The tumor cells in ALK-positive lymphoma (“ALKoma”) usually express the product of the NPM-ALK chimeric gene, generated by the t(2;5) chromosomal translocation. However, 10% to 20% of ALK-positive lymphomas express ALK fusion protein(s) other than NPM-ALK, and in this report, we describe the immunohistologic and clinicopathologic features of 15 such cases. The absence of theNPM-ALK fusion gene was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) in 8 cases and by fluorescence in situ hybridization (FISH) analysis in a further 2 cases. In each case, ALK staining was restricted to the cytoplasm and the N-terminus of NPM to the nucleus (contrasting with lymphomas expressing NPM-ALK in which cytoplasmic as well as nuclear labeling is seen). However, in the course of screening 53 ALK-positive lymphomas, 2 biopsies were found that had a “cytoplasm-only” ALK staining pattern but that nevertheless were shown to carry the (2;5) (by NPM staining and RT-PCR). The 15 cases resembled typical NPM-ALK–positive lymphomas in that all were of T or null phenotype, usually occurred in young male patients, and frequently presented with advanced disease associated with systemic symptoms and extranodal involvement. Moreover, their prognosis was excellent and indistinguishable from that of classical t(2;5)-positive tumors, but was clearly different from that of ALK-negative anaplastic large-cell lymphomas. These results suggest that lymphomas carrying variants of the NPM-ALK fusion protein can be detected by immunostaining for ALK and NPM and also that they can be grouped with classical t(2;5)-positive tumors as a single entity (ALK-positive lymphoma or “ALKoma”) that shows a better prognosis than ALK-negative anaplastic large-cell lymphoma.
A DISTINCT CLINICOPATHOLOGIC entity (“ALK-positive lymphoma” or “ALKoma”),1,2defined by expression of the anaplastic lymphoma kinase (ALK), has emerged from the heterogeneous category of CD30+ anaplastic large-cell lymphomas.3-9 ALK-positive lymphomas show a wide morphologic spectrum1,2 ranging from the common type to the small-cell or lymphohistiocytic variant,10-13 but share the same phenotype (T or null),1,2,14 show a male predominance, and tend to occur in the first 3 decades of life.1,2,14-16These lymphomas often present as rapidly growing and advanced tumors that are frequently associated with systemic symptoms and extranodal involvement, especially of skin and bone.16 However, their prognosis is good compared with ALK-negative anaplastic large-cell lymphomas.14-16
ALK expression in these cases is usually due to the presence of the t(2;5)(p23;q35) chromosomal translocation,17 which causes fusion of ALK and NPM genes.18,19 The resultant chimeric gene encodes an 80-kD fusion protein18-20 containing the N-terminal portion of NPM (amino acids 1-117)18,19 linked to the entire cytoplasmic domain of the neural-associated receptor tyrosine kinase ALK.21,22 The NPM-ALK hybrid protein is thought to play a direct role in lymphomagenesis,23,24 probably by aberrant phosphorylation of intracytoplasmic substrates.18
The presence of NPM-ALK chimeric transcripts or proteins can be detected in anaplastic large-cell lymphoma samples by the reverse transcriptase-polymerase chain reaction (RT-PCR).25 In addition, ALK protein is not expressed in lympho-hematopoietic tissues, and immunostaining with antibodies specific for the cytoplasmic portion of ALK2 26-28 has therefore been widely used to provide evidence for the t(2;5) translocation.
In ALK-positive lymphomas, strong immunocytochemical labeling for ALK protein is seen not only in the cytoplasm of neoplastic cells, but also within their nuclei.2,16 This is due to dimerization of NPM-ALK fusion protein with wild-type NPM, which carries nuclear localization motifs.29 However, not all ALK-positive lymphomas of T-cell or null cell phenotype carry the NPM-ALKfusion gene. The ALK gene can be involved in chromosomal anomalies other than the t(2;5), such as t(1;2)(q25;p23)19 or inv(2)(p23;q35),30 and in these ALK-positive lymphomas the ALK protein tends to accumulate only in the cytoplasm.28,30In 2 large studies,1 2 15% to 20% of ALK-positive lymphomas of T-cell or null cell phenotype showed cytoplasmic-restricted expression of ALK, suggesting that variants of the NPM-ALK fusion gene are not uncommon.
In a recent study,31 we reported that antibodies specific for the N-terminal region of the NPM molecule showed cytoplasmic labeling in 3 classical ALK-positive lymphomas. We speculated that this could be used as a means of confirming whether a lymphoma in which ALK is apparently restricted to the cytoplasm does indeed carry a variant of the NPM-ALK fusion gene. In this report, we have studied a series of such rare cases and report that in most but not all of them the staining pattern of the N-terminus of NPM provided confirmation of a variant translocation. This is the first series of such tumors containing variant ALK fusion proteins to be identified, and we have reviewed not only clinical and histologic features at presentation, but also patient survival (after treatment by conventional chemotherapy) to assess the prognostic significance of these genetic anomalies.
MATERIALS AND METHODS
Patients and Tissue Samples
A series of 84 T/null anaplastic large-cell lymphomas were identified for study, comprising 78 previously reported cases (53 ALK-positive and 25 ALK-negative)16 and 6 cases in which the presence of an ALK-containing fusion protein other than NPM-ALK was suggested by cytoplasmic-restricted expression of ALK protein, which was associated with a negative RT-PCR test for NPM-ALK, and (in 3 cases) the finding of variant translocations by cytogenetic analysis, namely t(1;2)(q21;p23) and t(2;3)(p23;q21). The latter cases were identified in the following institutions: the Laboratory of Pathology, University of Barcelona (Barcelona, Spain; 1 case); the Department of Pathology, University of Würzburg (Würzburg, Germany; 2 cases); and the Laboratory of Anatomic Pathology, C.H.U. Purpan (Toulouse, France; 3 cases).
The following samples were used as controls: normal lympho-hematopoietic tissues (thymus [n = 3], tonsil [n = 6], spleen [n = 3], and bone marrow [n = 3]), 50 lymph nodes showing reactive changes, and 485 ALK-negative lymphomas (other than anaplastic large-cell lymphomas) representative of the different categories of the REAL classification.5 All tissue samples were studied as paraffin-embedded biopsies that had been fixed in formalin (the majority) and B5 or Bouin fixatives.
Anti-ALK and Anti-NPM Monoclonal Antibodies
Monoclonal antibodies against ALK (ALK1 and ALKc) and against NPM (NPMa, NA24, and NPMc) were produced in 2 of the investigators’ laboratories (B.F. and D.Y.M.).2,16,28,31 Two of the anti-NPM antibodies (NPMa and NA24) recognize epitopes found on the N-terminus of this molecule (and on NPM-ALK). The third (NPMc) recognizes a C-terminal epitope present only on wild-type NPM.31 In normal human tissues, all anti-NPM antibodies immunostain wild-type NPM in cell nuclei. In addition, cytoplasmic reactivity is observed in mitotic cells, cerebral neurons, and megakaryocytes.31
Immunocytochemical Labeling
Paraffin sections on silane-coated slides were dewaxed and then subjected to microwave heating (750 W for 3 cycles of 5 minutes each) in 1 mmol/L EDTA buffer, pH 8.0.2,32 Sections were allowed to cool at room temperature for approximately 20 minutes, were washed in Tris-buffered-saline, and were immunostained by an immuno-alkaline phosphatase (APAAP) technique,33 as previously described.2 Endogenous alkaline phosphatase was blocked with 1 mmol/L levamisole34 and antibodies were used as undiluted tissue culture supernatants. Slides were counterstained for 5 minutes in Gill’s hematoxylin and mounted in Kaiser’s glycerol gelatin (Merck, Darmstadt, Germany).
RT-PCR Analysis
RT-PCR studies were performed as previously described.35
Clinical Data
The clinical findings and outcome of the 84 T/null anaplastic large-cell lymphomas (59 ALK-positive and 25 ALK-negative) were reassessed, and the following parameters were reviewed: sex, date of birth, date and site of the diagnostic biopsy, sites of nodal and extranodal involvement (as assessed by physical examination, computerized tomography [CT] scan, and marrow biopsy), Ann Arbor Stage at the time of diagnosis, performance status, maximum diameter of the largest tumor mass, and serum lactate dehydrogenase (LDH). Patients were stratified according to a simplified age-adjusted International Prognostic Index.36 Overall survival was measured from diagnosis to death from any cause, with surviving patient follow-up recorded at the last contact date. Estimates of overall survival distribution were calculated using the method of Kaplan and Meier.37 Time-to-event distributions were compared using the log-rank test.38 The Cox regression model39was used to correlate survival with prognostic factors and with the 3 different lymphoma categories defined by ALK and NPM immunostaining (NPM-ALK–positive, carrying a variant ALK fusion protein, and ALK-negative).
RESULTS
Identification of ALK-Positive Lymphomas Expressing Variants of NPM-ALK
Immunostaining.
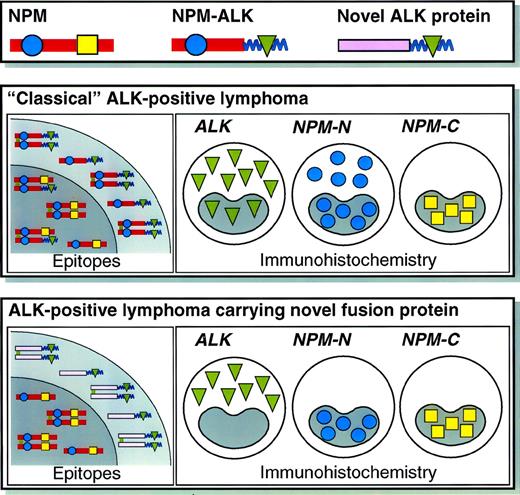
Two patterns of immunostaining for ALK protein were observed in our series of 59 ALK-positive lymphomas of T-cell or null cell phenotype (Table 1). In 42 biopsies (36 common type and 6 lymphohistiocytic/small cell type), ALK labeling was seen within the nuclei of large neoplastic cells (Fig1, top left), and this was typically accompanied by diffuse cytoplasmic reactivity (although in smaller ALK-positive neoplastic cells, labeling was restricted to the nucleus). The other labeling pattern, seen in 17 of the 59 cases (Table 1), comprised ALK immunostaining restricted to the cell cytoplasm. We interpreted this distinctive staining pattern as possibly indicating the presence of ALK fusion protein(s) that lacked NPM and that was therefore not transported to the nucleus (through heterodimerization with wild-type NPM).29
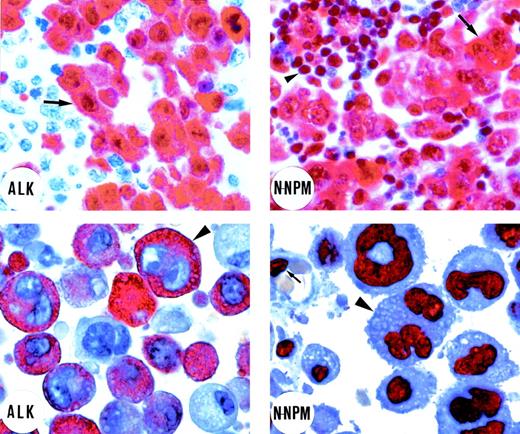
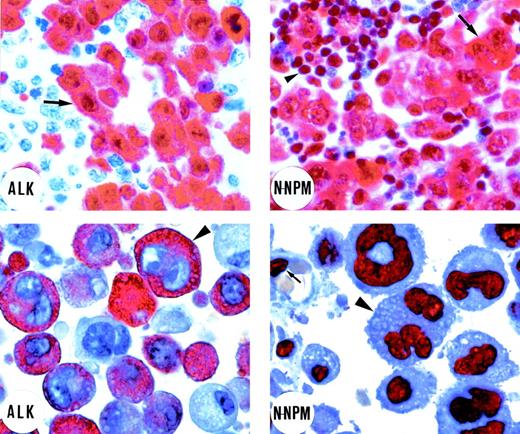
Immunostaining of an ALK-positive lymphoma bearing the t(2;5) translocation (top left and right). (Top left) Immunostaining for ALK protein. Tumor cells (arrow) show strong cytoplasmic and nuclear ALK expression. Residual lymphoid tissue is ALK-negative (original magnification × 800). (Top right) Tumor cells (arrow) show cytoplasmic and nuclear positivity for the N-terminus of NPM (N-NPM). Cytoplasmic positivity reflects immunostaining of the NPM moiety of the NPM-ALK fusion protein. Normal residual lymphoid cells (arrowhead) show wild-type nucleus-restricted NPM staining (original magnification × 800). In contrast, immunostaining for the C-terminus of NPM showed a nucleus-restricted expression of the protein (not shown). (Bottom left and right) An example of ALK-positive lymphoma expressing variant ALK fusion protein. (Bottom left) Positivity for the ALK protein is restricted to the cytoplasm of the large anaplastic tumor cells (arrowhead; original magnification × 1,000; right). The N-terminus of NPM (N-NPM) shows nucleus-restricted expression both in tumor cells (arrowhead) and in a normal endothelial cell (arrow), reflecting immunostaining of wild-type NPM (original magnification × 1,000). An identical staining pattern (not shown) was observed for the C-terminus of NPM. (APAAP immunostaining on lymph node paraffin sections; hematoxylin counterstaining.)
Immunostaining of an ALK-positive lymphoma bearing the t(2;5) translocation (top left and right). (Top left) Immunostaining for ALK protein. Tumor cells (arrow) show strong cytoplasmic and nuclear ALK expression. Residual lymphoid tissue is ALK-negative (original magnification × 800). (Top right) Tumor cells (arrow) show cytoplasmic and nuclear positivity for the N-terminus of NPM (N-NPM). Cytoplasmic positivity reflects immunostaining of the NPM moiety of the NPM-ALK fusion protein. Normal residual lymphoid cells (arrowhead) show wild-type nucleus-restricted NPM staining (original magnification × 800). In contrast, immunostaining for the C-terminus of NPM showed a nucleus-restricted expression of the protein (not shown). (Bottom left and right) An example of ALK-positive lymphoma expressing variant ALK fusion protein. (Bottom left) Positivity for the ALK protein is restricted to the cytoplasm of the large anaplastic tumor cells (arrowhead; original magnification × 1,000; right). The N-terminus of NPM (N-NPM) shows nucleus-restricted expression both in tumor cells (arrowhead) and in a normal endothelial cell (arrow), reflecting immunostaining of wild-type NPM (original magnification × 1,000). An identical staining pattern (not shown) was observed for the C-terminus of NPM. (APAAP immunostaining on lymph node paraffin sections; hematoxylin counterstaining.)
In the present study, we therefore tested this hypothesis by immunostaining all 59 cases with antibodies directed against the N-terminus of NPM. We have recently reported31 that typical ALK-positive lymphomas carrying the t(2;5) translocation show diffuse cytoplasmic labeling for the N-terminus of NPM (as well as nuclear labeling) (Fig 1, top right), reflecting immunostaining of the NPM moiety of the NPM-ALK fusion protein present at this site. This is in clear contrast to the labeling pattern for wild-type NPM seen with antibody to its C-terminus (absent from the fusion protein) that is found only in the nucleus (not shown).31 The latter staining represents wild-type NPM, and we have confirmed by extensive testing of normal, reactive, and neoplastic lymphoid tissue (see Materials and Methods) that labeling for NPM is restricted to nuclei in the great majority of cells.31
Fifteen of the 17 cases that showed purely cytoplasmic staining for ALK (Fig 1, bottom left) gave a distinctive NPM immunostaining pattern (ie, nucleus-restricted for N-terminal epitopes), favoring the hypothesis that they contained variant ALK-fusion proteins. This immunostaining is shown in Fig 1 (bottom right) and the differing reactions of classical ALK-positive lymphoma and of cases containing variant ALK fusion proteins are summarized schematically in Fig 2. In the remaining 2 cases, staining for the N-terminus of NPM was cytoplasmic and comparable to that seen in cases carrying the classical NPM-ALK fusion protein. This was in keeping with the results of RT-PCR analysis (see below).
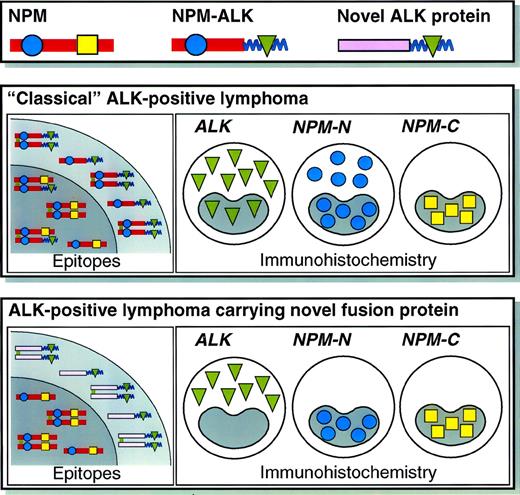
Schematic illustration of the staining patterns for ALK and the N- and C-terminal portions of NPM in lymphomas carrying NPM-ALK or variant ALK fusion protein(s). Further details of the reactivity of the anti-NPM monoclonal antibodies have been published elsewhere.31
Schematic illustration of the staining patterns for ALK and the N- and C-terminal portions of NPM in lymphomas carrying NPM-ALK or variant ALK fusion protein(s). Further details of the reactivity of the anti-NPM monoclonal antibodies have been published elsewhere.31
In contrast, all of the 42 cases with cytoplasmic plus nuclear labeling for ALK (Fig 1, top left) showed the predicted differing labeling patterns for the 2 portions of NPM, ie, cytoplasmic and nuclear for the N-terminus (Fig 1, top right) and nucleus-restricted for the C-terminus (not shown).31 It should be noted that larger neoplastic cells showing this differential pattern were obvious among the 36 cases with common type or giant cell-rich morphology, but less frequent (and often grouped around vessels) in the cases no. 37 through 42 (classified as having lymphohistiocytic or small cell variant morphology), in which small neoplastic cells predominated.
RT-PCR and fluorescence in situ hybridization (FISH) analysis.
A total of 28 cases were investigated directly for the presence of theNPM-ALK gene by RT-PCR or FISH analysis (Table 1). Each of the 16 cases with the classical immunostaining patterns for ALK and NPM tested in this way were positive, as were the 2 cytoplasmic ALK only cases in which NPM labeling had not provided evidence of a variant ALK-protein. In contrast, 10 cases with nuclear-restricted labeling for the N-terminal epitopes of NPM all lacked, as predicted, evidence of the NPM-ALK gene: in 8 cases, the RT-PCR reaction was negative, and in 3 of them, conventional cytogenetics showed variant translocations, ie, t(1;2)(q21p23) in 2 cases and t(2;3)(p23;q35) in 1 case. In the remaining 2 cases, a cryptic inv(2)(p23;q35) had been previously documented by FISH analysis.30
Clinical features of ALK-positive lymphomas carrying variants of NPM-ALK.
The 15 ALK-positive lymphomas identified in this way as carrying variants of the t(2;5) translocation showed clinical features similar to those of classical ALK-positive cases.16 In particular, the patients tended to be young (mean age, 19 years; age range, 4 to 45), with male predominance (male/female ratio, 1.5), and they frequently presented with advanced disease (9 of 15 cases in stage III-IV) and systemic symptoms (11 of 15 patients). Similarly to NPM-ALK–positive cases,16 these patients showed frequent involvement of extranodal sites (2 skin, 2 soft tissues, 3 lung, 2 central nervous system, 1 bone marrow, 1 pleura, and 1 gut). There was no statistically significant difference between NPM-ALK–positive and variant cases in terms of bulky disease, LDH levels, or performance status.
With the exception of 2 stage IA cases (treated with radiotherapy only), the 84 patients were treated with aggressive doxorubicin-containing regimens. Fourteen of the 15 cases bearing a variant ALK fusion protein, 32 of 44 NPM-ALK– positive, and 7 of 25 ALK-negative lymphomas are still alive. The median follow-up in the 3 groups was 2.26 years (range, 0.07 to 14.18 years), 2.37 years (range, 0.10 to 13.17 years), and 1.84 years (range, 0.16 to 9.92 years), respectively.
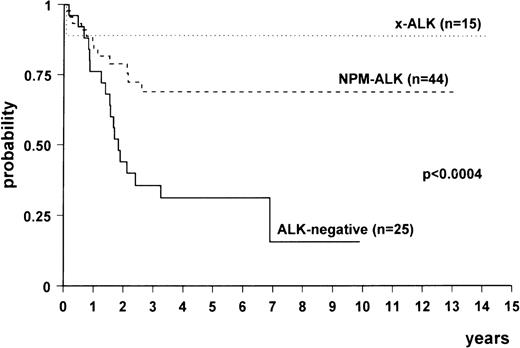
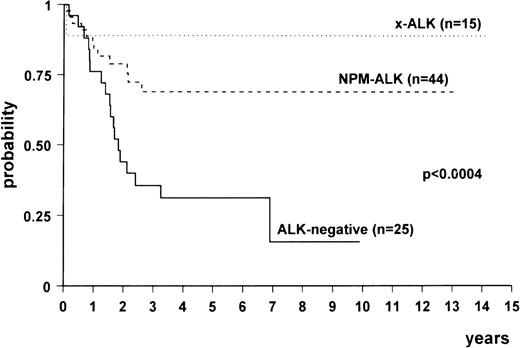
Comparison among the survival curves (Fig3) gave a statistically significant P value (<.0004). Multivariate analysis showed that there was a difference between NPM-ALK–positive and ALK-negative lymphomas (relative risk [RR], 0.24; 95% confidence interval [CI], 0.11 to 0.52; P < .0003) and between variant ALK-positive (x-ALK) and ALK-negative lymphomas (RR, 0.08; 95% CI, 0.01 to 0.6; P < .01). No difference was detected between the 2 subgroups of ALK-positive lymphomas. The age-adjusted International Prognostic Index also proved to be an independent risk factor (high risk v low risk: RR, 3.27; 95% CI, 1.54 to 6.92; P < .0001).
Overall survival of ALK-positive lymphomas expressing NPM-ALK (n = 44), variant ALK fusion protein(s) (“x-ALK”) (n = 15), and ALK-negative anaplastic large-cell lymphomas (n = 25).
Overall survival of ALK-positive lymphomas expressing NPM-ALK (n = 44), variant ALK fusion protein(s) (“x-ALK”) (n = 15), and ALK-negative anaplastic large-cell lymphomas (n = 25).
DISCUSSION
In this report, we describe the immunohistologic and clinicopathologic features of 15 cases of ALK-positive lymphomas that appear to express ALK fusion protein(s) other than NPM-ALK. Seventeen cases showed a cytoplasmic-only pattern of labeling when paraffin sections were immunostained for ALK protein, and this was associated with a negative RT-PCR test result for NPM/ALK in 8 cases (some of them bearing variant translocations at conventional cytogenetics or showing ALK proteins lacking NPM at Western blotting)40 and the presence of cryptic inv(2) (p23;q35) by FISH in an additional 2. Subsequently, monoclonal antibodies directed against the N-terminal portion of NPM31 were found to label only the nuclei of tumor cells (reflecting reactivity with wild-type NPM) in 15 of these 17 atypical cases, a staining pattern that differs from that of classical NPM/ALK-positive lymphoma, ie, cytoplasmic and nuclear.
These findings are of interest because they confirm the prediction that immunostaining for the N-terminal portion of NPM can identify lymphomas carrying variants of the NPM-ALK fusion protein.31 However, 2 cases that were initially suspected, because of their cytoplasm-only labeling pattern for ALK, to contain variant ALK fusion proteins were eliminated on the basis of the NPM labeling (and shown by RT-PCR analysis to contain NPM-ALK gene). A combination of NPM and ALK labeling thus appears necessary to identify cases carrying variants of NPM-ALK. Despite its recognized function as a phosphoprotein that shuttles between the nucleolus and the cytoplasm41 acting as a carrier for host42 and viral proteins,43,44 the level of NPM in the cytoplasm appears to be below the threshold for detection in subcellular fractionation studies or by immunocytochemistry.41 45 It is this fortuitous fact that allows immunocytochemical labeling for the N-terminus of NPM to be exploited as a means of specifically detecting NPM-ALK.
Having identified these 15 cases in which the cytoplasmic domain of ALK was presumably fused to a partner other than NPM, the obvious question is whether they differed in morphology or clinical behavior from classical t(2;5)-positive lymphomas. Our preliminary study suggests that variant ALK-positive lymphomas may not show the morphologic spectrum found in typical ALK-positive lymphomas,1 2because we found no cases in which a population of small neoplastic cells was present. However, this possibility that the absence of small tumor cells is a consistent feature of lymphomas expressing variant ALK-fusion protein(s) needs to be assessed in a larger number of cases.
Regardless of whether lymphomas carrying variants of NPM-ALK differ in morphologic terms from classical ALK-positive lymphomas, the clinical features of the cases in this report were essentially indistinguishable. They mostly occurred in children or young adults who presented with advanced disease associated with extranodal involvement and B symptoms (especially fever). Moreover, the prognosis of these variant ALK-positive lymphomas was excellent and indistinguishable from that of classical t(2;5)-positive tumors, but was clearly different from that of ALK-negative anaplastic large-cell lymphomas and also from that of the rare poor prognosis B-cell neoplasms that express full-length ALK protein and tend to occur in adults.46 This has theoretical and practical implications. If all ALK-positive tumors are indeed very similar in clinical terms, it may indicate that the expression of any ALK fusion protein in a T cell (or possibly NK cell), regardless of the molecular mechanism, sets in motion the same process of oncogenic transformation. A similar argument could be advanced for tumors such as Burkitt’s lymphoma in which cases carrying variant translocations involving the c-MYC gene behave identically to those of classical genotype.
The practical implication of the cases reported here is that, for clinical purposes, all lymphomas carrying ALK fusion proteins should continue to be grouped together under the term of ALK-positive lymphomas. These tumors appear together to constitute a homogeneous group of good prognosis neoplasms that should be distinguished from ALK-negative anaplastic large-cell lymphoid neoplasms.
ACKNOWLEDGMENT
The authors thank Barbara Bigerna, Roberta Pacini, Barbara Ugolini, Cristina Alunni, Laura Natali Tanci, and Gisberto Loreti for the skillful technical assistance. We also thank Claudia Tibidò for her excellent secretarial assistance.
Supported by A.I.R.C. (Associazione Italiana per la Ricerca sul Cancro) and the Leukaemia Research Fund of Great Britain. M.F. is supported by the Fondazione Antonio Castelnuovo, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Brunangelo Falini, MD, Istituto di Ematologia, Policlinico, Monteluce, 06100 Perugia, Italy.