Aggregation of blood platelets contributes to the arrest of bleeding at sites of vascular injury, but it can occlude atherosclerotic arteries and precipitate diseases such as myocardial infarction. The bonds that link platelets under flow conditions were identified using confocal videomicroscopy in real time. Glycoprotein (GP) Ib and von Willebrand factor (vWF) acted in synergy with IIbβ3 and fibrinogen to sustain platelet accrual at the apex of thrombi where three-dimensional growth resulted in increasing shear rates. The specific function of distinct adhesion pathways in response to changing hemodynamic conditions helps to explain hemostatic and thrombotic processes.
THE CONTROL OF HEMORRHAGE at wound sites requires platelet adhesion and aggregation,1,2 particularly when bleeding involves arterioles, where rapid blood flow results in high wall shear rates.3,4 In pathologic conditions, such as in atherosclerotic arteries, ruptured plaques and elevated shear rates at sites of stenosis5 may induce formation of platelet-rich thrombi that become life-threatening when they occlude the vascular lumen blocking blood flow.6,7 The first response to vascular injury, whether in normal hemostasis or pathologic thrombosis, consists of platelet adhesion to the damaged vessel wall or to exposed tissue components, and is mediated by interactions that have a key influence on subsequent thrombus growth.8 At high shear rates, binding of the membrane glycoprotein (GP) Ibα to immobilized von Willebrand factor (vWF)9 is the necessary initial step tethering platelets to reactive surfaces.10,11 However, stable attachment also requires the synergistic binding of integrins α2β1 and αIIbβ3(GP IIb-IIIa complex) to their respective substrates.8 12
Notwithstanding the importance of initiating events, a thrombus is constituted essentially of platelets linked to one another, not directly to subendothelial components; thus, its development is strictly dependent on the formation of interplatelet bonds. At present, it is generally accepted that fibrinogen bound to activated αIIbβ3 is the predominant adhesive bridge between aggregating platelets.13 Only at abnormally elevated shear rates, typically greater than 5,000 s−1, is vWF thought to participate in the process by binding to αIIbβ3, in addition to GP Ibα, in alternative to fibrinogen.14-16 However, these concepts have been suggested by the results of experiments with platelets in suspension not interacting with a reactive substrate,15-17thus under fluid dynamic conditions different from those that exist when aggregating platelets are attached to a surface exposed to flowing blood. To address this issue, we have studied the bonds that link platelets during thrombus development in real time, using collagen type I fibrils as a model substrate8 and confocal videomicroscopy for the three-dimensional quantitative evaluation of aggregation in a flow chamber. Our findings demonstrate that vWF and fibrinogen have distinct but synergistic roles in promoting interplatelet bonds at all but low venous shear rates. This novel understanding of the adhesive mechanisms involved in platelet aggregation during hemostatic and thrombotic processes suggests new strategies for antithrombotic intervention.
MATERIALS AND METHODS
Preparation of blood specimens and perfusion studies.
Blood was collected from healthy volunteers using as anticoagulantD-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone dihydrochloride (PPACK) at a final concentration of 80 μmol/L.8 Glass coverslips forming the bottom of the perfusion chamber exposed to blood were coated with acid-insoluble fibrillar type I collagen from bovine Achilles tendon (Sigma, St Louis, MO) prepared in 0.5 mol/L acetic acid, pH 2.8, as previously described.18 A silicone rubber gasket maintained a flow path height of 254 μm,8,19 and flow rates were selected to obtain the desired shear rates near the inlet of a variable linear shear stress chamber.20 Blood was treated with mepacrine (quinacrine dihydrochloride) at a final concentration of 10 μmol/L to render platelets fluorescent, and was aspirated with a syringe pump through the chamber mounted on the stage of a Zeiss Axiovert 135M/LSM 410 invert laser scan confocal microscope (Carl Zeiss, Oberkochen, Germany). The chamber was maintained at 37°C with controlled airflow. When indicated, the platelet membrane receptors GP Ibα and αIIbβ3 were blocked with the previously characterized monoclonal antibodies LJ-Ib1 and LJ-CP8, respectively.9,21,22 IgG was purified from ascitic fluid.23 Confocal optical sections were obtained with a 488-nm laser and a scanning time of 2 seconds on an area of 102,236 μm2 during blood flow, with a 1-μm distance between adjacent sections throughout the height of thrombi. All experiments were recorded in real time.
Preparation of washed blood cells.
Blood cells were washed free of plasma constituents by repeated centrifugation and resuspension in buffer using a method previously described in detail,21 but with two modifications: (1) cells were sedimented at 2,100g for 15 minutes at 22°C to 25°C; and (2) the final suspension was prepared with all blood cells at counts within 5% of the values in the original specimen using HEPES-Tyrode buffer, pH 7.4, containing 50 mg/mL bovine serum albumin, as well as 1.0 mmol/L Ca2+, 0.5 mmol/L Mg2+, and 0.02 mmol/L Mn2+. Fibrinogen22 and vWF24 were purified as previously described.
Image analysis and volume measurement.
Images were analyzed with a commercial software package (Metamorph; Universal Imaging Corp, West Chester, PA). A threshold was applied to distinguish platelets from the background, and the same value was then used for analyzing all the stacks of confocal images collected for a given experiment. Thrombus volume was calculated as the summation of partial volumes measured from the area occupied by platelets in each optical plane multiplied by height, ie, the z interval (1 μm) between adjacent planes.8
RESULTS
Role of two membrane receptors in platelet aggregation at different shear rates.
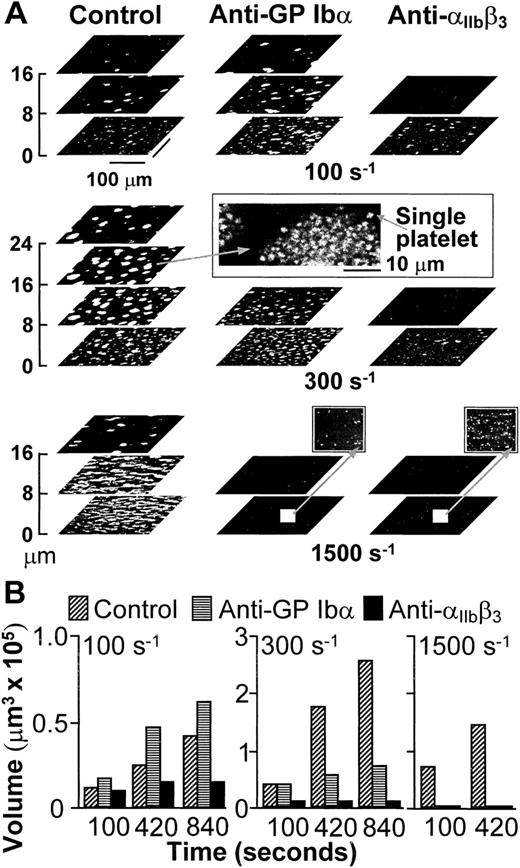
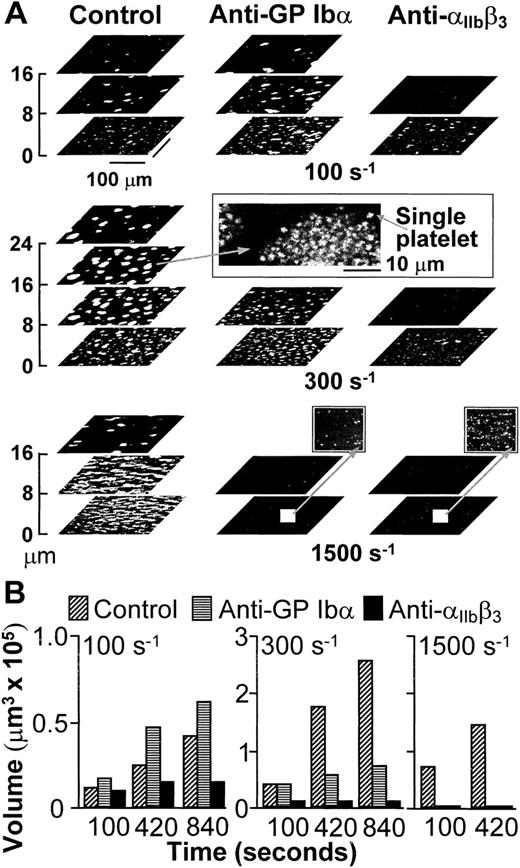
Platelets interacted with collagen within seconds from the beginning of blood perfusion, and then rapidly with one another, accumulating into thrombi firmly attached to the surface. In agreement with the results obtained in stirred platelet suspensions,17,25 aggregation in blood flowing with wall shear rate of 100 s−1, a value within the range that may be found in veins,3 was dependent on the function of integrin αIIbβ3, but not of GP Ibα (Fig1). The participation of αIIbβ3 in aggregation was still apparent when the shear rate was increased to 300 s−1, but selective inhibition of GP Ibα under these conditions limited the height reached by thrombi in spite of normal initial growth (Fig 1). It is known8 that vWF is not required to initiate platelet adhesion to collagen fibrils at wall shear rates as high as 500 s−1, which suggests that the effect of blocking GP Ibα on thrombus development at 300 s−1 was not likely to be the consequence of impaired platelet-surface interactions. With further increase of the wall shear rate to 1,500 s−1, a value within the range found in arterioles,3,4 individual blockade of either GP Ibα or αIIbβ3 completely hindered aggregation on the surface (Fig 1). However, under these conditions, vWF and GP Ibα are absolutely required to initiate adhesion8; thus, conclusions on their role in supporting platelet aggregation cannot be drawn from such results.
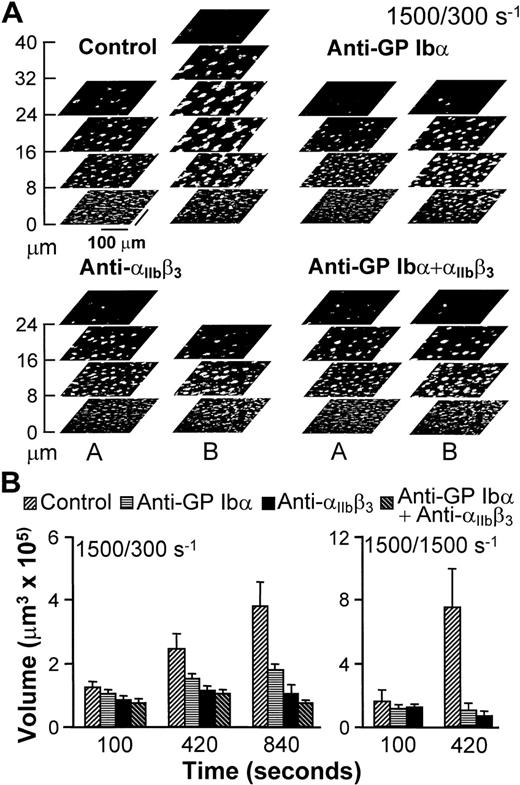
(A) Structure of thrombi formed at different shear rates in the absence (Control) or presence of monoclonal antibodies blocking GP Ib (LJ-Ib1, 100 μg/ml) or IIbβ3(LJ-CP8, 100 μg/ml), visualized by confocal optical sections at 1-μm intervals in the z-axis. Each plane shows an area of 52,345 μm2. For clarity, the z scale is expanded and only sections at selected distances from the surface are presented. Confocal sections were obtained after 840 seconds of flow at either 100 or 300 s−1, or after 420 seconds at 1,500 s−1. The inset in the middle panel displays the partial reproduction with less size reduction of a thrombus formed at wall shear rate of 300 s−1, demonstrating the resolution of single platelets in the confocal sections. The two insets in the bottom panel show with greater magnification part of the surfaces exposed to antibody-containing blood flowing with wall shear rate of 1,500 s−1. No thrombi were formed, but single platelets could easily be detected. Note that the confocal sections shown here are intended to give a global view of thrombus formation and not to visualize single platelets. (B) Total volume of thrombi calculated from confocal sections. Representative experiment of two that gave comparable results. Experiments at the shear rate of 1,500 s−1 were terminated after collecting z sections at 420 seconds when thrombi had already reached a large volume. Prolonging perfusion at this shear rate resulted in markedly abnormal flow patterns owing to nearly complete occlusion of the chamber.
(A) Structure of thrombi formed at different shear rates in the absence (Control) or presence of monoclonal antibodies blocking GP Ib (LJ-Ib1, 100 μg/ml) or IIbβ3(LJ-CP8, 100 μg/ml), visualized by confocal optical sections at 1-μm intervals in the z-axis. Each plane shows an area of 52,345 μm2. For clarity, the z scale is expanded and only sections at selected distances from the surface are presented. Confocal sections were obtained after 840 seconds of flow at either 100 or 300 s−1, or after 420 seconds at 1,500 s−1. The inset in the middle panel displays the partial reproduction with less size reduction of a thrombus formed at wall shear rate of 300 s−1, demonstrating the resolution of single platelets in the confocal sections. The two insets in the bottom panel show with greater magnification part of the surfaces exposed to antibody-containing blood flowing with wall shear rate of 1,500 s−1. No thrombi were formed, but single platelets could easily be detected. Note that the confocal sections shown here are intended to give a global view of thrombus formation and not to visualize single platelets. (B) Total volume of thrombi calculated from confocal sections. Representative experiment of two that gave comparable results. Experiments at the shear rate of 1,500 s−1 were terminated after collecting z sections at 420 seconds when thrombi had already reached a large volume. Prolonging perfusion at this shear rate resulted in markedly abnormal flow patterns owing to nearly complete occlusion of the chamber.
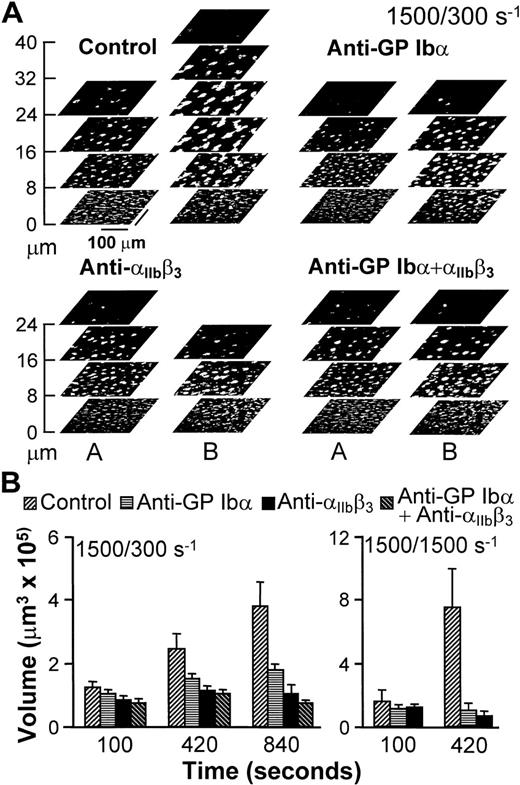
An experimental approach was devised to analyze the role of adhesion receptors in mediating interplatelet contact without interference from possible effects on initial surface interactions influencing subsequent aggregation.26 27 For this purpose, untreated blood was perfused over collagen fibrils for 100 seconds at the wall shear rate of 1,500 s−1, and was then followed by blood that contained function-blocking antibodies, or buffer as a control, at either 1,500 or 300 s−1. Thrombi, therefore, started to form with normal anchorage to the surface (Fig2), such that any consequence of receptor blockade could only result from effects on platelet-platelet interactions. Continuing perfusion with untreated blood at 300 or 1,500 s−1 resulted in progressive thrombus growth (Fig 2). As seen in Fig 2A for one experiment at 300 s−1, this growth in volume resulted from increasing height, as well as increasing cross-sectional area in planes at a distance from the surface. On the other hand, the base of thrombi did not change appreciably from the development attained at the end of the initial perfusion at 1,500 s−1 for 100 seconds (compare the bottom sections in stacks A and B of the control experiment in Fig 2A), which implies that surface coverage was already maximal or nearly maximal at that time. In contrast to these results, selective blockade of either GP Ibα or αIIbβ3 reduced the continuing accrual of platelets by 50% and 80%, respectively, when blood was perfused at 300 s−1 (Fig 2). It should be noted that this effect resulted from limiting the increase in height, as well as the cross-sectional area of thrombi above a threshold distance from the surface (Fig 2), with both factors contributing to reduced volume increase. Thrombus growth was completely prevented when blood that contained either antibody was perfused at 1,500 s−1(Fig 2), which confirms the role of both receptors in linking platelets to one another.
Progression of thrombus growth in 2-stage experiments, first with perfusion of untreated blood over collagen type I fibrils at 1,500 s−1 for 100 seconds, then continuing without interruption at 300 or 1,500 s−1 with blood containing either buffer (Control) or specific monoclonal antibodies, as indicated. Antibodies were incubated in blood for 10 minutes before initiating perfusion. (A) Stacks of z sections labeledA show thrombi formed after 100 seconds of perfusion with blood containing no antibody at wall shear rate of 1,500 s−1; those labeled B show the growth of thrombi after an additional 740 seconds of perfusion at 300 s−1with blood containing either buffer or the indicated antibodies (total perfusion time, 840 seconds). The effect of the antibodies in limiting increase in thrombus height, as clearly seen, is representative of the results observed in all experiments. (B) Thrombus volume was measured at the indicated cumulative perfusion times in these two-stage experiments. Bars are identified according to the type of inhibitor used in the second stage, but perfusion for the first 100 seconds was always performed with untreated blood. The results are expressed as mean ± SEM of between 4 and 10 experiments for the different conditions tested. Note that experiments at the shear rate of 1,500 s−1 were terminated after collecting z sections at 420 seconds, since thrombi had already reached a large volume in control blood. Experiments with the combination of anti-GP Ib and anti-IIbβ3 monoclonal antibodies were not performed at 1,500 s−1 since at this shear rate each individual antibody completely prevented thrombus growth as compared to the volume attained after the initial perfusion for 100 seconds with untreated blood.
Progression of thrombus growth in 2-stage experiments, first with perfusion of untreated blood over collagen type I fibrils at 1,500 s−1 for 100 seconds, then continuing without interruption at 300 or 1,500 s−1 with blood containing either buffer (Control) or specific monoclonal antibodies, as indicated. Antibodies were incubated in blood for 10 minutes before initiating perfusion. (A) Stacks of z sections labeledA show thrombi formed after 100 seconds of perfusion with blood containing no antibody at wall shear rate of 1,500 s−1; those labeled B show the growth of thrombi after an additional 740 seconds of perfusion at 300 s−1with blood containing either buffer or the indicated antibodies (total perfusion time, 840 seconds). The effect of the antibodies in limiting increase in thrombus height, as clearly seen, is representative of the results observed in all experiments. (B) Thrombus volume was measured at the indicated cumulative perfusion times in these two-stage experiments. Bars are identified according to the type of inhibitor used in the second stage, but perfusion for the first 100 seconds was always performed with untreated blood. The results are expressed as mean ± SEM of between 4 and 10 experiments for the different conditions tested. Note that experiments at the shear rate of 1,500 s−1 were terminated after collecting z sections at 420 seconds, since thrombi had already reached a large volume in control blood. Experiments with the combination of anti-GP Ib and anti-IIbβ3 monoclonal antibodies were not performed at 1,500 s−1 since at this shear rate each individual antibody completely prevented thrombus growth as compared to the volume attained after the initial perfusion for 100 seconds with untreated blood.
Synergistic interplatelet adhesive functions of fibrinogen and vWF at arterial shear rates.
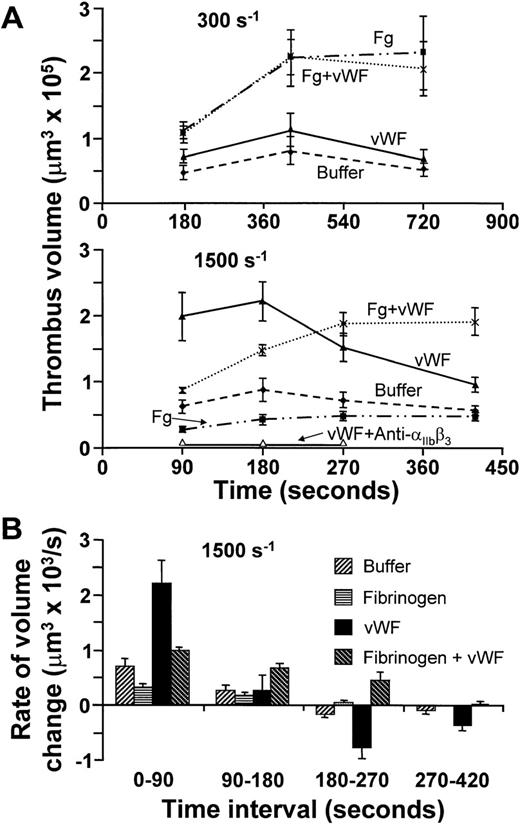
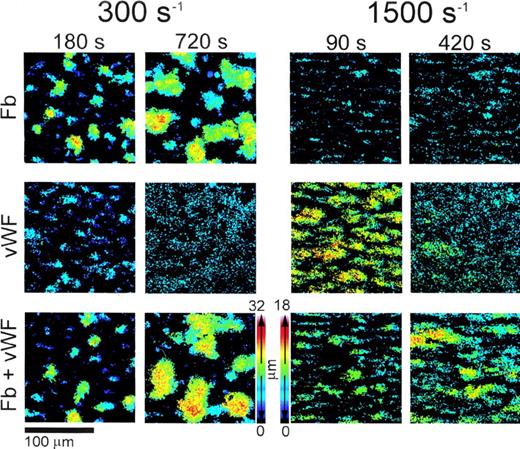
The demonstration that GP Ibα, a vWF receptor,9participates in platelet aggregation along with αIIbβ3, a fibrinogen as well as vWF receptor,24 prompted an investigation on the roles of these ligands in linking platelets to one another. At all shear rates tested, only small thrombi formed in the absence of plasma proteins. Addition of fibrinogen alone considerably increased thrombus volume at 300 s−1, whereas vWF by itself was minimally effective at this shear rate (Fig 3A). When the two proteins were present together, the total volume of aggregated platelets on the surface was similar to that seen with fibrinogen alone (Fig 3A). Different results were observed at 1,500 s−1, since not only did fibrinogen fail to support platelet aggregation, but also vWF by itself promoted the development of large thrombi in spite of increased shear stress (Fig 3A). The apparent enhancement of vWF-mediated platelet cohesion with increasing shear rate is consistent with previous findings demonstrating that vWF binding to platelets, involving both GP Ibα and αIIbβ3, is positively modulated by shear stress.21 However, the platelet aggregates supported by vWF were unstable, reaching their peak volume between 1 and 3 minutes and then decreasing in size (Fig 3A and B). Real-time monitoring and three-dimensional analysis showed that the reduction in volume after formation of large thrombi in the presence of vWF at 1,500 s−1 resulted from progressive detachment of single platelets or small aggregates, causing decreasing height but leaving a platelet monolayer on the collagen substrate (Fig4). Only the concurrent presence of both fibrinogen and vWF resulted in thrombi that resisted the forces generated by rapid flow for at least 7 minutes (Figs 3 and 4). These findings demonstrate that fibrinogen is required for stable platelet aggregation even when hemodynamic conditions prevent the molecule from exerting its adhesive functions directly. The efficient, albeit transient, interplatelet cohesion initiated by vWF provides the synergistic mechanism enabling fibrinogen to play its role at high shear rates.
(A) Time course of platelet thrombus development at wall shear rate of 300 s−1 (top) or 1,500 s−1(middle), obtained using washed blood cells with or without the addition of fibrinogen (Fg) or vWF, either individually or concurrently as indicated. The concentration of fibrinogen was 2 mg/mL, corresponding to the lower limit in normal plasma, but in excess of the amount needed to saturate IIbβ3; that of vWF was 20 μg/mL, approximately twofold higher than in normal plasma to compensate for the loss of larger multimers during purification. All results are reported as mean ± SEM of between four and six experiments for the different conditions analyzed. Open triangles in the middle panel indicate the results obtained with washed cells plus vWF in the presence of 100 μg/mL of the anti-IIbβ3 monoclonal antibody, LJ-CP8. (B) The results at wall shear rate of 1,500 s−1 shown in (A), but expressed as the mean ± SEM of the rate of change in thrombus volume during successive time intervals.
(A) Time course of platelet thrombus development at wall shear rate of 300 s−1 (top) or 1,500 s−1(middle), obtained using washed blood cells with or without the addition of fibrinogen (Fg) or vWF, either individually or concurrently as indicated. The concentration of fibrinogen was 2 mg/mL, corresponding to the lower limit in normal plasma, but in excess of the amount needed to saturate IIbβ3; that of vWF was 20 μg/mL, approximately twofold higher than in normal plasma to compensate for the loss of larger multimers during purification. All results are reported as mean ± SEM of between four and six experiments for the different conditions analyzed. Open triangles in the middle panel indicate the results obtained with washed cells plus vWF in the presence of 100 μg/mL of the anti-IIbβ3 monoclonal antibody, LJ-CP8. (B) The results at wall shear rate of 1,500 s−1 shown in (A), but expressed as the mean ± SEM of the rate of change in thrombus volume during successive time intervals.
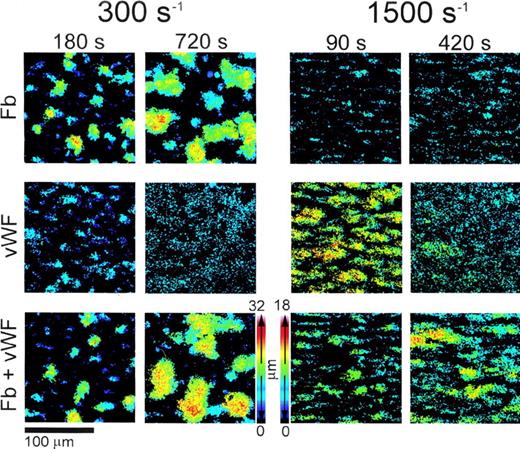
Color rendition of the shape and height of thrombi formed on type I collagen fibrils at different wall shear rates. Washed blood cells and adhesive proteins were used as described in the legend to Fig3. Note that both at 300 s−1 and 1,500 s−1, larger thrombi were present at later perfusion times in the presence of vWF and fibrinogen (Fb) combined than either of the two ligands alone. To obtain these pseudocolor images, stacks of confocal sections taken throughout the height of thrombi at 1-μm intervals in thez-axis were processed after fixing brightness and contrast. Noise was reduced by applying a 3 × 3 median filter, and images were binarized so that the gray scale value of all the pixels above a set threshold was 255. The value of each pixel composing individual images in a shear rate group was then multiplied by a factor, p/N, where p is the plane number (distance from the surface in μm) corresponding to the image, and N is the number of images in the stack including the thrombus of maximum height in that group. After this operation, the value of each pixel in a stack of confocal sections was proportional to the position in the z axis, ie, to height, and could be used to generate single pseudocolor images using for each pixel the maximum calculated intensity value in the stack. Thrombi were larger in the experiments performed at 300 s−1 than at 1,500 s−1 owing to longer perfusion times; thus, distinct height calibration color scales were calculated for the two groups, as shown.
Color rendition of the shape and height of thrombi formed on type I collagen fibrils at different wall shear rates. Washed blood cells and adhesive proteins were used as described in the legend to Fig3. Note that both at 300 s−1 and 1,500 s−1, larger thrombi were present at later perfusion times in the presence of vWF and fibrinogen (Fb) combined than either of the two ligands alone. To obtain these pseudocolor images, stacks of confocal sections taken throughout the height of thrombi at 1-μm intervals in thez-axis were processed after fixing brightness and contrast. Noise was reduced by applying a 3 × 3 median filter, and images were binarized so that the gray scale value of all the pixels above a set threshold was 255. The value of each pixel composing individual images in a shear rate group was then multiplied by a factor, p/N, where p is the plane number (distance from the surface in μm) corresponding to the image, and N is the number of images in the stack including the thrombus of maximum height in that group. After this operation, the value of each pixel in a stack of confocal sections was proportional to the position in the z axis, ie, to height, and could be used to generate single pseudocolor images using for each pixel the maximum calculated intensity value in the stack. Thrombi were larger in the experiments performed at 300 s−1 than at 1,500 s−1 owing to longer perfusion times; thus, distinct height calibration color scales were calculated for the two groups, as shown.
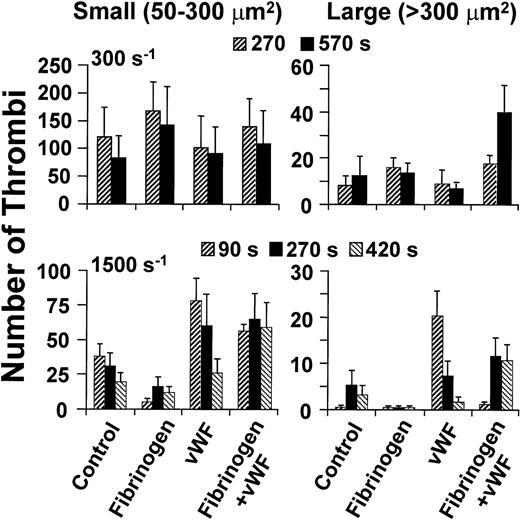
The ability of vWF to form bridges across platelets at wall shear rate of 1,500 s−1 depended not only on GP Ibα (Figs 1and 2), but also on αIIbβ3, as shown by the effect of a function-blocking antibody (Fig 3A) and in agreement with the notion that both receptors bind this ligand concurrently under flow.14,21 The rate of platelet aggregation during the first 90 seconds of perfusion was slower when fibrinogen was added to vWF in excess molar concentration to approximate the situation in normal plasma (Figs 3A and B, and 4). The latter finding reflects the known competition between the two ligands for αIIbβ3 occupancy,28 and confirms the lesser efficacy of fibrinogen in promoting platelet aggregation at high shear rates. At the physiologic concentrations in normal blood, therefore, fibrinogen may limit the amount of vWF bound to αIIbβ3, but the consequent effect of reducing the initial rate of thrombus growth is compensated by increased stability in time. On the other hand, vWF contributed to platelet aggregation in flowing blood even at the relatively low wall shear rates, such as 300 s−1, at which its presence did not increase total thrombus volume (Fig 3A). Indeed, a greater number of larger individual thrombi, rather than more numerous and smaller ones, formed at this shear rate when vWF was present in addition to fibrinogen (Figs 4 and 5). This result reflects enhanced efficiency of adhesive bonds, since platelets must oppose greater hydrodynamic forces (drag) to aggregate into thrombi of increasing size with higher shear rates at the growing edge. A similar function may be supported by vWF released from the α-granules of activated platelets,29 which explains why at the shear rate of 300 s−1 blocking GP Ibα was more inhibitory of thrombus formation (Figs 1 and 2) than removing vWF from the fluid phase (Fig 3A).
Size distribution of thrombi formed on type I collagen fibrils at different wall shear rates. Washed blood cells were perfused after suspension in buffer only (Control) or with the addition of fibrinogen (Fg) or vWF, individually or concurrently as indicated. The concentration of fibrinogen was 2 mg/mL, and the concentration of vWF was 20 μg/ml (see Fig 3). At the indicated time points, a single image was obtained from the arithmetic sum of all those in a confocal series, and the area of thrombi thus projected on the surface was determined. Thrombus size was arbitrarily classified as small when the latter value was less than 300 μm2, and large when it was greater than 300 μm2. Height and shape were not considered in these definitions; thus, the classification is not directly related to volume. The results shown are the mean ± SEM of four to seven experiments performed for each condition tested.
Size distribution of thrombi formed on type I collagen fibrils at different wall shear rates. Washed blood cells were perfused after suspension in buffer only (Control) or with the addition of fibrinogen (Fg) or vWF, individually or concurrently as indicated. The concentration of fibrinogen was 2 mg/mL, and the concentration of vWF was 20 μg/ml (see Fig 3). At the indicated time points, a single image was obtained from the arithmetic sum of all those in a confocal series, and the area of thrombi thus projected on the surface was determined. Thrombus size was arbitrarily classified as small when the latter value was less than 300 μm2, and large when it was greater than 300 μm2. Height and shape were not considered in these definitions; thus, the classification is not directly related to volume. The results shown are the mean ± SEM of four to seven experiments performed for each condition tested.
DISCUSSION
The results of these studies elucidate a novel interpretation of the mechanisms that mediate homotypic platelet aggregation during thrombus development in blood flowing at arterial shear rates. In fact, the long-held contention that fibrinogen and αIIbβ3 have an exclusive role in linking platelets to one another appears to be applicable only to the hemodynamic environment of veins in which, however, these processes may be less directly relevant for hemostasis.1,2,30 Moreover, our findings modify two additional concepts currently held as valid, namely, that vWF may have a role in platelet aggregation but limited to pathologically extreme shear rates,31 and that the functional involvement of fibrinogen decreases as that of vWF becomes manifest.16 On the contrary, our results demonstrate that the two adhesive proteins are complementary and synergistic, as they are both needed to support thrombus development at all levels of arterial flow. This proposed interpretation of the mechanisms of aggregation provides a plausible explanation for the altered hemostatic properties of platelets from patients with isolated congenital deficiency of either fibrinogen32 or vWF.33Because neither protein by itself can sustain the full development of stable thrombi within the range of pathophysiologically relevant flow conditions, hemostasis cannot be normal unless both are present and functional.
The synergy of fibrinogen and vWF in supporting platelet aggregation depends on the recognized ability of each of these molecules to establish bonds with distinct adhesive properties. The initial function of vWF may depend primarily on its rapid rate of association with GP Ibα.8,10 This is particularly important in hemodynamic conditions characterized by high flow rates that increase the velocity differential between adjacent platelets. Subsequent interaction of multimeric vWF with both GP Ibα and activated αIIbβ314,21 may favor optimal propagation of interplatelet contacts, allowing permanent bridging mediated by fibrinogen binding across activated platelet membranes. The latter interaction appears to be crucial owing to the intrinsic stability of the association between fibrinogen and αIIbβ3. In fact, the same distinctive characteristics of adhesive bonds are thought to explain the efficient but transient platelet tethering to immobilized vWF at high shear rates as opposed to the immediate arrest onto fibrinogen (or fibrin) that, however, involves fewer platelets with increasing flow velocity.10 In this respect, therefore, adhesive interactions between the platelet membrane and a thrombogenic surface or between two platelets may depend essentially on the same mechanisms to oppose fluid dynamic forces generated by blood flow. Thus, the threshold shear stress value above which the functions of vWF and GP Ibα become absolutely required for thrombus development may be similar for both heterotypic adhesion of platelets to extracellular matrix components and homotypic aggregation. In fact, the unique biomechanical properties of this bond may support the estimation of approximate shear rate values in areas of blood flow depending on whether blocking vWF binding to GP Ibα results in inhibition of platelet adhesion or aggregation. The results obtained by measuring single platelet adhesion in flow fields with minimal distortion8 10 suggest that a positive finding in this regard may indicate a shear rate value of 1,500 s−1or higher.
Such considerations may explain why blocking GP Ibα binding to vWF negatively affects thrombus growth even when blood is perfused with shear rates that do not prevent normal initiation of platelet adhesion and aggregation.8 Computing shear rate values around thrombi is complicated by distortions in the flow field caused by the growing mass of aggregating platelets. Nevertheless, it is intuitive that in areas at the edge of a thrombus the shear rate will increase as a function of distance from the wall because narrowing of the lumen will cause increased local flow velocity for a constant volumetric flow. In fact, the experimental results shown in Fig 1A indicate that blocking GP Ibα when the initial wall shear rate was 300 s−1 started to impair platelet aggregation at the tip of thrombi 5 to 8 μm high. By analogy with the value at which vWF function is needed to initiate interactions of single platelets on a substrate,8 10 it seems reasonable to assume as a first approximation that inhibition of thrombus growth happened when the local shear rate reached the value of 1,500 s−1.
The results presented here support the conclusion that only the functional integration of bonds with distinct biomechanical properties may provide efficient and stable platelet adhesion with subsequent thrombus development through interplatelet cohesion at normal and pathologic arterial shear rates. These findings, moreover, identify the vWF-GP Ibα interaction as a target for the selective inhibition of platelet aggregation aimed at preventing and treating arterial thrombotic diseases.
ACKNOWLEDGMENT
The authors thank James R. Roberts and Benjamin Gutierrez for their assistance with the productions and characterization of monoclonal antibodies; Richard A. McClintock for the purification and characterization of proteins; and Rachel A. Braithwaite for expert secretarial help.
Supported in part by Grants No. HL-31950, HL-42846, and HL-48728 from the National Institutes of Health (NIH). Additional support provided by NIH Grant No. RR0833 to the General Clinical Research Center of Scripps Clinic and Research Foundation and by the Stein Endowment Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Zaverio M. Ruggeri, MD, The Scripps Research Institute, MEM-175, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail: ruggeri@scripps.edu.