proteins associated with drug resistance in CLL. In relation to the translatable component of these findings to the clinic, our data demonstrate that a short (4 hours) exposure time to depsipeptide is effective for inducing apoptosis in vitro in human CLL cells. These data provide support for using a 4-hour schedule for phase I-II studies with depsipeptide in CLL. Such a short exposure has proven to be the best tolerated schedule investigated in preclinical toxicology studies with depsipeptide and is currently being used in phase I trials. Based on these data, inclusion of CLL patients on these phase I studies appears to be warranted.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John C. Byrd, MD, Hematology-Oncology Service, Ward 78, Walter Reed Army Medical Center, Washington, DC 20307.