Abstract
Antibodies against CD3ɛ are widely used as immunosuppressive agents. Although it is generally assumed that these reagents exert their immunomodulatory properties by inducing T-cell deletion and/or inactivation, their precise mechanism of action remains to be elucidated. Using a murine model, we demonstrate in this report that administration of anti-CD3ɛ antibodies causes the migration and maturation of dendritic cells (DC) in vivo, as determined by immunohistochemical analysis. This maturation/migration process was followed by selective loss of splenic DC, which resulted in a selective inhibition of antigen-presenting cell (APC) functions in vitro. Spleen cells from anti-CD3ɛ–treated animals were unable to productively stimulate naive alloreactive T cells and Th1-like clones in response to antigen, while retaining the ability to present antigen to a T-cell hybridoma and Th2 clones. Anti-CD3ɛ treatment was found to induce a selective deficiency in the ability of spleen cells to produce bioactive interleukin-12 in response to CD40 stimulation. APC dysfunction was not observed when nonmitogenic forms of anti-CD3ɛ antibodies were used, suggesting that splenic DC loss was a consequence of in vivo T-cell activation. Nonmitogenic anti-CD3ɛ monoclonal antibodies were found to be less immunosuppressive in vivo, raising the possibility that APC dysfunction contributes to anti-CD3ɛ–induced immunomodulation. Collectively, these data suggest a novel mechanism by which mitogenic anti-CD3ɛ antibodies downregulate immune responses.
THE INITIATION OF an adaptive immune response to foreign antigen (Ag) is dependent on the interaction between Ag-specific T lymphocytes and appropriate Ag-presenting cells (APC). Dendritic cells (DC) have been recognized as the major APC population able to optimally activate naive T lymphocytes both in vitro and in vivo. This priming property is related to their (1) ability to take up, process, and present Ags; (2) high expression of adhesive and costimulatory molecules able to interact with T-cell borne counterreceptors; and (3) ability to migrate from peripheral tissues to T-cell areas in lymphoid organs (for review, see Hart1 and Bancherau and Steinman2). Recent studies have demonstrated that these functional features were differentially expressed according to tissue location and maturation state. Immature DC are mostly found in peripheral tissues, where they capture and process Ags while displaying poor T-cell stimulatory properties. Conversely, mature DC in lymphoid organs have reduced Ag-presenting capacities, but are potent stimulators of naive T cells, a property related to their higher expression of costimulatory and adhesive molecules. This developmental sequence, originally demonstrated to occur spontaneously in vitro,3,4 has been recently studied in vivo.5 Collectively, these studies have led to the concept that immature DC in peripheral organs capture Ag locally and then migrate via afferent lymphatics to draining lymph nodes, where they acquire the capacity to activate Ag-specific naive T cells.
Studies related to transplantation have provided many insights into DC biology and function in vivo. Major histocompatibility complex (MHC) class II+ cells with DC morphology can be found in virtually all organs.6 Tissue DC migrate out of transplanted tissues into the circulation and reach lymphoid organs such as the spleen.7,8 It is noteworthy that depletion of donor DC has been shown to correlate with increased kidney and heart allograft survival.9 10 Taken together, these observations suggest that migration and maturation of DC represent critical steps for the initiation of an immune response to a transplanted organ.
Recent observations have demonstrated that, in addition to Ag-specific stimuli such as allogeneic grafts or contact allergens,11 a variety of factors, including microbial products (lipopolysaccharide [LPS]) or inflammatory mediators (tumor necrosis factor-α [TNF-α] and, to a lesser extent, interleukin-1 [IL-1]) can induce DC migration in vivo. Injection of LPS causes a massive migration of DC from skin, heart, kidney, and intestine, as well as maturation and movement of spleen DC from the marginal zone into the T-cell area.5 12
The observation that inflammatory stimuli mobilize DC from tissues led us to investigate the effect of in vivo administration of anti-CD3ε monoclonal antibodies (MoAbs) on APC distribution and function .13,14 Anti-CD3ε antibodies have been successfully used to prevent or treat allograft rejections. However, before exerting their immunosuppressive properties, mitogenic anti-CD3ε antibodies trigger the in vivo release of multiple cytokines, including IL-2, IL-4, interferon-γ (IFN-γ), and TNF-α.15-17 In this study, we demonstrate that administration of anti-CD3ε MoAbs induce profound changes in the APC population in vivo, suggesting a novel mechanism by which these antibodies may downregulate immune responses.
MATERIALS AND METHODS
Animals, Cell Lines, and Reagents
Six- to 8-week-old female BALB/c (H-2d), CBA (H-2k) CB17, and CB17 SCID mice were purchased from Charles River Wiga (Sulzfeld, Germany). Six- to 8-week-old A/J and B10.A mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained in pathogen-free conditions in our animal facility.
The OVA-specific T-cell hybridoma DO.11.1018 was kindly provided by P. Marrack (Howard Hughes Medical Institute, Denver, CO). The A.E7 Th1 clone, specific for pigeon cytochrome C (PCC)3+ Iek19 was a kind gift of R.H. Schwartz (National Institutes of Health, Bethesda, MD). The HG-4 Th2 clone was generated from the draining nodes of A/J (H-2a) mice primed with 300 μg human gamma globulins (HGG) in complete Freund adjuvant (CFA). The ovalbumine peptide (323-339)-specific Th clones were derived as described.20Briefly, spleen cells (5 × 106) from naive DO11.10 mice (kindly provided by Anne O'Garra, DNAX, Palo Alto, CA) were cultured in 24-well plates with 0.5 μg/mL of OVA(323-339) in the presence of recombinant IL-12 (rIL-12) and 10 μg/mL anti–IL-4 (clone 11B11; ATCC, Rockville, MD) to promote the development of Th1-like cells or 200 U/mL IL-4 and 100 ng/mL anti–IL-12 (clone C17.8; kindly provided by G. Trinchieri, Wistar Institute, PA21) to favor the differentiation toward a Th2 phenotype. T-cell clones (106/well) were restimulated biweekly in a 24-well plate with 107 irradiated syngeneic splenocytes/well and the relevant Ag. At 48 hours, the cells were expanded 5-fold into medium containing 10 U/mL rIL-2 and rested for at least 7 days. The clones used in this study were referred to as Th1 or Th2 based on the cytokine profile (IFN-γ or IL-4/IL-5/IL-10) secreted in response to Ag stimulation.
The antibodies 145-2C11 (hamster IgG, anti-CD3ε; ATCC) and PARSI-19 (hamster IgG, anti–p-azophenylarsonate; generated in our laboratory) were purified from ascitic fluids. The mγ3-2C11 MoAb22 is a chimeric anti-CD3ε antibody comprising the variable region of the 145-2C11 clone and the heavy and light constant regions of murine γ3 and k origin.
In Vivo Treatment
BALB/c mice were injected intravenously (IV) into the lateral tail vein with anti-CD3ε antibodies solubilized in pyrogen-free NaCl (0.9%). Control animals were injected with the same volume of diluent or with hamster control IgG.
In Vitro Responses
The complete medium used in all experiments was RPMI 1640 (Seromed Biochem KG, Berlin, Germany) supplemented with 2% HY ULTROSER (a serum-free media purchased from GIBCO BRL, Merelbeke, Belgium), penicillin, streptomycin, nonessential aminoacids, sodium pyruvate, 2-mercaptoethanol (2-ME), and L-glutamine (Flow ICN Biomedicals, Bucks, UK). Sephadex G10-depleted responder cells and T-cell hybridomas were stimulated in vitro as described in the figure legends. Supernatants were collected after 24 or 48 hours of culture, frozen, and assayed for IL-2 content by a bioassay using a subclone of the CTL.L cell line insensitive to murine IL-4. T-cell clones (0.3 to 1.0 × 105/well) were incubated in the presence of irradiated spleen cells (3.0 to 6.0 × 105/well) and nominal Ag [0.3 μmol/L of PCC, 1 mg of HGG, or 0.2 μg/mL of OVA (323-339)] in 96-well plates, and cell proliferation was determined by 3H-thymidine incorporation.
IL-12 determinations were performed using a bioassay based on the ability of IL-12 to induce IFN-γ secretion. Briefly, spleen cells isolated from control or anti-CD3–treated animals were incubated with graded doses of an activating anti-CD40 MoAb (clone 3/23; kindly provided by G. Klaus, National Institute for Medical Research, London, UK23). Supernatants were collected after 48 hours, and the amount of IFN-γ was determined by enzyme-linked immunosorbent assay (ELISA) as previously described. Standard curves were generated by incubating both control and anti-CD3–treated cell populations with recombinant murine IL-12 (kindly provided by S. Wolf, Genetics Institute, Cambridge, MA). The addition of neutralizing anti–IL-12 MoAbs (clone C17.8) was found to completely inhibit anti-CD40–induced IFN-γ secretion, thus establishing the specificity of the assay for IL-12. Control rat IgG2a antibodies (clone IR418) were kindly provided by H. Bazin (Université Catholique de Louvain, Brussels, Belgium).
Purification of Low-Density Spleen Cells
Spleens were digested with collagenase (CLSIII; Worthington Biochemical Corp, Freehold, NJ) and separated into low- and high-density fractions on a BSA gradient (Bovuminar Cohn fraction V powder; Armour Pharmaceutical Co, Tarrytown, NY), as previously described.24
Cytofluorometric Analysis
Cells were analyzed by flow cytometry with a FACScan cytometer (Becton Dickinson, Mountain View, CA). The cells were preincubated with 2.4G2 (a rat antimouse Fc receptor MoAb; ATCC) for 10 minutes before staining to prevent antibody binding to FcR and further labeled with phycoerythrin (PE)-coupled B220 (Pharmingen, San Diego, CA). The antibodies 145-2C11, 14.4.4-S (anti-I-Ek,d; ATCC), and N418 (anti-CD11c; ATCC) were purified from ascitic fluids and coupled to fluorescein isothiocyanate (FITC) or biotin in our laboratory. Cells were gated according to size and scatter to eliminate dead cells and debris from analysis.
Immunohistochemistry
Cryosections.
Tissue samples were frozen in isopentane (−80°C) and 8-μm cryostat sections were prepared. Samples were fixed in acetone for 10 minutes, air-dried, treated with 3% H2O2 in phosphate-buffered saline (PBS) for 30 minutes to block endogenous peroxydase, and incubated in PBS containing 0.5% of blocking reagent (Roche Diagnostics, Belgium, Brussels) for 30 minutes.
Immunohistowax process.
Spleens were fixed for 3 days in Immunohistofix (Sanver TECH, Boechout, Belgium) followed by dehydratation in acetone for 6 hours. Tissues were embedded in Immunohistowax (Sanver TECH), sectioned at 3 to 6 μm, deembedded by washing in acetone for 10 minutes, and transferred to PBS.
Immunostaining.
Sections were incubated for 20 minutes with the monoclonal rat antimouse antibody 2.4G2 to Fc receptors to prevent nonspecific staining. Slides were washed in PBS, incubated for 1 hour at room temperature with biotinylated MoAbs (10 μg/mL) in 0.5% PBS-BR, and washed in PBS: M1/70 (anti-CD11b/Mac-1; ATCC), N418 (anti-CD11c), RM4-5 (anti-mouse-CD4; Pharmingen), NLDC-145 (anti–DEC-205; ATCC), GL1 (anti-CD86; ATCC), and LO-MD6 (a kind gift of H. Bazin). Slides were then incubated in avidin-biotin-peroxidase complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) and stained with a solution of diaminobenzidine tetrahydrochloride with metal enhancer (DAB tablets, SigmaFAST; Sigma, St Louis, MO) or incubated in avidin-biotin-alkalin phosphatase complex (Vectastain ABC kit, AK-5000; Vector Laboratories) and stained with alkaline phosphatase substrate kit I (SK-5100, red; Vector Laboratories) or kit III (SK-5300, blue; Vector Laboratories). The sections were counterstained with methyl green, dehydrated, and mounted in Poly-Mount (Polysciences, Warrington, PA).
Digitized images were captured using a Ikegami CCD color camera (Ikegami Tsushinki, Tokyo, Japan) and analyzed using CorelDraw 7 software (Corel, Ottawa, Ontario, Canada).
Skin Grafting
BALB/c mice were grafted with tail skin from A/J (class I and class II MHC mismatch) as follows. Graft beds were created by excising approximately 0.5 cm2 of skin on the right thoracic wall and skin grafts were covered by a double layer of Vaseline gauze and plaster bandage, which were removed on day 8 after skin grafting. Grafts were considered rejected when no more viable tissue was visible.
RESULTS
Anti-CD3ε Administration Induces Dendritic Cell Movement and Maturation
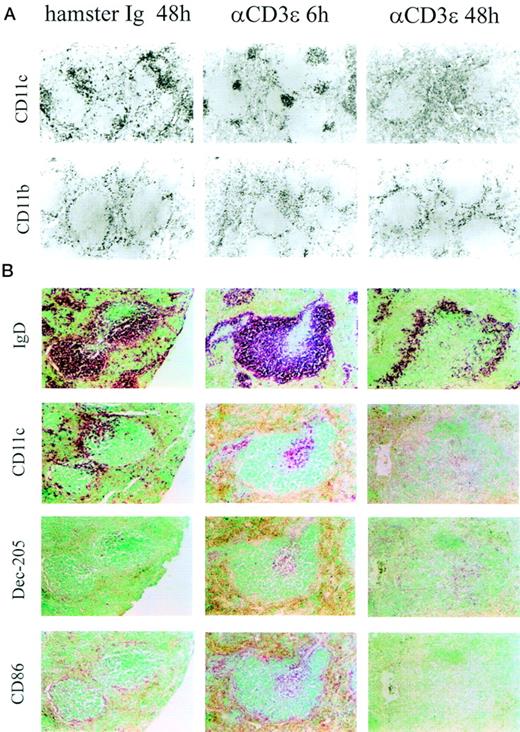
Anti-CD3ε MoAbs (50 μg) were injected IV into naive adult mice and APC populations visualized in serial sections using specific antibodies (Fig 1A). As expected,5CD11c+ DC and macrophage-like, CD11b+ cells were found in the marginal zone, between the red and white pulp in naive or saline-treated animals (Fig 1A, left panels). Injection of anti-CD3ε MoAbs led to the rapid (6 hours posttreatment) redistribution of some CD11b- and most CD11c-expressing cells to the white pulp in the T-cell area surrounding the central arteriole (Fig1A, central panels). Forty-eight hours after anti-CD3ε administration, the overall number of splenic DC was strongly reduced both in the marginal zone and in the T-cell area, whereas the number and location of CD11b-expressing cells was found unaltered compared with control spleens (Fig 1A, right panels). Few cells in the marginal zone were labeled with antibodies to the costimulatory molecule CD86 before treatment, whereas the expression of the DEC-205 marker was barely detectable (Fig 1B, left panels). Anti-CD3ε administration led to a redistribution of DC markers in the white pulp in the area surrounding the central arteriole (where most CD4+ T cells are located, data not shown). However, note that cells expressing CD11c and CD86 could still be found in the marginal zone 6 hours posttreatment (Fig 1B, central panels). Concomitantly, anti-CD3ε injection led to the increased expression of CD86 and DEC-205, 2 markers known to be upregulated during DC maturation2 (Fig1B, central panels). These observations strongly suggest that anti-CD3ε MoAbs induced the maturation and migration of DC from the marginal zone to the T-cell area. As previously noted, a strong reduction in the expression of all DC markers was observed in the spleen 48 hours after anti-CD3ε administration (Fig 1, right panels). The distribution of IgD+ cells did not change in a significant manner during the first 24 hours after treatment (Fig 1 and our own unpublished observations). However, note that the distribution of B cells was affected at 48 hours because of the increased size of the T-cell area after anti-CD3ε administration (Fig1).
Anti-CD3ɛ administration induces DC movement and maturation. (A) Immunoperoxidase staining of cryostat sections or (B) alkaline phosphatase staining of embedded serial sections of spleens from control (hamster Ig) or anti-CD3ɛ injected mice (50 μg IV), 6 and 48 hours posttreatment. Sections were stained with antibodies to CD11b, CD11c, IgD, Dec-205, and CD86 as indicated in the figure.
Anti-CD3ɛ administration induces DC movement and maturation. (A) Immunoperoxidase staining of cryostat sections or (B) alkaline phosphatase staining of embedded serial sections of spleens from control (hamster Ig) or anti-CD3ɛ injected mice (50 μg IV), 6 and 48 hours posttreatment. Sections were stained with antibodies to CD11b, CD11c, IgD, Dec-205, and CD86 as indicated in the figure.
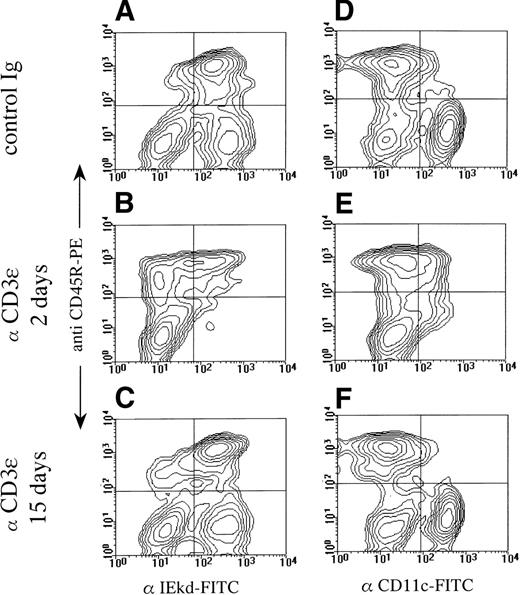
The selective loss of splenic DC induced by anti-CD3ε at 48 hours was further confirmed by flow cytometry (Fig2). DC-enriched, low-density spleen cells isolated from control and treated animals were double-stained with antibodies to a B-cell marker (CD45R), a DC marker (CD11c), and MHC class II molecules (I-Ed). The data confirmed that administration of anti-CD3ε antibodies led to a selective depletion of DC from the low-density splenic population (see the lower right panel in each contour plot) identified as I-Ed+, CD45R−(Fig 2A through C) or CD11c+, CD45R− (Fig2D through F). The effect of anti-CD3ε administration was reversible, because control levels of DC were found in mice tested 15 days after treatment.
Selective reduction of DC number after anti-CD3ɛ administration. BALB/c mice were treated on day 0 with control antibody or anti-CD3ɛ (50 μg IV) and analyzed on days 2 and 15 for I-Ed, CD45R, and CD11c expression. Pooled low-density spleen cells from 3 individuals in each group were analyzed by 2-color immunofluorescence after staining with PE-labeled anti-CD45R and FITC-labeled anti–I-Ed (A through C) or PE-labeled anti-CD45R and FITC-labeled anti-CD11c (D through F).
Selective reduction of DC number after anti-CD3ɛ administration. BALB/c mice were treated on day 0 with control antibody or anti-CD3ɛ (50 μg IV) and analyzed on days 2 and 15 for I-Ed, CD45R, and CD11c expression. Pooled low-density spleen cells from 3 individuals in each group were analyzed by 2-color immunofluorescence after staining with PE-labeled anti-CD45R and FITC-labeled anti–I-Ed (A through C) or PE-labeled anti-CD45R and FITC-labeled anti-CD11c (D through F).
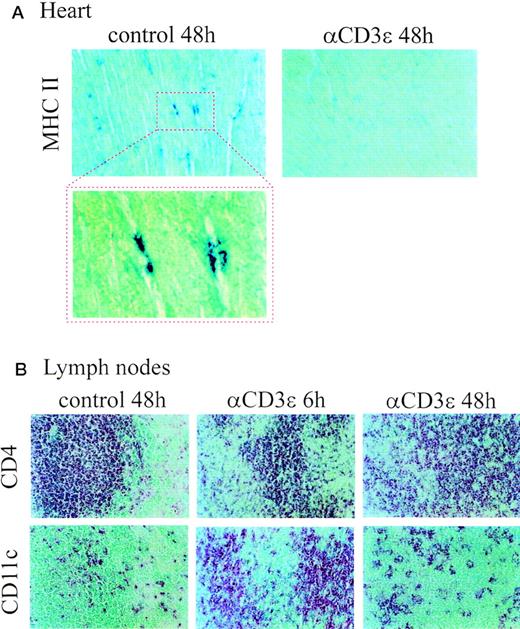
Because DC in nonlymphoid organs often fail to express the CD11c marker, we monitored the presence of MHC class II+ cells in the hearts of control and anti-CD3–treated mice (Fig 3A). Cells expressing MHC class II molecules in control hearts displayed a DC-like morphology, in agreement with a previous finding suggesting that DC represent the major MHC class II+ APC population in this organ.25 Anti-CD3ε administration led to a marked reduction in DC-like numbers in the heart 48 hours posttreatment (Fig3A). When sections of lymph nodes were analyzed, a marked increase in CD11c+ cells was observed in sections from mice injected with anti-CD3ε MoAbs 6 hours earlier (Fig 3B). DC numbers returned to near control levels 48 hours after treatment (Fig 3B). Collectively, these data suggest that anti-CD3ε MoAb-treatment profoundly affects the tissue distribution and phenotype of DC, with different effects depending on timing and tissue location.
Altered DC distribution in peripheral lymphoid and nonlymphoid organs after anti-CD3ɛ administration. Alkaline phosphatase staining of cryostat section of (A) hearts and (B) mesenteric lymph nodes (serial sections) from control and anti-CD3ɛ–injected mice (50 μg IV). Sections were stained with antibodies to MHC class II, CD4 and CD11c, as indicated in the figure. A higher magnification of MHC II+ cells in the heart of control mice is also shown.
Altered DC distribution in peripheral lymphoid and nonlymphoid organs after anti-CD3ɛ administration. Alkaline phosphatase staining of cryostat section of (A) hearts and (B) mesenteric lymph nodes (serial sections) from control and anti-CD3ɛ–injected mice (50 μg IV). Sections were stained with antibodies to MHC class II, CD4 and CD11c, as indicated in the figure. A higher magnification of MHC II+ cells in the heart of control mice is also shown.
Anti-CD3ε Injection Results in Reduced Ag-Presenting Capacities
Because DC represent the major APC population able to activate naive T cells and CD4+ Th1 clones,1 2 we evaluated the Ag-presenting capacity of unselected spleen cell populations from control (normal hamster Ig-treated) and anti-CD3ε–treated animals 48 hours postinjection.
As shown in Fig 4, spleen cells from anti-CD3ε–treated animals failed to activate naive alloreactive T cells to proliferation (Fig 4A) and IL-2 secretion (Fig 4B). A mix of equal numbers of spleen cells from control and antibody-treated mice stimulated T cells at intermediate levels, suggesting that defective accessory functions were not maintained through active suppression (Fig4A and B). By contrast, both APC populations were able to present an agonist peptide to an Ag-specific T-cell hybridoma (Fig 4C), showing that anti-CD3ε–treated spleen cells retained the ability to activate a costimulatory-independent T-cell line.
Defective APC function in anti-CD3ɛ–treated mice. Spleen cells from control antibody-treated or anti-CD3ɛ–treated BALB/c mice (50 μg IV) were irradiated and used as accessory cells to stimulate (A and B) G10-nonadherent responding cells from CBA mice (3 × 105/well) or (C) ovalbumin-specific T-cell hybridoma mice (3 × 105/well) in the presence of 1 μg/mL of ovalbumin peptide. Proliferation (A) and IL-2 production (B and C) were assayed as described in Materials and Methods. Results are expressed as counts per minute (cpm) of [3H] TdR incorporation by responder cells (A) or IL-2–dependent cell line CTL.L (B and C). These results are representative of 3 independent experiments.
Defective APC function in anti-CD3ɛ–treated mice. Spleen cells from control antibody-treated or anti-CD3ɛ–treated BALB/c mice (50 μg IV) were irradiated and used as accessory cells to stimulate (A and B) G10-nonadherent responding cells from CBA mice (3 × 105/well) or (C) ovalbumin-specific T-cell hybridoma mice (3 × 105/well) in the presence of 1 μg/mL of ovalbumin peptide. Proliferation (A) and IL-2 production (B and C) were assayed as described in Materials and Methods. Results are expressed as counts per minute (cpm) of [3H] TdR incorporation by responder cells (A) or IL-2–dependent cell line CTL.L (B and C). These results are representative of 3 independent experiments.
Murine Th1 and Th2 clones display distinct accessory cell requirements. In particular, Th1 clones have been shown to require an adherent cell population to optimally proliferate in response to Ag, whereas Th2 clones can be stimulated by Ag presented by purified B cells.26 We have confirmed these observations in our laboratory and found a good correlation between the T-helper phenotype of murine clones and their APC requirement (as determined on 5 Th1 and 5 Th2 clones, data not shown). To further characterize the effect of anti-CD3ε treatment on splenic APC function, 4 representative murine helper clones (identified as Th1 and Th2 based on their pattern of cytokine secretion) were stimulated by splenic accessory cells from anti-CD3ε–treated and control mice. The Th1 and Th2 helper clones used in this study displayed distinct APC requirements, as shown by the selective capacity of G10-passed splenic accessory cells to optimally activate Th2-type lymphocytes to proliferation. Spleen cells from animals previously injected with anti-CD3ε antibodies failed to optimally stimulate both murine Th1 clones in the presence of nominal Ag, while retaining the ability to efficiently present Ag to Th2 clones (Fig 5).
APC from anti-CD3ɛ–treated mice support the growth of the Th2, but not Th1, Ag-specific clones. Spleen cells from control antibody-treated or anti-CD3ɛ–treated mice (50 μg IV) were irradiated and used as accessory cells to stimulate the Th1 clones AE.7 and CLOVA 1.1 or the Th2 clones HG.4 and CLOVA 2.9 in the presence of nominal Ag. Clones were also stimulated by Ag presented by G10-depleted splenic accessory cells for the sake of comparison. Results are expressed as cpm of [3H] TdR incorporation by responder cells. These results are representative of 3 independent experiments.
APC from anti-CD3ɛ–treated mice support the growth of the Th2, but not Th1, Ag-specific clones. Spleen cells from control antibody-treated or anti-CD3ɛ–treated mice (50 μg IV) were irradiated and used as accessory cells to stimulate the Th1 clones AE.7 and CLOVA 1.1 or the Th2 clones HG.4 and CLOVA 2.9 in the presence of nominal Ag. Clones were also stimulated by Ag presented by G10-depleted splenic accessory cells for the sake of comparison. Results are expressed as cpm of [3H] TdR incorporation by responder cells. These results are representative of 3 independent experiments.
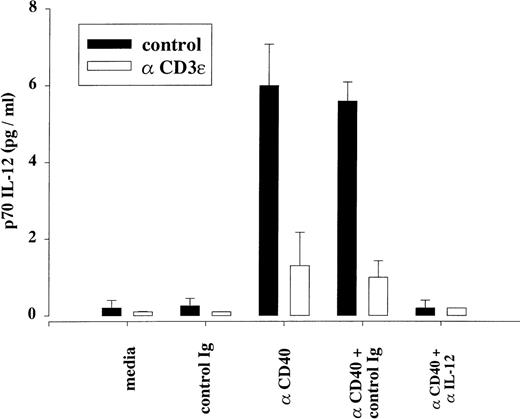
DC represent the major cell population able to secrete IL-12, a cytokine known to favor the differentiation of uncommitted T-cell precursors into Th1-like cells and to sustain the in vitro proliferation of Ag-stimulated Th1 clones.27 28 We therefore tested the ability of unselected spleen cells from control and treated mice to produce IL-12 in response to CD40 signaling, as determined by a sensitive bioassay. As shown in Fig 6, spleen cells from anti-CD3ε–treated mice displayed reduced secretion of bioactive IL-12 molecules when stimulated by activating anti-CD40 antibodies in vitro. The specificity of the assay for IL-12 was demonstrated using a blocking antibody to IL-12. Although we were unable to detect the production of immunoreactive p70 heterodimeric form of IL-12 under these experimental conditions, anti-CD3 treatment was found to inhibit the in vitro production of p40-containing molecules (as assayed by ELISA) in response to anti-CD40 stimulation (data not shown). Collectively, these data suggest that spleen cells from anti-CD3ε–treated mice retain the ability to capture, process, and present protein Ags in association with MHC class II molecules but are unable to provide the accessory signals required for the in vitro stimulation of naive and Th1-type murine T cells.
Downmodulation of anti-CD40–induced IL-12 secretion after anti-CD3ɛ treatment. Unselected spleen cells (106cells/well) from control and anti-CD3ɛ–injected mice (48 hours posttreatment, 50 μg IV) were stimulated in vitro by an activating rat IgG2a anti-CD40 MoAb (3 μg/mL) or an equivalent amount of an isotype-matched, control antibody (clone IR418). When indicated, cultures were supplemented with a neutralizing antibody to the IL-12 subunit p40 (rat IgG2a C17.8, 5 μg/mL) or with an equivalent amount of an isotype-matched, control antibody (clone IR418). Supernatants were harvested 48 hours later and assayed as described in Materials and Methods. The results represent the mean of 3 independent cultures (assayed in duplicate) and are representative of 3 independent experiments. The detection limit of the assay was 0.2 pg/mL.
Downmodulation of anti-CD40–induced IL-12 secretion after anti-CD3ɛ treatment. Unselected spleen cells (106cells/well) from control and anti-CD3ɛ–injected mice (48 hours posttreatment, 50 μg IV) were stimulated in vitro by an activating rat IgG2a anti-CD40 MoAb (3 μg/mL) or an equivalent amount of an isotype-matched, control antibody (clone IR418). When indicated, cultures were supplemented with a neutralizing antibody to the IL-12 subunit p40 (rat IgG2a C17.8, 5 μg/mL) or with an equivalent amount of an isotype-matched, control antibody (clone IR418). Supernatants were harvested 48 hours later and assayed as described in Materials and Methods. The results represent the mean of 3 independent cultures (assayed in duplicate) and are representative of 3 independent experiments. The detection limit of the assay was 0.2 pg/mL.
Only Mitogenic Anti-CD3ε MoAbs Affect Splenic Accessory Cell Functions
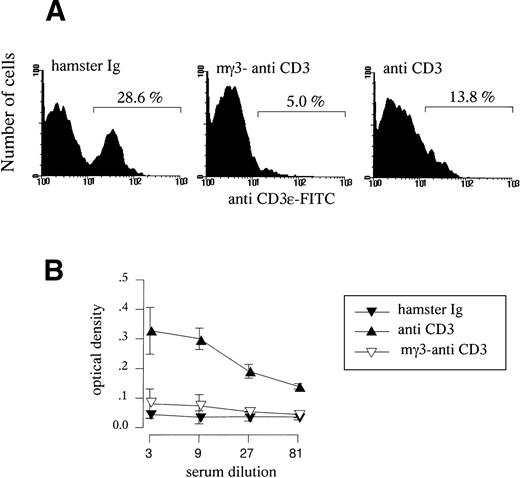
To evaluate the role of T-cell activation in the downregulation of splenic APC functions, mice were injected with a single dose of mitogenic hamster anti-CD3ε antibodies (clone 145-2C11) or chimeric anti-CD3ε antibodies comprising the variable regions of the 145-2C11 clone and the heavy and light constant regions of murine γ3 andk origin. This chimeric molecule displays very low affinity for murine FCRγ, resulting in marked reduction of its mitogenic properties.22 As expected, injection of the mγ3 isotype induced a strong downmodulation of TCR cell surface expression as detected using both anti-CD3ε (Fig 7A) and anti-TCRVβ reagents (data not shown), but failed to induce the systemic release of serum IL-2 observed in response to hamster anti-CD3ε antibodies (Fig 7B). To investigate the in vivo immunosuppressive properties of mitogenic and nonmitogenic anti-CD3ε MoAbs under the experimental conditions used throughout this study (a single injection regimen), we monitored the survival of MHC mismatched A/J tail skin grafts that were transplanted on the day of antibody treatment onto BALB/c mice. The administration of mitogenic anti-CD3ε MoAbs significantly prolonged the mean graft survival (Fig 8), whereas animals injected with the nonmitogenic anti-CD3ε MoAb displayed a normal rejection response when compared with control, untreated mice. We next established the effect of mitogenic and nonmitogenic anti-CD3ε MoAbs on APC functions. As shown in Fig 9, spleen cells isolated from animals injected with nonmitogenic forms of anti-CD3ε antibodies were able to efficiently stimulate alloreactive naive T cells to proliferate, whereas spleen cells from mice treated with the mitogenic form of anti-CD3ε MoAbs displayed reduced APC functions. Flow cytometry studies confirmed that nonmitogenic anti-CD3ε antibodies failed to downregulate CD11c+ splenic DC numbers (data not shown). Finally, no effect of mitogenic anti-CD3ε antibodies on APC function was observed in SCID mice (data not shown), in agreement with the idea that APC dysfunction induced by in vivo administration of anti-CD3ε antibodies is a consequence of T-cell activation.
In vivo administration of mγ3-anti CD3ɛ and mitogenic anti-CD3ɛ administration. (A) BALB/c mice were treated on day 0 with control antibody, mγ3-anti-CD3ɛ, or anti-CD3ɛ (30 μg IV) and analyzed on day 1 for CD3ɛ expression. Pooled spleen cells from 3 individuals in each group were analyzed by immunofluorescence after staining with FITC-labeled anti-CD3ɛ. (B) BALB/c mice (5 per group) were treated with control antibody, mγ3-anti CD3ɛ, or anti-CD3ɛ. Mice were bled 2 hours later and the serum IL-2 activity was tested by ELISA. Results are expressed as the mean absorbance (492 nm) ± SD of individual determinations.
In vivo administration of mγ3-anti CD3ɛ and mitogenic anti-CD3ɛ administration. (A) BALB/c mice were treated on day 0 with control antibody, mγ3-anti-CD3ɛ, or anti-CD3ɛ (30 μg IV) and analyzed on day 1 for CD3ɛ expression. Pooled spleen cells from 3 individuals in each group were analyzed by immunofluorescence after staining with FITC-labeled anti-CD3ɛ. (B) BALB/c mice (5 per group) were treated with control antibody, mγ3-anti CD3ɛ, or anti-CD3ɛ. Mice were bled 2 hours later and the serum IL-2 activity was tested by ELISA. Results are expressed as the mean absorbance (492 nm) ± SD of individual determinations.
Effect of mγ3-anti CD3ɛ and hamster anti-CD3ɛ administration on allogeneic skin graft survival. BALB/c mice were injected with 30 μg of anti-CD3ɛ MoAbs and grafted with MHC-mismatched A/J tail skin on the day of antibody treatment. Control animals were injected with PBS. P < .001 for hamster versus mγ3 anti-CD3ɛ or control, PBS-treated animals; P is not significant for mγ3 anti-CD3ɛ versus control, PBS-treated animals.
Effect of mγ3-anti CD3ɛ and hamster anti-CD3ɛ administration on allogeneic skin graft survival. BALB/c mice were injected with 30 μg of anti-CD3ɛ MoAbs and grafted with MHC-mismatched A/J tail skin on the day of antibody treatment. Control animals were injected with PBS. P < .001 for hamster versus mγ3 anti-CD3ɛ or control, PBS-treated animals; P is not significant for mγ3 anti-CD3ɛ versus control, PBS-treated animals.
Effect of mγ3-anti-CD3ɛ and mitogenic anti-CD3ɛ administration on APC functions. Spleen cells from control antibody-treated mice, mγ3-anti-CD3ɛ–treated mice, or anti-CD3ɛ–treated mice (50 μg IV) were irradiated and used as accessory cells to stimulate G10-nonadherent responding cells from CBA mice (3 × 105/well). Results are expressed as cpm of [3H] TdR incorporation by responder cells.
Effect of mγ3-anti-CD3ɛ and mitogenic anti-CD3ɛ administration on APC functions. Spleen cells from control antibody-treated mice, mγ3-anti-CD3ɛ–treated mice, or anti-CD3ɛ–treated mice (50 μg IV) were irradiated and used as accessory cells to stimulate G10-nonadherent responding cells from CBA mice (3 × 105/well). Results are expressed as cpm of [3H] TdR incorporation by responder cells.
DISCUSSION
Despite extensive clinical and animal experience, the precise mechanisms by which anti-CD3ε antibodies inhibit in vivo transplantation responses have not yet been firmly established. Because CD3ε, the molecular target of most therapeutic anti-CD3ε antibodies, is a 17- to 20-kD transmembrane molecule that is a part of a multimolecular, Ag-specific receptor complex selectively expressed by T cells, inhibition of T-cell functions has been proposed as the key mechanism of action of these antibodies in vivo.
Accordingly, in vivo administration of anti-CD3ε MoAbs has been shown to induce (1) peripheral T-cell depletion, due to receptor-mediated opsonization and killing,29-32 increased adhesion to vascular endothelium,33 and activation-induced cell death34; (2) Ag-specific receptor blockade and/or downmodulation35; and (3) induction of a state of cell unresponsiveness related to T-cell anergy.36 37
The present study demonstrates that administration of anti-CD3ε antibodies caused a marked but selective inhibition of splenic accessory cell functions, suggesting an additional mechanism by which these antibodies can downmodulate in vivo immune responses.
Injection of anti-CD3ε MoAbs appears to induce the same phenomenon of DC maturation and peripheral cell loss previously observed in response to LPS.5 Anti-CD3ε MoAbs induced the migration of splenic DC from the marginal zone to the T-cell area and the simultaneous upregulation of the activation-associated markers DEC-205 and CD86 (Fig1). By analogy with studies performed in LPS-treated mice, we interpret the loss of DC from the heart (Fig 3A) and the increase in DC numbers observed in lymph nodes (Fig 3B) as the consequence of anti-CD3ε–induced migration of DC from nonlymphoid organs to lymph nodes via the lymphatic route.
Although the molecular mechanisms that control migration and recruitment of DC are not well defined, it has been recently demonstrated that systemic administration of recombinant TNF-α, and to a lesser extent of IL-1, induces movement of DC from nonlymphoid organs into lymph nodes.12 Because anti-CD3ε MoAbs cause the in vivo production of TNF-α and other inflammatory mediators,16 it is reasonable to assume that DC migration after anti-CD3ε administration is secondary to the in vivo release of inflammatory mediators by polyclonally activated T cells. In support of this conclusion, no effect on APC function was observed in SCID mice injected with mitogenic anti-CD3ε MoAbs (data not shown) or in immunocompetent mice treated with nonmitogenic forms of anti-CD3ε (Fig 7C).
It has been recently suggested that DC undergo 3 discrete sequential stages of differentiation, identified as immature, mature, and apoptotic.38 In particular, in vitro studies have suggested that functional maturation of DC into potent APC is ended by apoptotic cell death and that no reversion to the immature phenotype was observed. We have recently confirmed this hypothesis in vivo and shown that, after LPS-induced maturation, DC undergo apoptotic cell death in situ.39 Therefore, loss of splenic DC observed 48 hours after anti-CD3ε administration probably reflects the apoptotic cell death that follows DC maturation in vivo.
In keeping with the phenotypical analysis, the functional studies presented in this study indicate that anti-CD3ε treatment induces a selective APC deficiency characterized by the loss of cells able to provide costimulation to naive T cells and Th1 clones and to secrete the proinflammatory cytokine IL-12. The ability of spleen accessory cells to capture and efficiently present Ag to a Th2 clone is not affected by anti-CD3ε treatment (Fig 5). Accordingly, we have recently demonstrated that administration of anti-CD3ε MoAbs selectively downregulates the secretion of Th1-type cytokines (IL-2 and IFN-γ) upon in vivo restimulation, whereas the production of Th2-type cytokines was only marginally affected (IL-4) or upregulated (IL-10).40 Thus, both the relative resistance of Th2-like T cells to anergy induction in vitro41 and in vivo40,42 and the selective APC deficiency induced in vivo by anti-CD3ε MoAbs may explain why, despite intense immunosuppression, mitogenic anti-CD3ε antibodies often induce a humoral response comprising both anti-xenotypic and anti-idiotypic antibodies that neutralize the drug and abrogate its immunosuppressive activity.43 44
Recently, nonmitogenic MoAbs have been produced and shown in animal models to retain immunosuppressive properties while minimizing toxicity.22,45 However, nonmitogenic antibodies to CD3ε displayed reduced immunomodulatory potential both in vivo and in vitro when compared with mitogenic forms.45 Indeed, as demonstrated in the present study, only mitogenic anti-CD3ε MoAbs were able to achieve immunosuppression after a single, moderate dose injection regimen.45 Moreover, and of potential relevance for this study, nonmitogenic anti-CD3ε MoAbs forms were found unable to suppress generation of cytotoxic T cells in vitro36,45,46 or to cause long-term anergy in the CD8+ T-cell subset.47 Based on the well-described capacity of DC2 and IL-1248,49to potentiate cytotoxic T lymphocytes (CTL) development, it is tempting to speculate that downregulation of DC numbers contributes to the long-term efficacy of mitogenic anti-CD3ε–based therapy. Finally, although the precise contribution of APC cell dysfunction to the anti-CD3ε–induced immunosuppression in vivo is difficult to establish, numerous studies have suggested that inhibition of accessory cell functions through disruption of the CD28 and CD40L signaling has strong therapeutic potential.50,51 The observation that both cyclosporine52 and steroids4 affect DC activity and/or survival further suggests that the clinical efficacy of several immunosuppressive therapies may in part be due to their ability to affect accessory cell functions in vivo.
Maturation and migration of DC from the periphery into T-cell areas have been recognized as important steps leading to T-cell sensitization and therefore initiation of an Ag-specific immune response.1,2 The observation that these phenotypic and migratory changes are induced in vivo by anti-CD3ε Abs can be viewed as serious constraint to the use of mitogenic anti-TCR complex MoAbs as immunosuppressive agents. However, as demonstrated by early studies, a strong correlation between interstitial DC depletion and delayed graft rejection has been established,10 suggesting that DC loss from peripheral organs is beneficial to allograft survival. The findings reported herein suggest therefore the possibility of anticipating the first anti-CD3ε administration 24 to 48 hours before organ transplantation to avoid DC maturation in the presence of alloAgs.
Finally, inhibition of APC functions may contribute to the long-term efficacy of anti-CD3ε–based therapies or even play a role in the induction of immune tolerance/suppression after discontinuation of antibody therapy. Indeed, DC loss/redistribution may favor stimulation of alloreactive T cells by accessory cells presenting alloAgs in an inadequate, costimulatory-deficient fashion, leading to T-cell unresponsiveness. Further studies are thus required to better evaluate the importance of APC downmodulation in the achievement of in vivo immunosuppression.
ACKNOWLEDGMENT
The authors thank P. Veirman, G. Dewasme, M. Swanepoel, and F. Tielemans for technical assistance and thank H. Bazin (UniversitéCatholique de Louvain), J.A. Bluestone (University of Chicago, Chicago, IL), G. Klaus (National Institute for Medical Research, London, UK), P. Marrack (University of Colorado Health Sciences Center, Denver, CO), A. O'Garra (DNAX, Palo Alto, CA), R.H. Schwartz (National Institutes of Health, Bethesda, MD), and G. Trinchieri (Wistar Institute, Philadelphia, PA) for providing reagents used in this study. The scientific responsibility is assumed by the authors.
This work was founded by the Belgian Program in Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming and by a Research Concerted Action of the Communauté Française de Belgique. Additional financial support was given by the Fonds Emile Defay. M.M. is a Research Associate from the FNRS (Belgium).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Oberdan Leo, PhD, Laboratoire de Physiologie Animale, Université Libre de Bruxelles, Rue Pr Jeneer et Brachet 12, 6041 Gosselies, Belgium.

![Fig. 4. Defective APC function in anti-CD3ɛ–treated mice. Spleen cells from control antibody-treated or anti-CD3ɛ–treated BALB/c mice (50 μg IV) were irradiated and used as accessory cells to stimulate (A and B) G10-nonadherent responding cells from CBA mice (3 × 105/well) or (C) ovalbumin-specific T-cell hybridoma mice (3 × 105/well) in the presence of 1 μg/mL of ovalbumin peptide. Proliferation (A) and IL-2 production (B and C) were assayed as described in Materials and Methods. Results are expressed as counts per minute (cpm) of [3H] TdR incorporation by responder cells (A) or IL-2–dependent cell line CTL.L (B and C). These results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4347/5/m_blod42431004x.jpeg?Expires=1769732000&Signature=cV0WYz9uSyiFBff-k0519vrRcLfpq7QpXVMqPXHN3sm58pwIHBYYCpRdLpBUSJm7lLY~Af5e3xGjS25VEQ9AVvq0f4Hf7Dw9KEqBiY2AbYjztzGN9HfOZnDt7LK2sha-xIsi6fM8BKjs8rsdOLRy3d0GGLLAcg-XF5zYzvytue6cLvvNtk4GhIumhu6rjd9aQslasX7YCnXfK0qOXJyocHyzuUblml9xaujrRHwL5wZP8CtHWcsb~MHfnz474~UHhx9nm~aZmgDbTUCg4G1tvePZxCSINw-3Y0AK57h6cU-ZK7zXbwedPEUyFUshs-HQyqQUOXpP-Ujqz-D94y8IhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. APC from anti-CD3ɛ–treated mice support the growth of the Th2, but not Th1, Ag-specific clones. Spleen cells from control antibody-treated or anti-CD3ɛ–treated mice (50 μg IV) were irradiated and used as accessory cells to stimulate the Th1 clones AE.7 and CLOVA 1.1 or the Th2 clones HG.4 and CLOVA 2.9 in the presence of nominal Ag. Clones were also stimulated by Ag presented by G10-depleted splenic accessory cells for the sake of comparison. Results are expressed as cpm of [3H] TdR incorporation by responder cells. These results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4347/5/m_blod42431005x.jpeg?Expires=1769732000&Signature=uQkioz5b8jYs-cwvUhu9pPwL28HTEV~h-CoEg1PXjrqWp7xkfpf5ifIKUd2iXvvFkKu2iT2Gps-aeHzUbJ1AINwVH5v746KUWvQaiVgSmr8r1oXIJ9OVVxDBTpHDFxzYbzns-xGMdUarv~qF5y615AZGBHg-4hu6DXy3~LDlAY1ON00hRdxfgTsxhzs~-gtUxH6bTrINUEgQ1bJzbqOllf62hBmhH4yukOXpHuSPdPKoF~AKQ~bchRRg0Omuk~b87pIZelSLekLRUdx6p0ldcikaab98dQ98r4ygoPXLjqg~eHVP5FJLWc9CaTZV~~F45X48vBsaq8EbmV3HDTlsKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 9. Effect of mγ3-anti-CD3ɛ and mitogenic anti-CD3ɛ administration on APC functions. Spleen cells from control antibody-treated mice, mγ3-anti-CD3ɛ–treated mice, or anti-CD3ɛ–treated mice (50 μg IV) were irradiated and used as accessory cells to stimulate G10-nonadherent responding cells from CBA mice (3 × 105/well). Results are expressed as cpm of [3H] TdR incorporation by responder cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4347/5/m_blod42431009x.jpeg?Expires=1769732000&Signature=er~AkKzQAisAyU6o9ArbdmrOOx-rufTxtoKmQdU7xSmWUF7tN3NN5YZNQnTIUCdBGRkZaZWjUeEy8WbL3dR69URzguPYu6EDeKuJVv886LwHccjjI6AWqymxlbvH-2kAOiMvMS9P9PRzsf1r2hIaLreHSQfaCyJtZu3AGrpuk9AMfQtZzI16g0b~dfFqnLqDJRxN8sr721jQCvRV7DvhPbM46e9wShSzIRKD88-Z3vHPbpLwf9WX7bxL-vJvX2FAuafiLGb1wV3HmDvDH9bAobd6FhguT1nh5jXAiGFyHN2CKF0K-MFNhpVAkrNKevBHJ9ehN66sIBZeObqcZuZDBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
