Abstract
Chronic lymphocytic leukemia (CLL) shows a remarkably heterogeneous clinical outcome; survival ranges from several months in advanced stages to more than 10 years in early stages. The Binet and Rai staging systems distinguish three major prognostic subgroups, but do not accurately predict the individual risk of disease progression in early CLL (Binet stage A or Rai stage 0 to II). Because most newly diagnosed CLL patients present with early disease, it seems desirable to search for additional prognostic factors to identify early CLL patients at high risk of rapid progression. It has been shown that elevated serum thymidine kinase (s-TK) levels predict disease progression in CLL. Therefore, this study aimed to assess the prognostic value of s-TK in 122 previously untreated patients with Binet stage A CLL (mean age ± SD, 58.7 ± 8.5 years). In univariate analyses, 18 of the 22 parameters investigated predicted progression-free survival (PFS). In a stepwise multiple regression analysis, only three parameters provided independent prognostic information on PFS: s-TK greater than 7.1 U/L; presence of lymphadenopathy; and white blood cell (WBC) count greater than 75,000/μL. When added to the classification of smoldering versus nonsmoldering CLL, s-TK levels separated two groups within the group of nonsmoldering stage A patients: patients with s-TK values greater than 7.1 U/L had a median PFS of 8 months, whereas patients with s-TK values ≤ 7.1 U/L expected a much longer PFS (49 months; P < .001), similar to smoldering CLL (42 months). The results demonstrate that s-TK is a prognostic parameter that adds independent prognostic information to the definitions of smoldering and nonsmoldering CLL in Binet stage A.
CHRONIC LYMPHOCYTIC LEUKEMIA (CLL) is a markedly heterogeneous disease with regard to its prognosis and clinical course. Several staging systems have been proposed1-3 that identify three major prognostic subgroups and guide the treatment decision. There is general agreement that only patients with advanced Binet or Rai stages require chemotherapy.4-6 Patients with early CLL, ie, Binet stage A or Rai stages 0 to II, do not usually receive treatment until progression. However, approximately 30% to 40% of patients with early CLL show a short progression-free survival (PFS)5 and might benefit from early and/or intensified treatment. Unfortunately, the current staging systems for CLL are not able to identify these early CLL cases at high risk for progression.7 Therefore, this study aimed to investigate whether novel prognostic factors might assist in the identification of a high-risk category of early CLL.
It has been shown that elevated levels of serum thymidine kinase (s-TK) predict a high risk of disease progression in low-grade non-Hodgkin’s lymphoma and CLL.8-14 In CLL and immunocytoma, s-TK seems to provide prognostic information that is independent of the Binet staging system.15 The present prospective clinical study investigated the prognostic value of s-TK in 122 patients with previously untreated early CLL (Binet stage A, Rai stage 0 to II). The results demonstrate that s-TK is a parameter that adds independent prognostic information to the definitions of smoldering and nonsmoldering CLL in Binet stage A.
MATERIALS AND METHODS
Patients.
Between January 1987 and October 1992, 122 previously untreated patients with CLL Binet stage A who presented to one of the four participating study centers (Medizinische Klinik, Universität München, and I. Medizinische Klinik, Technische Universität München; Städtisches Krankenhaus München-Schwabing; Klinikum Benjamin Franklin, Freie Universität Berlin, Germany) were included. Informed consent was obtained from all patients. Histopathologic diagnosis of CLL was established from bone marrow or lymph node biopsies according to the Kiel classification.16-18 Bone marrow histology was obtained from 106 patients at diagnosis. Several patients were included in the study some time after the diagnosis was established. The mean interval ± SD from diagnosis to inclusion in the study was 30.8 ± 5.75 months. There was a male preponderance of 71:51 (Table1). Mean age ± SD was 58.7 ± 8.5 years (Table 1). Staging was performed according to the Binet staging system, and only patients with Binet stage A were included in the study.1 Eighteen patients (15%) were treated with interferon-α after inclusion in the study on a randomized multicenter trial.19 The median observation time of all patients evaluable by October 1996 was 36 months. Further characteristics of the patients are listed in Table 1.
Staging and other tests.
Routine laboratory studies of these patients were performed at inclusion in the study and consisted of complete blood cell count with differential, platelet count, and blood chemistry, including serum lactate dehydrogenase (s-LDH). Peripheral blood mononuclear cells were analyzed by immunophenotyping to establish the diagnosis of typical CD5+ CLL. At inclusion in the study, a complete physical examination, chest x-ray, and abdominal ultrasound were performed. A bone marrow biopsy was performed at study entry in 106 patients (Table1). Computed tomographic scans of the abdomen and pelvis were performed if clinical symptoms required a more thorough investigation of thoracic or abdominal organs. The physical performance status was determined according to the Eastern Cooperative Oncology Group (ECOG) score. Sera from patients obtained at inclusion in the study were stored at −20°C until further analysis for s-TK and serum β2-microglobulin (s-β2M). The TK assay was performed with a commercially available radioenzyme assay (Prolifigen; Sangtec Medical, Bromma, Sweden).20 The TK activity is expressed in units per liter. Control values of s-TK as determined in 22 healthy young adults were 3.8 ± 0.9 U/L (mean ± SD; range, 2.2 to 6.0). For TK values, the intraassay variability was between 5.4% and 7.5%, and the interassay variability between 5.7% and 8.3%. S-β2m levels were determined with a radioimmunoassay (Isotopen Diagnostik CIS GmbH, Dreieich, Germany).
Prognostic parameters.
The following prognostic parameters were evaluated at inclusion in the study: age, sex, performance status (ECOG score), white blood cell (WBC) count, peripheral blood lymphocyte count, peripheral blood neutrophil count, platelet count, blood hemoglobin, s-LDH, s-β2M, s-TK, serum creatinine, presence of lymphadenopathy, time from diagnosis to inclusion in the study, serum immunoglobulin levels (IgA, IgM, and IgG), presence of hepatomegaly, presence of splenomegaly, bone marrow histology (nodular vnonnodular infiltration), Rai stage, and lymphocyte doubling time according to the method of Montserrat et al.21 Because some patients were treated with interferon-α 2b in a randomized trial,19 interferon-α treatment was also analyzed as a prognostic variable.
Evaluation of disease progression.
It has been shown that the time to disease progression has an important impact on survival of early-stage CLL patients.22Therefore, this parameter can be used to shorten the duration of clinical studies in early CLL (Binet stage A or Rai stages 0 to II), because it is a valuable surrogate end point for overall survival. Disease progression was assessed as described.19Progressive disease was defined by an increase of the size of lymph nodes or liver or spleen by greater than 50%, the appearance of new enlarged nodes or new hepatomegaly/splenomegaly, the increase of peripheral lymphocyte counts to greater than 100,000/μL, transition from stage A to B or C, or transformation to prolymphocytic leukemia or high-grade lymphoma (Richter’s syndrome). Follow-up examinations were scheduled every 3 months in the first year after inclusion in the study or more often if the clinical situation indicated a rapid disease progression. After the first year, the follow-up interval was 6 months.
Statistics.
Statistical analysis was performed with the SPSS program version 7.5 (SPSS Inc, Chicago, IL). PFS was estimated by the method of Kaplan and Meier.23 Continuous variables were dichotomized with optimal cut-off values using the classification and regression trees (CART) method.24 Cox multiple regression analysis was performed to determine the independent contribution of the variables.25 Correlations were assessed by the Spearman rank test.
RESULTS
s-TK levels, presence of lymphadenopathy, and WBC counts independently predict time to disease progression.
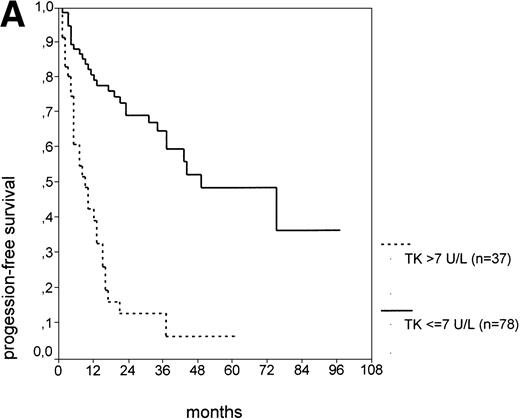
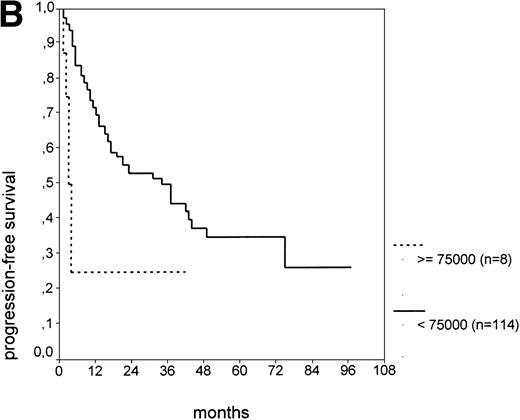
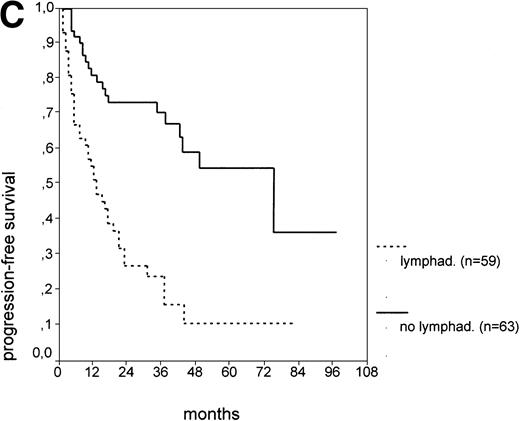
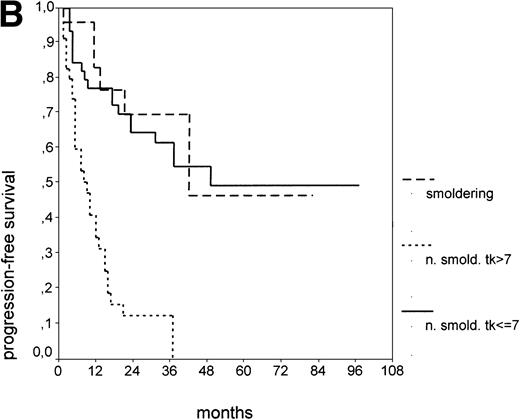
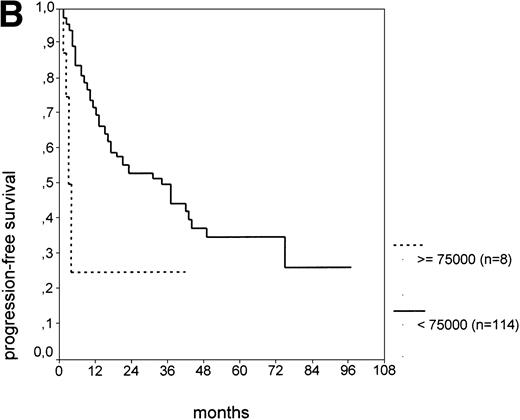
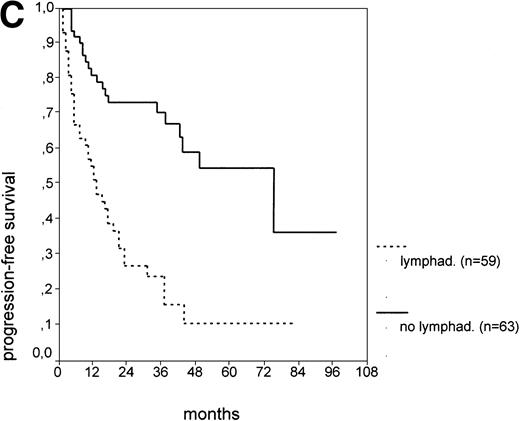
The cut-off values of 22 prognostic parameters investigated were determined by the CART analysis (Table 2). All parameters except age, sex, ECOG score, and presence of hepatomegaly showed a statistically significant relationship with PFS (P < .05). Using the cut-off values determined by the CART analysis, a stepwise multiple regression analysis was performed. Only three parameters provided independent prognostic information: (1) s-TK greater than 7.1 U/L, (2) presence of lymphadenopathy, and (3) WBC count greater than 75,000/μL (Table 2). s-TK values showed a weak correlation with lymphocyte counts or WBC counts (ρ = .28; P< .05; Spearman rank test). Figure 1demonstrates that each of the three independent prognostic variables was able to separate two subgroups within Binet stage A that had a different time to disease progression. For patients with high versus low s-TK values, the median PFS duration was 9 (range, 5 to 13) versus 49 (range, 24 to 74) months (P < .001; Fig 1A). For patients with high versus low leukocyte counts, PFS was 3 (range, 2 to 4) versus 34 (range, 9 to 49) months (P < .001; Fig 1B). For patients with versus without lymphadenopathy, PFS was 5 (range, 1 to 9) versus 37 (range, 26 to 48) months (P < .001; Fig 1C).
PFS of CLL Binet stage A patients in relation to different prognostic parameters: (A) s-TK, with a cut-off level of 7.1 U/L; (B) peripheral blood leukocyte count with a cut-off level of 75,000/μL; (C) presence or absence of lymphadenopathy. Differences were statistically significant (P < .001) for all three parameters.
PFS of CLL Binet stage A patients in relation to different prognostic parameters: (A) s-TK, with a cut-off level of 7.1 U/L; (B) peripheral blood leukocyte count with a cut-off level of 75,000/μL; (C) presence or absence of lymphadenopathy. Differences were statistically significant (P < .001) for all three parameters.
Elevated s-TK and presence of lymphadenopathy define a subgroup at high risk of disease progression within nonsmoldering Binet stage A CLL.
The current risk-assessment strategies in CLL are relatively potent in identifying patients with a relatively poor prognosis (Binet stage C or Rai stage III and IV patients), as well as patients with a very good prognosis that is identical to the age-matched healthy population. The latter group of patients is considered to have smoldering CLL. It is a subgroup of Binet stage A patients identified by normal blood hemoglobin greater than 13.0 g/dL, a low absolute lymphocyte count (< 30,000/μL), a lymphocyte doubling time greater than 12 months, and a nondiffuse pattern of lymphoid bone marrow infiltration.26Similar criteria were developed by the French CLL Study Group27 and by the Rai staging system (Rai stage 0). While there is clear evidence of a very good prognosis for smoldering CLL, predicting the prognosis of the larger group of nonsmoldering CLL patients in Binet stage A is more difficult.
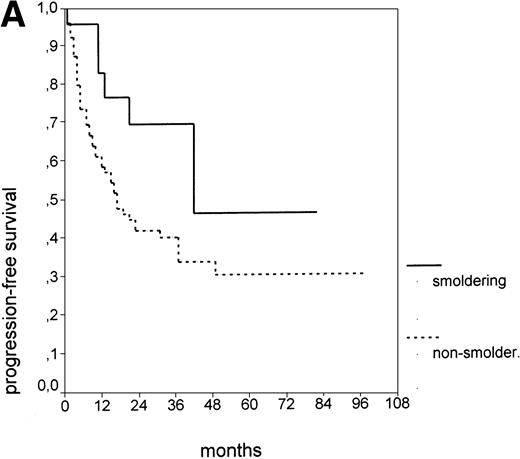
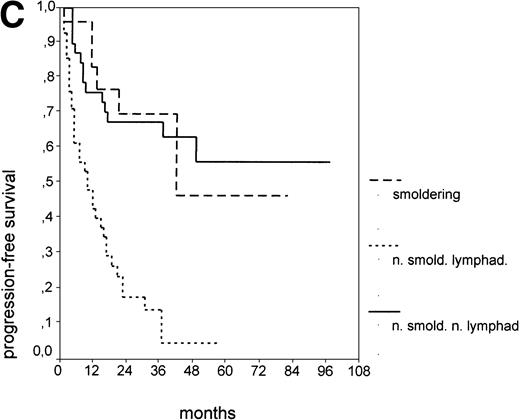
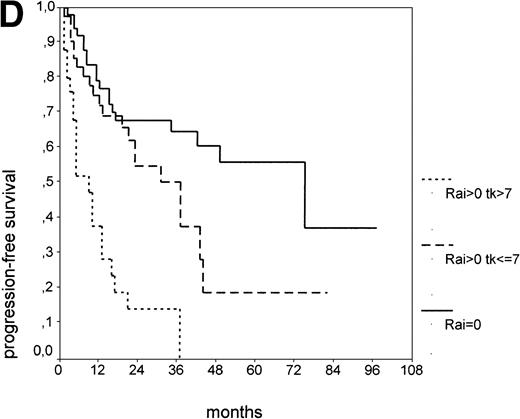
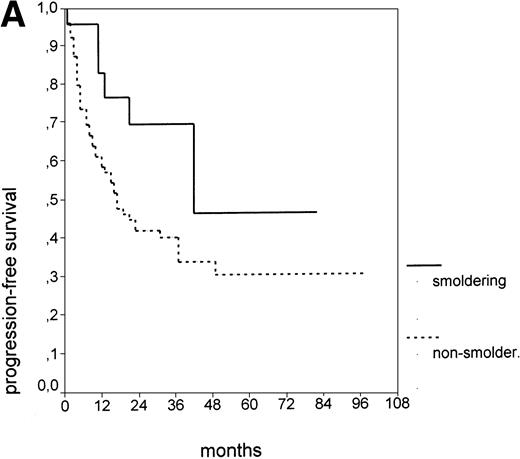
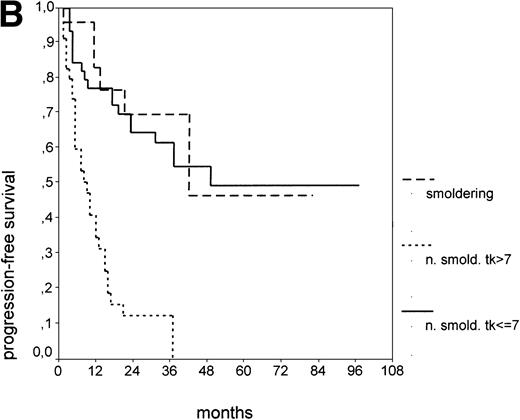
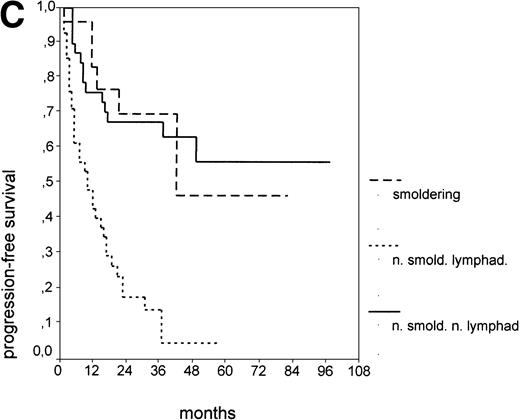
Since our analyses found two parameters, s-TK and presence of lymphadenopathy, that were not part of the above definition of smoldering CLL,26 we tested whether their inclusion was able to identify new prognostic subgroups. Figure2A shows the PFS of CLL stage A patients separated into those with smoldering and nonsmoldering disease. The 25 patients with smoldering CLL had a significantly longer PFS than the 81 patients with nonsmoldering CLL (P < .001; 42 v 18 months). In addition to the criteria defining smoldering CLL, elevated s-TK levels were able to identify 31 patients within the group of nonsmoldering Binet stage A patients who had a significantly shorter PFS than patients with low s-TK levels (P < .001; 8 v49 months; Fig 2B). Interestingly, only two of the patients with smoldering CLL had an elevated s-TK value greater than 7.1 U/L. A similar phenomenon was observed for the prognostic parameter “lymphadenopathy.” The PFS of nonsmoldering patients who presented with lymphadenopathy was significantly shorter than that of patients without lymphadenopathy (P < .001; 10 v 38 months; Fig 2C).
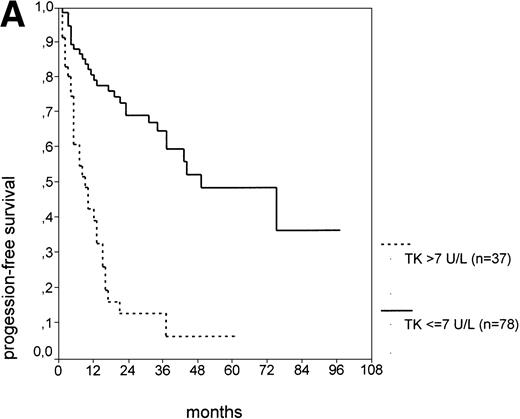
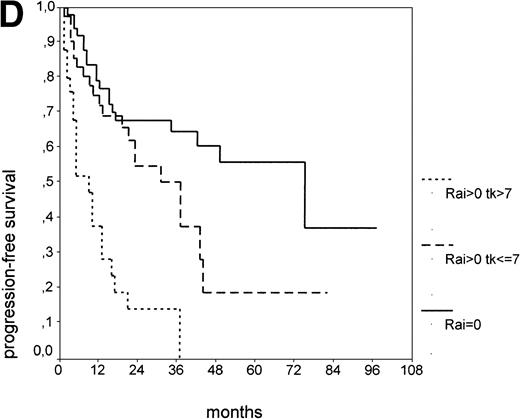
PFS of 106 CLL Binet stage A patients assigned to different prognostic subgroups (Kaplan-Meier plots). (A) 25 patients with smoldering CLL versus 81 with nonsmoldering CLL: median PFS, 42 versus 18 months (P < .001). (B) 79 patients with nonsmoldering CLL are further divided according to s-TK levels: 31 with high s-TK values (>7.0 U/L) versus 48 with low s-TK values (≤7.0 U/L) had a PFS of 8 versus 49 months (P < .001). (C) 81 patients with nonsmoldering CLL are further divided according to the presence or absence of lymphadenopathy: 42 with lymphadenopathy versus 39 without lymphadenopathy had a median PFS of 10 versus 38 months (P < .001). (D) 106 Binet stage A patients, now classified in 52 patients with Rai stage 0, 42 patients with Rai stage I to II and low s-TK values (≤7.0 U/L), and 25 patients with Rai stage I to II and high s-TK values (>7.0 U/L). Median PFS was 75 months for Rai stage 0 patients, 31 months for Rai stage I/II with low s-TK, and 9 months for Rai stage I/II with high s-TK (P < .001).
PFS of 106 CLL Binet stage A patients assigned to different prognostic subgroups (Kaplan-Meier plots). (A) 25 patients with smoldering CLL versus 81 with nonsmoldering CLL: median PFS, 42 versus 18 months (P < .001). (B) 79 patients with nonsmoldering CLL are further divided according to s-TK levels: 31 with high s-TK values (>7.0 U/L) versus 48 with low s-TK values (≤7.0 U/L) had a PFS of 8 versus 49 months (P < .001). (C) 81 patients with nonsmoldering CLL are further divided according to the presence or absence of lymphadenopathy: 42 with lymphadenopathy versus 39 without lymphadenopathy had a median PFS of 10 versus 38 months (P < .001). (D) 106 Binet stage A patients, now classified in 52 patients with Rai stage 0, 42 patients with Rai stage I to II and low s-TK values (≤7.0 U/L), and 25 patients with Rai stage I to II and high s-TK values (>7.0 U/L). Median PFS was 75 months for Rai stage 0 patients, 31 months for Rai stage I/II with low s-TK, and 9 months for Rai stage I/II with high s-TK (P < .001).
Binet stage A consists of Rai stage 0, I, and II. The major prognostic parameter distinguishing Rai 0 patients from Rai I and II patients is the presence of lymphadenopathy. Again, we asked whether elevated s-TK levels would add prognostic information to the Rai staging system at early stages (Rai 0 to II). When the subgroup of Rai stage I/II patients was further split by s-TK levels greater than 7.1 U/L, a group of patients with a very short PFS of 9 months was identified (Fig 2D). Rai stage I/II patients with s-TK levels ≤ 7.1 U/L had a longer PFS (31 months; P < .001). Rai stage 0 patients had the longest PFS (75 months). Finally, the addition of s-β2m values seemed to further enhance the distinction between nonsmoldering patients at high and low risk (data not shown).
DISCUSSION
This study shows that s-TK level provides independent prognostic information on PFS in Binet stage A CLL, in addition to the criteria defining smoldering CLL. Since the time to disease progression seems to predict the survival time of CLL patients,22 it was used as a surrogate end point in this study; it can be expected but needs to be proven that s-TK levels will also be relevant for predicting survival of Binet stage A CLL patients.
Thymidine kinase, adenosine triphosphate (ATP):thymidine 5′-phosphotransferase (EC 2.7.1.21), is a cellular enzyme known to be involved in a “salvage pathway” for DNA synthesis.28 Mammalian cells contain at least two TK isoenzymes, which differ in their biochemical properties and their cellular distribution.29 The cytosolic isozyme, TK1 (also known as fetal TK), is found in the G1/S phase of dividing cells, but is absent in resting cells.30 In contrast, the levels of the mitochondrial isozyme, TK2, remain stable throughout the cell cycle. TK1 accounts for 95% of the s-TK activity found in most normal and pathologic situations.29,31 The s-TK activity in CLL patients is probably related to the number of dividing tumor cells as a result of tumor mass and rate of tumor cell proliferation, since s-TK levels correlate with the proliferative activity of CLL cells.32 Moreover, the proportion of S-phase cells detectable in tumor biopsies was found to correlate with s-TK, but not with s-β2M levels.33 Finally, s-TK levels, but not the TK activity of normal peripheral blood mononuclear cells, correlate with the tumor stage of breast cancer patients.34Taken together, s-TK levels in cancer patients seem to reflect the proliferative activity of the tumor.
Since the commercial introduction of a reliable and technically easy assay (see Materials and Methods), s-TK levels have been investigated in a variety of malignancies. Elevated s-TK levels were found in Hodgkin’s disease, non-Hodgkin’s lymphoma, multiple myeloma, acute lymphatic and myeloid leukemia, breast cancer, prostate cancer, and non–small-cell lung cancer.35 In low-grade non-Hodgkin’s lymphoma, s-TK levels correlate with the disease stage and provide prognostic information on overall survival and PFS.8-15
Serum CD23 (s-CD23) is another serum parameter that has been shown to identify patients at risk in early CLL.36,37 In a series of untreated, newly diagnosed patients with Binet stage A, high levels of s-CD23 were found to correlate with elevated s-TK levels, a lymphocyte doubling time less than 12 months, and a nonnodular pattern of bone marrow infiltration. Moreover, elevated s-CD23 levels predicted early disease progression.36 Sarfati et al have shown that s-CD23 levels greater than 574 U/mL are associated with a rapid disease progression in Binet stage A CLL patients.37 However, at present, it is unknown which of the two parameters, s-TK or s-CD23, is more potent in predicting progression of early CLL and whether they provide prognostic information independently from one another. Both parameters may be used for a risk-adapted management of early CLL. In a recent trial testing the value of interferon-α treatment in Binet stage A patients, s-TK was used in combination with the type of bone marrow infiltration and the lymphocyte doubling time to stratify patients according to the risk of progression. This strategy allowed prospective identification of a patient group with a significantly shorter time to disease progression.19
Considerable progress has been made over the past 20 years in identifying new prognostic parameters in CLL. In addition to advanced Binet or Rai stages, at least four factors seem generally accepted to predict a poor prognosis in CLL: diffuse bone marrow infiltration, blood lymphocyte counts greater than 50,000/μL, a lymphocyte doubling time ≤ 12 months, and multiple or complex abnormalities of the karyotype.6 38 However, it should be emphasized that some of these four prognostic factors were tested in a limited number of studies or showed independent prognostic value in a small subgroup of studies only. Moreover, a major disadvantage of bone marrow histopathology as a prognostic marker may be its relatively low reproducibility when different histopathologists review the biopsy slides.
In comparison with the above “established” parameters, the value of serum parameters as prognostic factors for CLL may have been underestimated in the past. Recent studies indicate that at least three serum parameters—s-TK, s-β2m, and s-CD23—may add prognostic information to the current staging systems.15,19,36,37,39 40 The versatility and ease of serum tests, which yield quantitative rather than qualitative results, are important advantages, eg, in comparison to the laborious classic cytogenetics or the investigator-biased evaluation of bone marrow histology. The results of this study suggest that the use of s-TK levels might improve the assessment of the individual prognosis in patients with early CLL. It seems highly desirable to further define its value by large prospective studies.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael Hallek, MD, Medizinische Klinik III, Klinikum Groβhadern, Ludwig-Maximilians-Universität München, Marchioninistr. 15, D-81377 München, Germany.