Abstract
Photochemical treatment (PCT) with the psoralen S-59 and long wavelength ultraviolet light (UVA) inactivates high titers of contaminating viruses, bacteria, and leukocytes in human platelet concentrates. The present study evaluated the efficacy of PCT to prevent transfusion-associated graft-versus-host disease (TA-GVHD) in vivo using a well-characterized parent to F1 murine transfusion model. Recipient mice in four treatment groups were transfused with 108 splenic leukocytes. (1) Control group mice received syngeneic splenic leukocyte transfusions; (2) GVHD group mice received untreated allogeneic splenic leukocytes; (3) gamma radiation group mice received gamma irradiated (2,500 cGy) allogeneic splenic leukocytes; and (4) PCT group mice received allogeneic splenic leukocytes treated with 150 μmol/L S-59 and 2.1 J/cm2UVA. Multiple biological and clinical parameters were used to monitor the development of TA-GVHD in recipient mice over a 10-week posttransfusion observation period: peripheral blood cell levels, spleen size, engraftment by donor T cells, thymic cellularity, clinical signs of TA-GVHD (weight loss, activity, posture, fur texture, skin integrity), and histologic lesions of liver, spleen, bone marrow, and skin. Mice in the control group remained healthy and free of detectable disease. Mice in the GVHD group developed clinical and histological lesions of TA-GVHD, including pancytopenia, marked splenomegaly, wasting, engraftment with donor derived T cells, and thymic hypoplasia. In contrast, mice transfused with splenic leukocytes treated with (2,500 cGy) gamma radiation or 150 μmol/L S-59 and 2.1 J/cm2 UVA remained healthy and did not develop detectable TA-GVHD. Using an in vitro T-cell proliferation assay, greater than 105.1 murine T cells were inactivated by PCT. Therefore, in addition to inactivating high levels of pathogenic viruses and bacteria in PC, these data indicate that PCT is an effective alternative to gamma irradiation for prevention of TA-GVHD.
A PHOTOCHEMICAL TREATMENT process (PCT) using the psoralen S-59 and long wavelength ultraviolet light (UVA, 320 to 400 nm) has been developed to inactivate viral and bacterial pathogens in platelet concentrates (PC) while retaining in vitro and in vivo platelet function.1 Because S-59 is nucleic acid specific, T cells contaminating PC preparations are highly susceptible targets for inactivation. Human T cells have been shown to be extremely sensitive to S-59 PCT when evaluated by a sensitive in vitro limiting dilution assay (LDA) and other molecular analyses.2 The concentration of S-59 used to inactivate viruses and bacteria in PC is approximately 3,000-fold higher than that required to inactivate greater than 5 log10 of T cells in a clonogenic T-cell proliferation assay.2
Contaminating leukocytes in PC provide no hemostatic benefit and can cause a number of adverse immune reactions in PC recipients. Donor T cells are known to initiate transfusion-associated graft-versus-host disease (TA-GVHD).3 TA-GVHD, a life-threatening T-cell mediated immune reaction, is 80% to 90% fatal and there is no effective therapy.4 Many PC transfusion recipients are at risk for TA-GVHD. Although the risk is greater for immunocompromised patients, TA-GVHD has been reported in immunocompetent patients as well.5
Although we previously have shown the inactivation of T cells using sensitive in vitro assays, in vivo inactivation of T cells with PCT has not yet been established. In this study we evaluated the efficacy of PCT to prevent TA-GVHD in a well-characterized parent to F1murine transfusion model.6 Affected mice exhibit clinical signs and findings analogous to human TA-GVHD. In this model, parental A mice were used as donors and hybrid offspring B6AF1(C57BL/6 × A) mice as recipients. Strain A donor mice are homozygous at the H-2 locus and the recipient B6AF1 mice are heterozygous. Donor A cells recognize the B6 antigens on recipient cells as foreign and initiate acute TA-GVHD, while the recipient B6AF1 host cells recognize the donor cells as self and fail to reject them. Transfusion of 1.0 × 108 leukocytes results in acute TA-GVHD that closely resembles human TA-GVHD. PCT was compared with gamma radiation, the current method of TA-GVHD prophylaxis, for its ability to prevent TA-GVHD in this model. The dynamic range of murine T-cell inactivation also was determined using an in vitro T-cell proliferation assay.
MATERIALS AND METHODS
Mice.
Strain A and B6AF1 mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were housed in rodent HEPA-vented condominiums with filter top cages and autoclaved bedding (Northeastern Products Corp, Columbia City, OR). Mice were maintained on a diet of gamma radiation sterilized mouse chow (PMI Nutritional International, St Louis, MO) and autoclaved water. Bedding and water were changed daily. Donor (A) and recipient (B6AF1) mice were 8 to 12 weeks old at the time of transfusion.
Preparation of donor splenic leukocytes (splenocytes).
Donor mice, A or B6AF1, were anesthetized with metophane (Mallinkrodt, Chicago, IL) and killed by cervical dislocation. For each transfusion, 2 to 3 spleens from donor mice were used. The spleens were removed under aseptic conditions and teased through 70-μm nylon mesh filters in 5 mL of isotonic phosphate buffered saline (PBS) supplemented with 1% bovine serum albumin (PBS/1% BSA). Five mL of splenocyte suspension was overlayed onto 5 mL of lympholyte M (Accurate Chemical, Westbury, NY) and centrifuged at 1,000g for 20 minutes at 22°C. The mononuclear cells were isolated and washed twice with PBS 1% BSA (250g, 10 minutes).
Gamma irradiation.
One third of the donor A splenocytes were resuspended in 30 mL of PBS/1% BSA and transferred into small PL 2410 plastic containers (Baxter Healthcare Corp, Fenwal Division, Deerfield, IL). The splenocytes were transported on ice (4°C) to the Alameda Contra Costa Blood Bank and irradiated with 2,500 cGy (Nordion Gamma Cell-1000 Irradiator; Nordion Inc, Kanata, Ontario, Canada). The irradiated cells were pelleted by centrifugation at 250g for 10 minutes at 22°C and resuspended in PBS/1% BSA to a final concentration of 1.0 × 108 cells in 200 μL to 400 μL.
Photochemical treatment.
One third of the donor A splenocytes were resuspended in 30 mL of PBS/1% BSA and transferred into small PL 2410 plastic containers. The volume to surface ratio resulted in a fluid layer of approximately 1 cm. The psoralen S-59 (Cerus Corp, Concord, CA) was added to the splenocyte suspension to a final concentration of 150 μmol/L. The structure and synthesis of S-59 has been described.7 The splenocytes were illuminated with 2.1 J/cm2 of UVA on a UVA illumination device (Baxter Healthcare Corp). The fluence of the UVA device was 15 to 20 mW/cm2. A dose of 2.1 J/cm2was delivered in approximately 2 to 3 minutes. After PCT, the treated cells were pelleted by centrifugation at 250g for 10 minutes at 22°C and resuspended in PBS/1% BSA to a final concentration of 1.0 × 108 cells in a final volume of 200 μL to 400 μL.
Transfusion.
The splenocytes were counted on a hematology analyzer (Biochem Immunosystems, Allentown, PA). Approximately 1.0 × 108cells were transfused through the lateral tail veins of recipient mice anesthetized with metophane. Donor splenocytes were transfused into recipients according to the four experimental groups (Table1).
Peripheral blood cell counts.
One day before transfusion and weekly after transfusion, blood samples were obtained from recipient mice by retro-orbital venipunture. Capillary tubes (Biochem Immunosystems) were used to collect 40 μL of blood that was immediately diluted into 10 mL Haema Line Diff Silos (Biochem Immunosystems). White blood cell (WBC), red blood cell (RBC), and platelet (PLT) levels were enumerated with an automated hematology analyzer (Biochem Immunosystems).
Clinical scores.
One day before transfusion and weekly after transfusion, recipient mice were weighed and scored for clinical signs of TA-GVHD as previously described.8 Body weight, posture, activity, fur texture, and skin integrity were scored from 0 to 2 for a total possible score of 10.
Assessment of splenomegaly.
Spleens of recipient mice were removed 2 to 3 weeks after transfusion. Spleens were weighed in preweighed tubes containing PBS. The spleen index is defined as (spleen weight/body weight) × 1,000.
Assessment of donor T-cell engraftment.
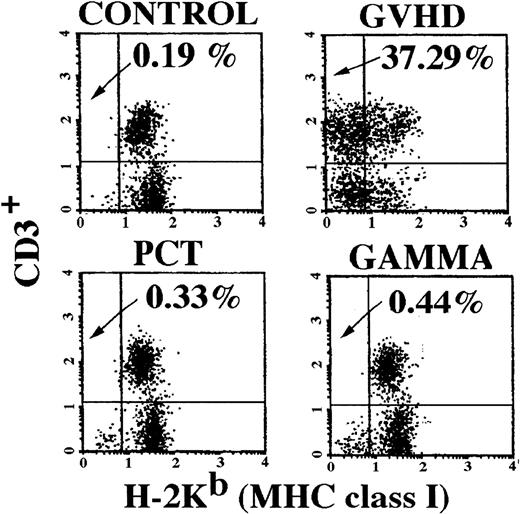
Recipient spleens were removed 2 weeks after transfusion, processed into single-cell suspensions by teasing through a 70-μm nylon mesh screen in PBS, and prepared for two-color flow cytometric analysis. Splenocytes (1.0 × 106 cells) were labeled with 1.0 μg of anti-CD3-PE (pan T cell) and anti-H-2Kb-FITC (recipient major histocompatibility complex [MHC] class I) antibodies (Pharmingen, San Diego, CA) for 30 minutes on ice. The cells were then washed by centrifugation (250g for 10 minutes at 22°C) and resuspended in 1.0 mL of PBS. The cells were analyzed on a flow cytometer with lysis II software (Becton Dickinson, San Jose, CA) using forward and side scatter to gate on the leukocytes. A dot plot of 1.0 × 104 cells was generated with FL1 (horizontal axis) measuring fluorescence intensity of recipient MHC class I H-2Kb+ cells and FL2 (vertical axis) measuring fluorescence intensity of CD3+ T cells (Fig 1). Cells in the upper left quadrant, positive for CD3 and negative for H-2Kb, were classified as donor T cells and were quantified as a percentage of the total number of cells analyzed.
Engraftment of donor T cells in spleens of recipient mice. Analysis of recipient spleens for the presence of donor-derived T cells was performed 2 weeks after transfusion. Splenocytes were analyzed using two-color flow cytometry with antibodies for CD3+ cells on the vertical axis and recipient specific MHC class I H-2b on the horizontal axis. Donor T cells appear in the upper left-hand quadrant. Spleens of two mice in each group were analyzed and the figure shown is representative of both mice in their respective group. As expected, mice in the control group had no allogeneic donor T cells present in their spleens 2 weeks after transfusion. Mice in the GVHD group had 37% donor-derived T cells. Mice in the PCT and Gamma groups had no evidence of donor-derived T cells in their spleens, similar to the control group.
Engraftment of donor T cells in spleens of recipient mice. Analysis of recipient spleens for the presence of donor-derived T cells was performed 2 weeks after transfusion. Splenocytes were analyzed using two-color flow cytometry with antibodies for CD3+ cells on the vertical axis and recipient specific MHC class I H-2b on the horizontal axis. Donor T cells appear in the upper left-hand quadrant. Spleens of two mice in each group were analyzed and the figure shown is representative of both mice in their respective group. As expected, mice in the control group had no allogeneic donor T cells present in their spleens 2 weeks after transfusion. Mice in the GVHD group had 37% donor-derived T cells. Mice in the PCT and Gamma groups had no evidence of donor-derived T cells in their spleens, similar to the control group.
Assessment of TA-GVHD induced immune suppression.
GVHD-induced immune suppression, measured by thymic cellularity, was quantified 2 to 3 weeks after transfusion. At the time of death, thymus glands were removed from recipient mice and macerated between the frosted surfaces of two glass microscope slides. The thymocytes were resuspended in 5 mL of PBS with 1% BSA and thoroughly mixed. The cells were filtered through 70-μm nylon filters to obtain single cell suspensions. The number of thymocytes was quantified using an automated hematology analyzer (Biochem Immunosystems).
Histology.
Sections of liver, spleen, skin, and bone marrow were removed at various times after transfusion. Each section was preserved in formalin (Shandon, Pittsburgh, PA) embedded in paraffin, mounted on slides, and stained with hematoxylin and eosin. The slides were scored in blinded fashion by a single observer using a scoring system (Table2). Histology data were obtained from three separate experiments (Table 3) over 6 months using a total of 30 mice. The number of mice in each treatment group ranged from 6 to 11.
Statistical analysis.
Data for the spleen index, WBC counts, RBC counts, PLT counts, donor T-cell engraftment, and thymic counts were analyzed by Student’st-test for significant differences between the control group and the test groups. A P value of <.05 was considered significant.
In vitro inactivation of murine T cells.
Using the same murine strain combinations as for the preceding in vivo experiments, T-cell inactivation by PCT was determined by an in vitro T-cell proliferation assay. Assays were performed in U-bottom 96-well microtiter plates. Mixed lymphocyte cultures were prepared using splenocytes from B6AF1 mice as stimulators and splenocytes from A mice as responders. Donor splenocytes (strain A) were prepared and subjected to PCT as described above.
Two experiments were conducted. Stimulator cells were irradiated with 2,000 cGy to prevent proliferation and 2 × 105 cells were plated per well. In one experiment (experiment 4), untreated donor responder cells were plated at levels of 20, 40, and 80 cells per well (24 wells per cell level) containing stimulator cells. Approximately 1 × 105 PCT donor responder cells were plated into 80 wells containing stimulator cells. In another experiment (experiment 5), untreated donor responder cells were plated at levels of 200, 400, and 800 cells per well (32 wells per cell level). PCT donor responder cells were plated at levels of 5 × 104 and 1 × 105 per well (32 wells per cell level).
The mixed splenocytes were cultured in a volume of 200 μL of RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, nonessential amino acids, 5 × 10−5 mol/L 2-mercaptoethanol, penicillin and streptomycin, lipopolysaccharide (Sigma Chemical Co, St Louis, MO), 2.5 μg/mL Con A, and 40 units/mL hIL2. Cells were cultured in humidified 5% CO2 at 37°C for 7 days and pulsed with 1 μCi/well of 3H-thymidine (5 Ci/mg; Amersham) for the last 12 to 18 hours of culture. On day 8, cells were harvested onto glass fiber filters with an automated cell harvester (Cambridge Technology, Cambridge, MA). Incorporated radioactivity was measured by scintillation counting. Wells were scored either positive or negative. Only wells that were three standard deviations above the background were scored positive. The T-cell frequencies and the log10T-cell reduction were calculated by minimum chi-square analysis based on a Poisson distribution as previously described.2
RESULTS
Overview.
Three separate experiments were performed using a total of 40 mice as transfusion recipients. Separate experiments were conducted to assess the spectrum of parameters associated with TA-GVHD. Both biological and clinical parameters were measured to monitor the development and severity of TA-GVHD in transfusion recipients. The number of recipient mice used per study group and the time post-transfusion when each biological measurement was made were summarized (Table 3).
Experiment 1 was designed to measure biological parameters and clinical scores for up to 3 weeks after transfusion. Peripheral blood cell levels (WBC, RBC, and PLT) were monitored weekly. Clinical scores including body weight, skin integrity, fur texture, activity, and posture were scored weekly. On week 3, mice were killed. Thymic cellularity and splenomegaly were evaluated. Sections of liver, skin, spleen, and bone marrow were analyzed and scored for histopathologic evidence of TA-GVHD.
In experiment 2, the recipient mice were observed for up to 10 weeks after transfusion. All but two mice survived the 10-week evaluation period. Two mice in the GVHD group died 1 to 2 weeks after transfusion because of severe TA-GVHD. Weekly peripheral blood cell levels and clinical scores were obtained. Mice were killed on week 10 for preparation of tissue sections and scored for histopathologic evidence of TA-GVHD.
In experiment 3, two mice from each study group were killed on week 2 for analysis of splenomegaly, thymic cellularity, and engraftment of donor T cells. The remaining mice in each study group were monitored for a total of 9 weeks for weekly measurement of peripheral blood cell levels and clinical scores. Mice were killed on week 9 for tissue histopathologic evaluation of TA-GVHD.
The data from each experiment were pooled for analysis. Statistical comparisons were made among the four study groups (control, GVHD, gamma, and PCT).
Splenomegaly.
Splenomegaly has been shown to be a reliable measure of the severity of GVHD (Table 4).9Mice in the control group received syngeneic splenic leukocyte transfusions and had normal spleen sizes with an average spleen index of 3.67 ± 0.2 (SE). Mice in the GVHD group had grossly enlarged spleens with an average spleen index of 18.75 ± 1.3. Mice that received PCT or gamma-irradiated splenocyte transfusions, however, had spleen indices comparable with the control, 3.66 ± 0.3 and 3.64 ± 0.4, respectively. The spleen indices for the PCT, gamma, and control groups were not significantly different from each other (P > .05). The GVHD group was statistically different (P < .05) from the PCT, gamma, and control groups.
Donor T-cell engraftment.
Proliferation of donor T cells is a key initiating event in the onset of TA-GVHD.4 The number of donor T cells in recipient spleens was measured by flow cytometric analysis. This assay used the MHC class I difference between donor and recipient mice to identify cell phenotype. Cells in the upper left quadrant of the dot plot (Fig1) represent donor-derived T cells (CD3+, Kb−). Mice in the control group that received syngeneic splenocyte transfusions contained no evidence of donor T cells. Mice in the GVHD group that received untreated allogeneic splenocyte transfusions had an average ± standard error of 34.34% ± 2.2% (Table 4) donor T cells in their spleens 2 to 3 weeks after transfusion, indicative of GVHD.9 Additionally, they also contained donor-derived non-T cells typical of GVHD (lower left quadrant). The control group and mice that received PCT or gamma radiation-treated splenocytes did not have a significant percentage of donor T cells in their spleens. These results indicate that PCT or gamma irradiation protected mice from engraftment by viable donor T cells.
Thymic cellularity—Immune suppression.
Immune suppression is a well-documented sign of TA-GVHD.9Thymic cellularity has been used to measure the severity of GVHD in murine systems. For each experimental group, the average number of thymocytes ± the standard error was measured 2 to 3 weeks after transfusion. In this study, mice in the GVHD group had consistently low numbers of thymocytes (1.2 ± 0.2 × 107 cells) in their thymus glands 2 to 3 weeks after transfusion, indicative of an immunosuppressed state (Table 4). Mice in the control, PCT, or gamma groups had normal thymic cellularity, 5.5 ± 0.9 × 107 cells, 3.8 ± 0.6 × 107 cells, and 3.8 ± 0.6 × 107 cells, respectively. Thymic cellularity of mice in the GVHD group was statistically different from thymic cellularity in the PCT, gamma, and control groups when analyzed by Student’s t-test (P < .05). Although thymic cellularity of the PCT and gamma groups was less than that of the control group, this difference was not statistically significant (P > .05).
Peripheral blood cell levels.
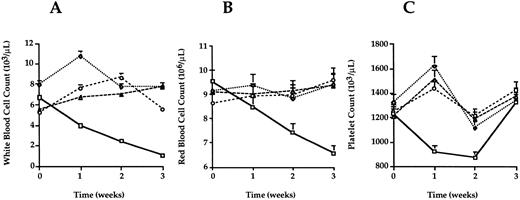
Peripheral blood samples of each mouse were obtained the day before donor leukocyte transfusion and on a weekly basis after transfusion. WBC counts, RBC counts, and PLT counts were monitored up to 10 weeks after transfusion or until the mice were killed for other analyses. Data for each group up to 3 weeks after transfusion are shown (Fig2). Peripheral blood cell levels in the syngeneic transfusion control mice remained stable for the course of the study. Mice that received PCT- or gamma-treated splenocyte transfusions had peripheral blood cell counts that were not significantly different from the controls (P > .05). In contrast, the peripheral blood cell counts of all three hematopoietic lineages were significantly less (P < .05) in the GVHD group. Three weeks after transfusion, PLT counts in the GVHD group recovered. This rebound coincided with an increase in megakaryocytes observed in spleen sections of these mice. In mice, when the bone marrow becomes aplastic, the spleen can become a site of hematopoiesis.10 This observation suggests the platelet counts in the GVHD group rebounded because of splenic hematopoiesis, but these mice had persistent acute GVHD documented in other tissues.
Peripheral blood cell counts. Peripheral blood was sampled from each mouse 1 day before and weekly after transfusion until death. Average counts for WBC (A) RBC (B) and PLT (C) were plotted for each week. Error bars represent the standard error for each group. Mice in the GVHD group (squares) developed pancytopenia 2 to 3 weeks after transfusion. Levels of WBC, RBC, and PLT for mice in the control (diamonds), PCT (triangles), and gamma (circles) groups were not statistically different at any time after transfusion (P > .05) when analyzed by Student’s t-test.
Peripheral blood cell counts. Peripheral blood was sampled from each mouse 1 day before and weekly after transfusion until death. Average counts for WBC (A) RBC (B) and PLT (C) were plotted for each week. Error bars represent the standard error for each group. Mice in the GVHD group (squares) developed pancytopenia 2 to 3 weeks after transfusion. Levels of WBC, RBC, and PLT for mice in the control (diamonds), PCT (triangles), and gamma (circles) groups were not statistically different at any time after transfusion (P > .05) when analyzed by Student’s t-test.
Body weight.
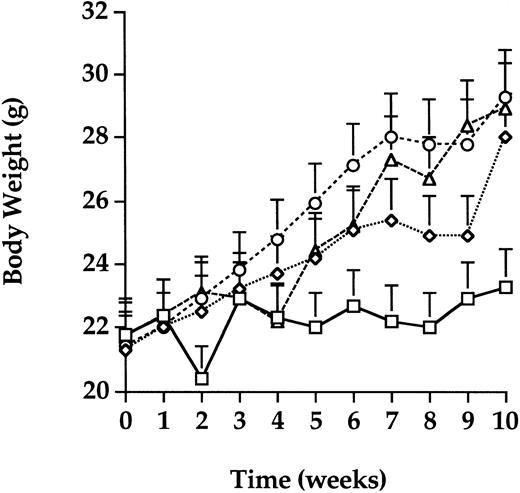
The average body weight of mice in each group over a 10-week period after transfusion, was determined (Fig 3). Mice in the GVHD group gained an average of only 1 g of body weight in the 10-week period consistent with active TA-GVHD. Mice in the control, PCT, and gamma groups gained an average of 7 g of body weight as expected for healthy mice of this age. The body weights of mice in the control, gamma, and PCT groups were not significantly different from each other (P > .05) throughout the observation period. In contrast, after week 4, mice in the GVHD group weighed significantly less (P < .05) than all other groups.
Body weight. Body weight was monitored on a weekly basis for each transfusion group. The average of body weight in the control group (diamonds) increased by 7 g as expected for healthy mice of this age. Mice in the PCT (triangles) and gamma (circles) groups also gained weight, similar to the control group. Mice in the GVHD (squares) group that received untreated transfusions failed to gain weight, an observation consistent with the development of TA-GVHD. Error bars represent the standard error for each group.
Body weight. Body weight was monitored on a weekly basis for each transfusion group. The average of body weight in the control group (diamonds) increased by 7 g as expected for healthy mice of this age. Mice in the PCT (triangles) and gamma (circles) groups also gained weight, similar to the control group. Mice in the GVHD (squares) group that received untreated transfusions failed to gain weight, an observation consistent with the development of TA-GVHD. Error bars represent the standard error for each group.
Clinical scores.
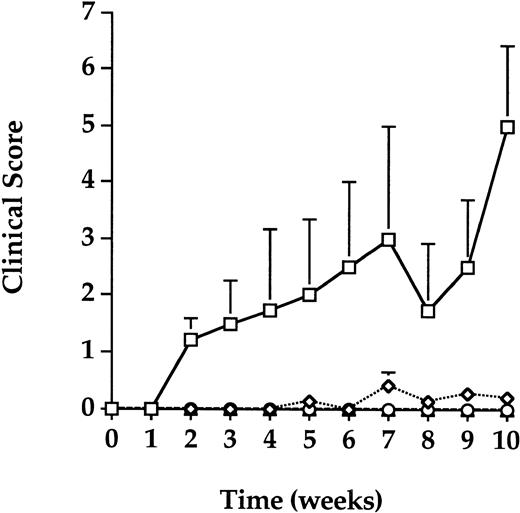
Recipient mice were monitored for clinical signs of TA-GVHD 1 week before transfusion and weekly after transfusion for up to 10 weeks. Body weight, posture, activity, fur texture, and skin integrity were scored on a scale from 0 to 2 for severity with a total possible cumulative score of 10 (Table 2). The average and standard error for each group at each week was calculated (Fig 4). Similar to the syngeneic controls, mice that received gamma- or PCT-treated splenocyte transfusions remained healthy and free of clinical signs of TA-GVHD. The average cumulative clinical scores for these groups remained below 1.0 for the duration of the study. Mice that received untreated allogeneic splenocyte transfusion in the GVHD group, however, displayed progressively more severe signs of TA-GVHD as indicated by the increase in clinical scores over time. Mice in the GVHD group had significantly higher scores (P < .05) than all other groups at several time points; progressive mortality in the GVHD group, however, reduced the number of evaluable mice over time and contributed to increased variance. The trend for mice in the GVHD was indicative of increasingly worse clinical scores while mice in all other groups exhibited no clinical deterioration. The average cumulative clinical score for the GVHD group was 5.0 (out of a maximal score of 10) 10 weeks after transfusion.
Clinical scores. Clinical assessments of body weight, activity, posture, fur texture, and skin integrity were scored weekly. The average and standard deviations of weekly clinical scores over a 10-week period after transfusion for each group were calculated. The error bars represent the standard error for each weekly average. Mice in the GVHD (squares) group had progressively higher clinical scores indicative of TA-GVHD, while mice in the control (diamonds), PCT (triangles), and gamma (circles) groups remained healthy and free of clinical signs of TA-GVHD.
Clinical scores. Clinical assessments of body weight, activity, posture, fur texture, and skin integrity were scored weekly. The average and standard deviations of weekly clinical scores over a 10-week period after transfusion for each group were calculated. The error bars represent the standard error for each weekly average. Mice in the GVHD (squares) group had progressively higher clinical scores indicative of TA-GVHD, while mice in the control (diamonds), PCT (triangles), and gamma (circles) groups remained healthy and free of clinical signs of TA-GVHD.
Histology.
The liver, spleen, bone marrow, and cheek (skin) tissue sections of selected animals from each group of transfusion recipients were evaluated for histologic evidence of GVHD (Table 5). Mice that received PCT- or gamma-irradiated splenocyte transfusions did not develop histologic lesions characteristic of TA-GVHD. They had average cumulative scores of 1.2 ± 0.5 and 1.6 ± 0.7, respectively, out of a total possible cumulative histologic score of 13. Histology scores of mice in the PCT and gamma groups were not significantly different when analyzed by Student’s t-test (P > .05) from mice in the control group (1.0 ± 0.3) that received syngeneic transfusions. In contrast, mice in the GVHD group that received untreated allogeneic spleen-cell transfusions developed severe histologic abnormalities indicative of TA-GVHD. The average cumulative score for the GVHD group was 9.0 ± 0.6. Scores of the GVHD group were statistically different from the control, PCT and gamma groups (P < .05).
In vitro murine T-cell inactivation.
The extent of T-cell inactivation by PCT was measured in two experiments (Table 6). Untreated donor T cells in both experiments showed viability at a frequency of 1/51 and greater than 1/61 T cells. After PCT, donor T cells showed viability at a frequency of less than 1/7,950,093 and less than 1/4,759,209 T cells, respectively. The levels of T-cell inactivation by PCT were estimated at greater than 105.2 for Experiment 4 and greater than 104.9 for Experiment 5, with a mean inactivation of greater than 105.1 T cells.
DISCUSSION
Gamma irradiation of PC is the current method used for TA-GVHD prophylaxis for platelet transfusion. Many years of clinical use have shown that gamma irradiation can prevent TA-GVHD. An in vitro LDA that measures T-cell viability indicated that 2,500 cGy was required to inactivate at least 105 T cells.11 The LDA has shown that the dose response curve for gamma irradiation T-cell inactivation is steep. A fivefold decrease in the dose of gamma (from 2,500 cGy to 500 cGy) affected the T-cell inactivation efficacy. Only 101.1-1.3 T cells were inactivated by treatment with 500 cGy of gamma.11 Although infrequent, failure of gamma irradiation to prevent TA-GVHD has been reported with doses of 1,500 to 2,000 cGy.12 In contrast, the S-59 concentration used in PCT can be reduced 3,000-fold while maintaining high levels of T-cell inactivation. The combination of 150 μmol/L S-59 and 3 J/cm2 UVA used to inactivate viruses and bacteria in PC was shown to inactivate greater than 105.4T-cells.2 A 3,000-fold decrease in the dose of S-59 (from 150 μmol/L to 0.05 μmol/L) did not affect the T-cell inactivation efficacy by PCT. The combination of 0.05 μmol/L S-59 and 1 J/cm2 UVA inactivated (to the limit of detection) greater than 104.1 T cells.2
PCT has the potential to greatly improve the safety of platelet transfusion by inactivating viruses and bacteria contaminating PC and potentially blood-borne pathogens that remain undetected. Clinical studies in humans have shown that PCT of PC results in adequate retention of in vivo platelet recovery and lifespan.13 The present study suggests that PCT can be used independently of gamma irradiation for the prevention of TA-GVHD. Because PCT has a large dose range for the inactivation of T cells, it offers the potential for a new robust means to prevent TA-GVHD. Thus, viral and bacterial inactivation by PCT, and TA-GVHD prophylaxis could be achieved with a single procedure.
Cytokines secreted by leukocytes that accumulate during PC storage have been shown to cause febrile nonhemolytic transfusion reactions.14 Using conditions described in this report, PCT inhibited cytokine accumulation during PC storage.2,15 This is not unexpected, given the mechanism of S-59 plus UVA photochemistry with modification of leukocyte DNA at a frequency of one adduct per 83 base pairs, effectively inhibiting nucleic acid synthesis and transcription.2 Gamma irradiation before PC storage, at the doses used for TA-GVHD prohylaxis, only partially inhibited cytokine accumulation during PC storage.15 Therefore, inhibition of cytokine synthesis during storage of PC is another potential clinical benefit that PCT can provide to PC transfusion recipients.
Donor leukocytes in PC potentiate HLA alloimmunization that can lead to platelet transfusion refractoriness in recipients of multiple PC transfusions.16-18 In vitro studies have shown that leukocytes treated with 8-methoxypsoralen (8-MOP) and UVA are unable to stimulate proliferation of untreated allogeneic leukocytes in mixed lymphocyte reaction assays.19 Other studies have shown that contaminating T cells in PC treated with 4′-aminomethyl 4,5′8-trimethylpsoralen and UVA cannot upregulate the early activation antigen CD69 when stimulated with phorbyl myristate ester.20 These results suggest that PCT treated leukocytes are unlikely to become activated or serve as effective antigen-presenting cells in vivo, a pathway thought to be important for donor recognition followed by host alloantibody production. Furthermore, in vivo studies have shown that treatment with 8-MOP and UVA of allogeneic mouse PC containing leukocytes reduced alloimmunization in recipient mice.21
The current study augments previous investigations using in vitro biological and molecular assays to show inactivation of leukocytes by S-59 and UVA.2 Mice that received lymphocyte transfusions treated with S-59 and UVA remained healthy and free of detectable TA-GVHD, comparable with mice that received gamma-treated lymphocyte transfusions. The dynamic range of T-cell inactivation using the in vivo experimental murine model was limited by the number of T cells that could be transfused intravenously. This limitation arose because of the increased viscosity of the highly concentrated leukocyte suspensions required for induction of TA-GVHD. To show a greater range of T-cell inactivation, we utilized an in vitro T-cell proliferation assay, similar to our prior study with human T cells, to expand the dynamic range of PCT inactivation. With this in vitro assay, we were able to show a greater than 105.1-fold reduction in viable murine T cells. This level of inactivation was similar to our previous data with human T cells. Thus, the in vitro inactivation data, in conjunction with the in vivo data, further indicate the potential of PCT for prophylaxis of TA-GVHD in addition to inactivation of contaminating viruses and bacteria in PC.
ACKNOWLEDGMENT
We thank Prof John E. Hearst for his constant encouragement and valuable suggestions throughout this study, Dr Chu Lin for his supervision of animal care, Margaret Rheinschmidt for her assistance in the fluorescence-activated cell sorter analysis of antibody-labeled mouse leukocytes, Dr Peyton Metzel for providing the UVA illumination device and disposables, Dr Don Buchholz for reviewing the manuscript, and John Hull for reviewing the experimental data. We also thank the Blood Bank of Alameda Contra Costa County for making the Nordion Gamma Cell-1000 Irradiator available for gamma irradiation of cellular samples.
Supported in part by Grants No. HL 3340 and CA 34952 from the National Heart, Lung and Blood Institute. Presented in part at the American Society of Hematology, December 1996.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Lily Lin, PhD, Cerus Corporation, 2525 Stanwell Dr, Concord, CA 94520.