Abstract
Embryonic stem cells can differentiate in vitro into hematopoietic cells through two intermediate stages; the first being FLK1+ E-cadherin− proximal lateral mesoderm and the second being CD45− VE-cadherin+endothelial cells. To further dissect the CD45−VE-cadherin+ cells, we have examined distribution of 4-integrin on this cell population, because 4-integrin is the molecule expressed on hematopoietic stem cells. During culture of FLK1+ E-cadherin− cells, CD45− VE-cadherin+4-integrin− cells differentiate first, followed by 4-integrin+ cells appearing in both CD45− VE-cadherin+ and CD45−VE-cadherin− cell populations. In the CD45−VE-cadherin+ cell population, 4-integrin+ subset but not 4-integrin− subset had the potential to differentiate to hematopoietic lineage cells, whereas endothelial cell progenitors were present in both subsets. The CD45−VE-cadherin− 4-integrin+ cells also showed hematopoietic potential. Reverse transcription-polymerase chain reaction analyses showed that differential expression of the Gata2 and Myb genes correlated with the potential of the 4-integrin+ cells to give rise to hematopoietic cell differentiation. Hematopoietic CD45−VE-cadherin+ 4-integrin+ cells were also present in the yolk sac and embryonic body proper of 9.5 day postcoitum mouse embryos. Our results suggest that the expression of 4-integrin is a marker of the earliest precursor of hematopoietic cell lineage that was diverged from endothelial progenitors.
THE MAJOR SITE OF hematopoiesis changes during the ontogeny of many vertebrate species. In the mouse embryo, hematopoiesis begins in the extra-embryonic yolk sac at day 7.5 of gestation.1 The visceral yolk sac mesodermal cells aggregate into clusters to form blood islands that consist of an inner core of blood cells and an external layer of endothelial cells. Large nucleated primitive erythrocytes that express embryonic hemoglobin develop in the blood islands and migrate into the embryo proper via circulation that commences on day 9 of gestation. The major hematopoietic site shifts to the fetal liver at day 10 of gestation,2 where definitive erythrocytes containing fetal/adult type hemoglobin, myeloid, and lymphoid cells first appear. The site of hematopoiesis further shifts from the fetal liver to the spleen and eventually settles in the bone marrow at day 15 to 16 of gestation.3 4
Although the yolk sac is the first hematopoietic site and the only site known to generate hematopoietic cells in situ, the first appearance of erythroid cells in the blood vessels was staged to day 8 to 8.5 of gestation.5 The mesodermal component of the para-aortic splanchnopleura at day 8.5 of gestation was identified as the site that contains hematopoietic precursors before liver colonization.6,7 At day 10 to 11 of gestation, the aorta, gonads, and mesonephros (AGM) region that originates from the para-aortic splanchnopleura contains multipotent hematopoietic cell progenitors with long-term repopulating activity.8,9Cytological analyses have detected two types of structures relevant to hematopoiesis in the embryo proper before fetal liver hematopoiesis begins. The first are intra-arterial hematopoietic cell clusters present in the omphalomesenteric and umbilical arteries and dorsal aorta.10,11 The second are blood islands in the mesentery of the hind gut, which is included in the para-aortic splanchnopleura of day 9.5 of gestation.11 The mesenteric blood island is similar to the yolk sac blood island in that it consists of a core of hemocytoblasts circumscribed by an endothelial envelope.
Although the close association of development of hematopoietic and endothelial cell lineages had raised a hypothesis that the two lineages are derived from a common progenitor referred as the hemangioblast, so far no direct evidence has been reported for the existence of a bipotential cell that gives rise to only hematopoietic and endothelial cell lineages. To understand the cellular basis underlying early development of hematopoietic cell lineage, we need to dissect the developmental pathway from lateral mesodermal cells to mature hematopoietic cells. Recently, in vitro differentiation systems of mouse embryonic stem (ES) cells identified early progenitors of hematopoietic cell lineage. The first demonstration of the induction of hematopoietic cells from ES cells in vitro was reported by Doetschman et al.12 Thereafter, most attempts have been made to extend the range of hematopoietic cell lineage induced from ES cells, to detect hematopoietic progenitor cells in various stages of differentiation, and to examine the kinetics of expression of the genes relevant to hematopoiesis.13-22 An attempt to detect early hematopoietic progenitors in the in vitro differentiation system of ES cells also pointed to a common precursor for hematopoietic and endothelial cells.23 FLK1, a tyrosine kinase receptor that is expressed on endothelial cells and their precursor, proximal lateral mesoderm, was used as a cell surface marker to purify the earliest progenitors of hematopoietic cell lineage from ES cell cultures as well as developing mouse embryos.24 25 However, the induction of in vitro hematopoiesis from ES cells normally required formation of cell aggregates termed embryoid bodies (EB) or coculture with a stromal cell layer, and this has largely restricted identification of cellular intermediates between mesodermal cells and committed precursors for hematopoietic and endothelial cell lineages.
Recently, we developed a novel culture system in which ES cells differentiate into hematopoietic and endothelial cell lineages through the proximal lateral mesoderm without formation of EB nor requirement of feeder cells.26 In this culture system, ES cells are cultured in a dish coated with type IV collagen so that progressive separation of differentiated cells by fluorescence-activated cell sorting (FACS) and reculture to induce further differentiation can be easily achieved. This culture system identified FLK1+VE-cadherin+ cells derived from FLK1+VE-cadherin− proximal lateral mesoderm as a population that contains endothelial cell precursors endowed with hematopoietic potential.26
The hemocytoblasts in the blood islands are interconnected to each other and to circumscribing endothelial cells through cell junctions of the zonula adherens and zonula occludens types.11 Cell adhesion molecules of the cadherin family might be involved in the zonula adherens type junctions. However, this type of cell junctions should disappear and be replaced by other types of cell adhesion machinery such as the integrin superfamily during development of hematopoietic cells. The α4-integrin has been shown to be expressed by the hematopoietic stem cell and plays an important role in its migration.27-29 Although several types of integrin molecules are also found on broad range of the endothelial cells, expression of α4-integrin is restricted to a small subset of endothelium.30 To further dissect the hematopoietic FLK1+ VE-cadherin+ cell population, we recruited α4-integrin as an additional surface marker. In this report, we examined expression of α4-integrin on the precursor cells for hematopoietic and endothelial lineages that were derived from ES cells and mouse embryonic tissues. Our results demonstrated that α4-integrin can be used as a valuable cell surface marker to focus on a diverging point of endothelial and hematopoietic cell lineages.
MATERIALS AND METHODS
Cell lines.
CCE ES cell line, which was a gift from Dr M. Evans31 (Wellcome/CRC Institute, Cambridge, UK), was maintained in a culture dish coated with gelatin (Type A from porcine skin; Sigma, St Louis, MO) using Dulbecco’s modified Eagle medium (DMEM; GIBCO BRL, Grand Island, NY) supplemented with 15% fetal calf serum (FCS; Whittaker Bioproducts, Walkersville, MD), 100 μmol/L 2-mercaptoethanol (2ME), 2 mmol/L L-glutamine, 10 mmol/L Minimum Essential Medium nonessential amino acids (GIBCO BRL), and 5,000 U/mL leukemia inhibitory factor (LIF; R&D Systems, Minneapolis, MN). OP9 stromal cell line32 was maintained in MEM Alpha medium (GIBCO BRL) supplemented with 20% FCS (HyClone Laboratories, Logan, UT).
Monoclonal antibodies (MoAbs), cell staining, and sorting.
The MoAb against E-cadherin, ECCD2,33 was a gift from Dr A. Nagafuchi (Kyoto University, Kyoto, Japan). The MoAbs AVAS12 (anti-FLK1),24 TER119 (erythroid lineage marker),34 and VECD1 (anti–VE-cadherin)35 were purified from hybridoma culture supernatants on a protein G-Sepharose column (Pharmacia, Uppsala, Sweden). These MoAbs were labeled with fluorescein isothiocyanate (FITC) or allophycocyanin (APC) by standard methods. The FITC-labeled MoAbs 30-F11 (anti-CD45), M1/70 (anti-CD11b/Mac-1), and biotin-conjugated MoAbs TER119 and 9C10 (anti-CD49d/α4-integrin) were purchased from PharMingen (San Diego, CA). The phycoerythrin (PE)-conjugated streptavidin was purchased from Southern Biotechnology Associates (Birmingham, AL).
Cells were blocked with normal mouse serum and stained with several combinations of labeled MoAbs. The biotin-conjugated MoAbs were shown by further staining with PE-streptavidin. Stained cells were resuspended in Hanks’ balanced salt solution (GIBCO BRL) containing 1% bovine serum albumin (Sigma) and 5 μg/mL propidium iodide (PI; Sigma) to exclude dead cells. Cells were analyzed and sorted by FACS Vantage (Becton Dickinson Immunocytometry Systems, San Jose, CA). Data were analyzed and printed out by using software CellQuest (Becton Dickinson Immunocytometry Systems).
In vitro differentiation of ES cells.
For induction of differentiation, 1 × 104 CCE cells were transferred to each well of a type IV collagen-coated 6-well plate (BIOCOAT; Becton Dickinson Labware, Bedford, MA) and incubated for 4 days in MEM Alpha medium (GIBCO BRL) supplemented with 10% FCS (GIBCO BRL) and 50 μmol/L 2ME (the induction medium) in the absence of LIF. Cultured cells were harvested with cell dissociation buffer (GIBCO BRL) and analyzed for expression of E-cadherin and FLK1 by flow cytometry. Cells (1.5 to 2 × 106) were routinely recovered from each well after 4 days of the first induction. We tested more than 30 batches of FCS by determining the percentages of FLK1+cells induced after CCE cells were plated on type IV collagen-coated dish under the same conditions. There was an extreme variability between batches, and we selected a good lot in which we can induce a high percentage of FLK1+ cells. FLK1+ E-cadherin− cells were sorted from the harvested cells for second induction. Sorted cells (3 × 105) were transferred to each well of a 6-well plate (Becton Dickinson) that was precoated with gelatin and incubated in the induction medium for 3 days. Cultured cells were harvested with cell dissociation buffer and analyzed for expression of VE-cadherin, α4-integrin, CD45, and TER119 by flow cytometry. Cells (3 to 5 × 105) were routinely recovered from each well after 3 days of the second induction. The CD45−TER119− cell population in the harvested cells was fractionated into several fractions by cell sorting and cultured for further induction of hematopoietic or endothelial cells.
For the induction of hematopoietic cells, sorted cells were transferred into a 35-mm dish coated with type IV collagen (Becton Dickinson Labware) and incubated for 7 days in the induction medium supplemented with a mixture of recombinant growth factors containing 200 U/mL murine interleukin-3 (IL-3), 2 U/mL human erythropoietin (Epo), 100 ng/mL murine granulocyte colony-stimulating factor (G-CSF), and 100 ng/mL murine mast cell growth factor (MGF). Recombinant Epo and G-CSF were purchased from R&D Systems. Recombinant murine IL-3 and MGF were prepared as described.36 Cultured cells were analyzed for expression of surface markers by flow cytometry and for cell morphology by May-Gruenwald Giemsa staining (Hemacolor; Merck, Darmstadt, Germany). For measurement of frequency of hematopoietic precursors, sorted cells were put into a 6-well plate that was preseeded with OP9 stromal cells and incubated in the induction medium supplemented with the mixture of growth factors described above. After 24 hours, medium was replaced with a fresh semisolid medium that consisted of the induction medium, the mixture of growth factors, and 1.2% methylcellulose (Muromachi Kagaku, Tokyo, Japan). Cells were further cultured for 6 days and hematopoietic cell colonies were scored under a microscope.
For induction of endothelial cell growth, sorted cells were put into a 6-well plate that was preseeded with OP9 cells and incubated in the induction medium. After 2 weeks, the cultures were fixed in situ by 4% paraformaldehyde and stained with either the anti-FLK1 or anti–VE-cadherin MoAbs and alkaline phosphatase-conjugated antirat IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). FLK1+/VE-cadherin+ endothelial cell colonies were shown by using NBT/BCIP substrate solution (Boehringer Mannheim, Mannheim, Germany) and scored under a microscope.
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was prepared from sorted cells or cultured cells using ISOGEN (Nippon Gene, Toyama, Japan). RNA was reverse-transcribed by using Superscript II reverse transcriptase (GIBCO BRL) and oligo (dT)12-18 primer (GIBCO BRL) according to the manufacturer’s instructions. PCR assays were performed in the reaction mixture containing 1× ExTaq Buffer (Takara Shuzo, Osaka, Japan), 200 μmol/L dNTPs (Pharmacia), 25 U/mL ExTaq DNA polymerase (Takara Shuzo), several dilutions of cDNA, and 2 μmol/L of the following oligonucleotide primers: Gata1, 5′ ACT CGT CAT ACC ACT AAG GT 3′, 5′ AGT GTC TGT AGG CCT CAG CT 3′;Tal1, 5′ CAT TGC AAG ATG TCT GTT GG 3′, 5′ GTG AAG CTG CAA AGC TGA TG 3′; Lmo2, 5′ AGA ACA TAG GGG ACC GCT AC 3′, 5′ GAT GAT CCC ATT GAT CTT GG 3′;Gapd (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]), 5′ ATG GTG AAG GTC GGT GTG AAC GGA TTT GGC 3′, 5′ GCA TCG AAG GTG GAA GAG TGG GAG TTG CTG 3′. Sequences of other primers were described elsewhere: Gata2 andMyb37; Hbb-bh1 (hemoglobin Z)17; and Hbb-y (hemoglobin Y) and Hbb-b1(hemoglobin β major).13 Amplification of the cDNA was performed with 1 cycle at 95°C for 5 minutes, 70°C for 30 seconds, 72°C for 30 seconds, 3 cycles at 95°C for 1 minute, 66°C for 30 seconds, 72°C for 30 seconds, 3 cycles at 95°C for 1 minute, 62°C for 30 seconds, 72°C for 30 seconds and 40 cycles at 95°C for 30 seconds, 56°C for 30 seconds, and 72°C for 1 minute. RT-PCR products were electrophoresed through 1% agarose gel and analyzed by staining with ethidium bromide.
Cell dissociation from embryos and colony formation analysis.
Pregnant ICR mice (9.5 day postcoitum) were purchased from Shimizu Co Ltd (Kyoto, Japan). The yolk sac and embryonic body were separated as described.3 Anterior and caudal parts were cut off from the embryo as described,7 and the embryonic trunk lower than the heart level was pooled. The pooled yolk sac and lower trunk were incubated in dispase II (Boehringer Mannheim) at 37°C for 20 minutes. Cell clump was washed and further dissociated by incubation in cell dissociation buffer (GIBCO BRL) at 37°C for 20 minutes. Finally, single-cell suspension was prepared by gentle pipetting.
Cells were stained with anti-CD45, anti-TER119, anti–VE-cadherin, and anti–α4-integrin MoAbs and CD45−TER119− population was fractionated into several fractions by cell sorting. Sorted cells were suspended in a matrix gel consisted of the induction medium, 0.08% type I collagen (Nitta Gelatin Co, Osaka, Japan), and the mixture of growth factors containing IL-3, Epo, G-CSF, and MGF and incubated in a 35-mm dish precoated with type IV collagen. Hematopoietic cell colonies were scored after 7 days. The culture was dehydrated by capillary action and stained with May-Gruenwald Giemsa solution for morphological examination of colonies.
RESULTS
Differentiation of α4-integrin+ cells from FLK1+ mesodermal cells induced in the ES cell culture.
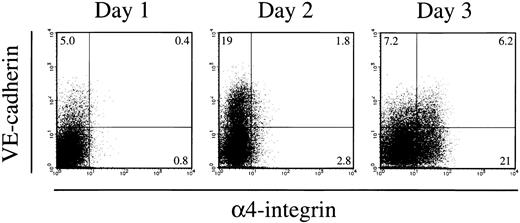
ES cells that were maintained in a gelatin-coated dish in the presence of LIF were transferred into a type IV collagen-coated dish and cultured in the absence of LIF to induce differentiation of mesodermal cells. FACS analysis of the cells induced for 4 days showed that nearly half of the cells downregulated the expression of E-cadherin and upregulated FLK1 (Fig 1A). Previous studies demonstrated that the FLK1 is a marker of proximal lateral mesoderm.24,26 38 The FLK1+E-cadherin− mesodermal cells were sorted by FACS as shown in Fig 1A and recultured in the same condition but in a gelatin-coated dish. The second culture induced the proliferation of VE-cadherin+ endothelial cells and CD45+ or TER119+ hematopoietic cells. FACS analysis of the cells cultured for 3 days showed that all CD45+ or TER119+ cells induced in the culture were α4-integrin+ (Fig 1B). Thirty percent of the cell population that was devoid of CD45+/TER119+committed blood cells also expressed α4-integrin on the surface (Fig1B). Expression of VE-cadherin was found on 13% to 17% of the CD45− TER119− cells. In the VE-cadherin+ CD45−TER119− cell fraction, nearly half of the cells were α4-integrin+ (Fig 1B). VE-cadherin−α4-integrin+ cells were also present in the CD45− TER119− population. We examined the kinetics of appearance of α4-integrin+ cells in the CD45− TER119− cell population in the culture induced from FLK1+E-cadherin− cells. Differentiation of VE-cadherin+ cells was already evident 1 day after the initiation of the culture, whereas only a few cells in the CD45− TER119− cell population expressed α4-integrin (Fig 2). On the second day, both the VE-cadherin+α4-integrin+ and VE-cadherin−α4-integrin+ cells appeared simultaneously in the CD45− TER119− cell fraction and the number of the α4-integrin+ cells continuously increased in the culture (Fig 2). We did not observe any situation in which the appearance of either the VE-cadherin+α4-integrin+ or VE-cadherin−α4-integrin+ cells precedes that of the other.
Induction of differentiation of ES cells in vitro. (A) CCE ES cells were allowed to differentiate in a type IV collagen-coated dish for 4 days. Expression of FLK1 and E-cadherin on the CCE cells before (left panel, day 0) and after (middle panel, day 4) the induction was analyzed by flow cytometry. FLK1+E-cadherin− cells were sorted and reanalyzed (right panel). (B) The ES cell-derived FLK1+E-cadherin− cells were cultured in a gelatin-coated dish for 3 days. Expression of CD45, TER119, and 4-integrin on the total cells (left panel) and VE-cadherin and 4-integrin on the CD45− TER119− cells (right panel) was analyzed. The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is a representative of more than five independent experiments.
Induction of differentiation of ES cells in vitro. (A) CCE ES cells were allowed to differentiate in a type IV collagen-coated dish for 4 days. Expression of FLK1 and E-cadherin on the CCE cells before (left panel, day 0) and after (middle panel, day 4) the induction was analyzed by flow cytometry. FLK1+E-cadherin− cells were sorted and reanalyzed (right panel). (B) The ES cell-derived FLK1+E-cadherin− cells were cultured in a gelatin-coated dish for 3 days. Expression of CD45, TER119, and 4-integrin on the total cells (left panel) and VE-cadherin and 4-integrin on the CD45− TER119− cells (right panel) was analyzed. The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is a representative of more than five independent experiments.
Kinetics of appearance of 4-integrin+cells in the culture initiated from ES cell-derived FLK1+cells. FLK1+ cells sorted from differentiating CCE cells were cultured in a gelatin-coated dish for 1 to 3 days and analyzed for expression of VE-cadherin and 4-integrin by flow cytometry. CD45+ cells and TER119+ cells were excluded from the analyses. The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is a representative of three independent experiments.
Kinetics of appearance of 4-integrin+cells in the culture initiated from ES cell-derived FLK1+cells. FLK1+ cells sorted from differentiating CCE cells were cultured in a gelatin-coated dish for 1 to 3 days and analyzed for expression of VE-cadherin and 4-integrin by flow cytometry. CD45+ cells and TER119+ cells were excluded from the analyses. The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is a representative of three independent experiments.
Hematopoietic potential of VE-cadherin+α4-integrin+ and VE-cadherin−α4-integrin+ cell fractions.
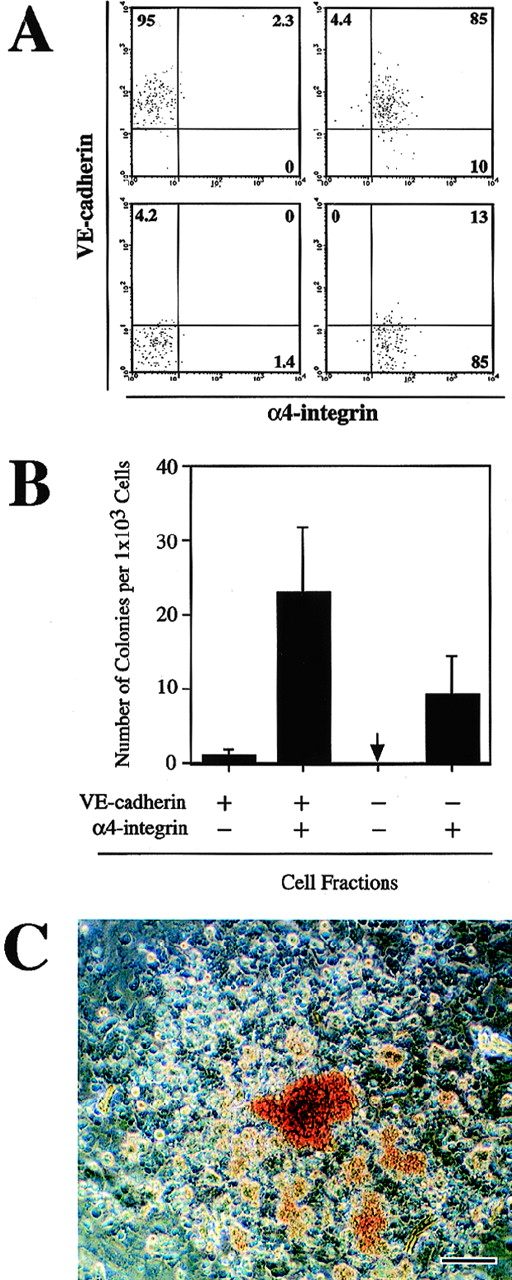
We previously showed that the CD45−TER119− VE-cadherin+ cells that were derived from ES cells in vitro included endothelial precursors with hematopoietic potential.26 To investigate whether the α4-integrin+ cells induced from ES cells have a capacity to generate hematopoietic lineage cells, the CD45−TER119− cells in the 3-day culture of FLK1+ cells were fractionated into VE-cadherin+α4-integrin−, VE-cadherin+α4-integrin+, VE-cadherin−α4-integrin−, and VE-cadherin−α4-integrin+ populations by FACS (Fig 3A). The sorted cells were put into a culture that contains OP9 stromal cell layer and were maintained for 7 days in the presence of recombinant IL-3, Epo, G-CSF, and MGF. To measure the frequency of the progenitors that give rise to clonal expansion of hematopoietic cells, we covered the cultures with a semisolid medium containing methylcellulose. One in 40 cells in the VE-cadherin+ α4-integrin+ fraction proliferated to form a hematopoietic cell colony (Fig 3B). Approximately 70% of the colonies contained hemoglobinized erythrocytes (Fig 3C). In contrast to the VE-cadherin+α4-integrin+ fraction, the VE-cadherin+α4-integrin− fraction contained much fewer hematopoietic cell progenitors (1 in 1,000 cells; Fig 3B). One in 100 cells in the VE-cadherin− α4-integrin+fraction also gave rise to a hematopoietic cell colony (Fig 3B). The hematopoietic cell colonies derived from the VE-cadherin− α4-integrin+ fraction could not be morphologically distinguished from the colonies obtained in the VE-cadherin+ α4-integrin+ cell culture (data not shown). The VE-cadherin−α4-integrin− fraction did not give rise to any hematopoietic cell colonies in the same culture condition (Fig 3B). These results demonstrated that both VE-cadherin+ and VE-cadherin− fractions derived from FLK1+mesodermal cells contained precursors of hematopoietic cell lineage and that those progenitors exclusively express α4-integrin.
Hematopoietic potential of the CD45−TER119− 4-integrin+ cells induced from ES cell-derived FLK1+ cells. FLK1+ cells sorted from differentiating CCE cells were cultured in a gelatin-coated dish for 3 days. Cultured cells were fractionated by FACS into VE-cadherin+ 4-integrin−, VE-cadherin+ 4-integrin+, VE-cadherin− 4-integrin−, and VE-cadherin− 4-integrin+ cells. CD45+ cells and TER119+ cells were excluded from the sorting gates. Sorted cells were cultured on OP9 stromal cell layer in the presence of IL-3, Epo, G-CSF, and MGF for 7 days. (A) Reanalyses of the sorted cells. A representative result of more than five independent experiments is shown. (B) Frequency of hematopoietic colony-forming cells in the indicated fractions. The arrow indicates that no colony-forming cell was detected. Error bars indicate standard deviations for four independent determinations. (C) Morphology of a hematopoietic cell colony formed in the culture of VE-cadherin+ 4-integrin+ cells. Hemoglobinized erythrocytes are observed. The bar represents 100 μm.
Hematopoietic potential of the CD45−TER119− 4-integrin+ cells induced from ES cell-derived FLK1+ cells. FLK1+ cells sorted from differentiating CCE cells were cultured in a gelatin-coated dish for 3 days. Cultured cells were fractionated by FACS into VE-cadherin+ 4-integrin−, VE-cadherin+ 4-integrin+, VE-cadherin− 4-integrin−, and VE-cadherin− 4-integrin+ cells. CD45+ cells and TER119+ cells were excluded from the sorting gates. Sorted cells were cultured on OP9 stromal cell layer in the presence of IL-3, Epo, G-CSF, and MGF for 7 days. (A) Reanalyses of the sorted cells. A representative result of more than five independent experiments is shown. (B) Frequency of hematopoietic colony-forming cells in the indicated fractions. The arrow indicates that no colony-forming cell was detected. Error bars indicate standard deviations for four independent determinations. (C) Morphology of a hematopoietic cell colony formed in the culture of VE-cadherin+ 4-integrin+ cells. Hemoglobinized erythrocytes are observed. The bar represents 100 μm.
To characterize the hematopoietic cells derived from the VE-cadherin+ α4-integrin+ and VE-cadherin− α4-integrin+ fractions, the cells sorted from these fractions were cultured for 7 days in a type IV collagen-coated dish in the presence of recombinant IL-3, Epo, G-CSF, and MGF. The number of cells per culture increased 20-fold and fourfold in the cultures initiated from the VE-cadherin+α4-integrin+ and VE-cadherin−α4-integrin+ fractions, respectively. May-Gruenwald Giemsa staining of the harvested cells showed that erythroblasts, polymorphonuclear cells, and monocytes/macrophages were induced in the cultures initiated from both fractions (Fig4A through C). Essentially the same frequencies of TER119+and Mac-1+ cells were detected in both cultures by FACS analyses (Fig 4D and E). We analyzed expression of the hemoglobin genes in the harvested cells by RT-PCR. The transcripts of the embryonic typeHbb-bh1 (hemoglobin Z), the embryonic/fetal type Hbb-y(hemoglobin Y), and the fetal/adult type Hbb-b1 (hemoglobin β major) genes39 were comparably detected in the hematopoietic cells derived from both fractions (data not shown), indicating that the α4-integrin+ fractions have a potential to produce both the primitive and definitive erythroid lineages.
Phenotype of hematopoietic cells differentiated from the VE-cadherin+ 4-integrin+ and VE-cadherin− 4-integrin+ cell fractions. The two fractions were sorted from differentiating CCE cells as shown in Fig 3A and cultured in a type IV collagen-coated dish in the presence of IL-3, Epo, G-CSF, and MGF for 7 days. (A through C) May-Gruenwald Giemsa staining of cytospots prepared from the cultured cells initiated from the VE-cadherin+4-integrin+ fraction. Erythroblasts (A), monocytes/macrophages (B), and polymorphonuclear cells (C) are observed. The bars represent 25 μm. (D and E) Expression of Mac-1 and TER119 on the cultured cells initiated from the VE-cadherin+ 4-integrin+ (D) and VE-cadherin− 4-integrin+ (E) cell fractions analyzed by flow cytometry. The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is a representative of two independent experiments.
Phenotype of hematopoietic cells differentiated from the VE-cadherin+ 4-integrin+ and VE-cadherin− 4-integrin+ cell fractions. The two fractions were sorted from differentiating CCE cells as shown in Fig 3A and cultured in a type IV collagen-coated dish in the presence of IL-3, Epo, G-CSF, and MGF for 7 days. (A through C) May-Gruenwald Giemsa staining of cytospots prepared from the cultured cells initiated from the VE-cadherin+4-integrin+ fraction. Erythroblasts (A), monocytes/macrophages (B), and polymorphonuclear cells (C) are observed. The bars represent 25 μm. (D and E) Expression of Mac-1 and TER119 on the cultured cells initiated from the VE-cadherin+ 4-integrin+ (D) and VE-cadherin− 4-integrin+ (E) cell fractions analyzed by flow cytometry. The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is a representative of two independent experiments.
Clonal proliferation of endothelial cells derived from VE-cadherin+ cell fractions.
We previously reported that the VE-cadherin+ cells derived either from embryonic tissues or ES cell culture proliferated in response to stromal cells to form endothelial cell colonies.26 The colonies consisted of FLK1+VE-cadherin+ CD31(PECAM-1)+ cells that have the capacity to take up acetylated low-density lipoprotein (Ac-LDL), indicating that the VE-cadherin+ cell fraction contains clonogenic precursors of endothelial cell lineage (Hirashima et al, manuscript submitted). To compare the capacity of the VE-cadherin+ α4-integrin− and VE-cadherin+ α4-integrin+ cells to form endothelial cell colonies, these cell fractions that were induced from FLK1+ cells in the ES cell culture were purified by FACS and cultured in the presence of OP9 stromal cell layer for 2 weeks. The formation of colonies that consist of FLK1+ endothelial cells was shown by immunohistochemical staining of the whole cultures with an anti-FLK1 MoAb (Fig 5A). Both the VE-cadherin+ α4-integrin− and VE-cadherin+ α4-integrin+ cell fractions gave rise to endothelial cell colonies. The frequency of colony-forming cells was almost comparable in both fractions (1 in 35 cells; Fig 5B). The VE-cadherin− α4-integrin+ fraction contained much fewer colony-forming cells (1 in 1,000 cells; Fig 5B). We obtained a comparable result by staining with the anti–VE-cadherin MoAb (data not shown).
Potential of CD45− TER119−VE-cadherin+ cells to form endothelial cell colonies. VE-cadherin+ 4-integrin−, VE-cadherin+ 4-integrin+, and VE-cadherin− 4-integrin+ cells were sorted from differentiating CCE cells as shown in Fig 3A and cultured on OP9 stromal cell layer for 2 weeks. The cultures were stained in situ with either anti-FLK1 or anti–VE-cadherin MoAbs. (A) Morphology of a FLK1+ endothelial cell colony formed in the culture of VE-cadherin+ 4-integrin+ cells. The bar represents 400 μm. (B) Frequency of cells capable of formation of endothelial cell colony in the indicated fractions. A representative result of three independent experiments is shown.
Potential of CD45− TER119−VE-cadherin+ cells to form endothelial cell colonies. VE-cadherin+ 4-integrin−, VE-cadherin+ 4-integrin+, and VE-cadherin− 4-integrin+ cells were sorted from differentiating CCE cells as shown in Fig 3A and cultured on OP9 stromal cell layer for 2 weeks. The cultures were stained in situ with either anti-FLK1 or anti–VE-cadherin MoAbs. (A) Morphology of a FLK1+ endothelial cell colony formed in the culture of VE-cadherin+ 4-integrin+ cells. The bar represents 400 μm. (B) Frequency of cells capable of formation of endothelial cell colony in the indicated fractions. A representative result of three independent experiments is shown.
Expression of transcription factors in the cells differentiated from ES cell-derived FLK1+ mesoderm.
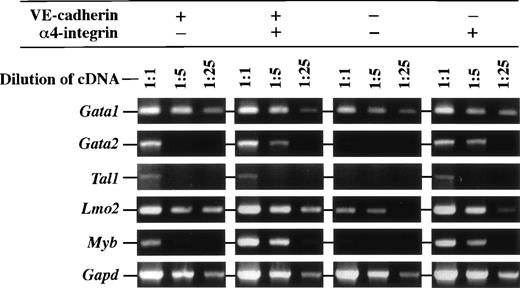
We next examined the expression of transcription factors that are known to be involved in regulation of hematopoietic cell development in the cell fractions differentiated from ES cell-derived FLK1+cells by RT-PCR. Figure 6 shows that expression level of the Gata2 gene that is essential for hematopoietic cell development was higher in the α4-integrin+ fractions that have a hematopoietic potential than in others. On the contrary, transcript of theLmo2 gene, whose product physically associates with GATA2, was abundantly detected in all fractions tested. The transcripts of theTal1 gene, whose product also associates with Lmo2, was detected in the VE-cadherin+α4-integrin−, VE-cadherin+α4-integrin+, and VE-cadherin−α4-integrin+ fractions that have a potential of either hematopoietic or endothelial cell differentiation. Transcripts of theGata1 gene that is essential for erythroid lineage development were abundantly detected in all fractions. Higher expression of theMyb gene that is essential for the development of definitive hematopoiesis in fetal liver was also detected in the α4-integrin+ fractions than in others.
Expression of mRNA of transcription factors in the VE-cadherin+ 4-integrin−, VE-cadherin+ 4-integrin+, VE-cadherin− 4-integrin−, and VE-cadherin− 4-integrin+ cells induced in vitro from ES cell-derived FLK1+ cells. These fractions were all CD45− TER119−. Different dilutions of cDNA prepared from sorted cells were subjected to PCR amplification specific for Gata1, Gata2, Tal1,Lmo2, Myb, and Gapd transcripts. PCR products were separated on 1% agarose gel stained with ethidium bromide. The result shown is a representative of two independent experiments.
Expression of mRNA of transcription factors in the VE-cadherin+ 4-integrin−, VE-cadherin+ 4-integrin+, VE-cadherin− 4-integrin−, and VE-cadherin− 4-integrin+ cells induced in vitro from ES cell-derived FLK1+ cells. These fractions were all CD45− TER119−. Different dilutions of cDNA prepared from sorted cells were subjected to PCR amplification specific for Gata1, Gata2, Tal1,Lmo2, Myb, and Gapd transcripts. PCR products were separated on 1% agarose gel stained with ethidium bromide. The result shown is a representative of two independent experiments.
Reanalyses of sorted fractions did not detect any CD45/TER119 fluorescence (data not shown). However, because the expression level of TER119 on primitive erythrocytes is low as compared with that on definitive erythrocytes,40 some mature primitive erythrocytes may contaminate into sorted fractions and might result in the overall detection of Gata1 and Lmo2 transcripts. To test this possibility, cytospots were prepared from the sorted cells and presence of mature primitive erythrocytes was examined by staining with an antiembryonic hemoglobin antibody. Substantial number of mature primitive erythrocytes were detected in all the fractions examined (10% in VE-cadherin+ α4-integrin−, 2% in VE-cadherin+ α4-integrin+, 10% in VE-cadherin− α4-integrin+, and 33% in VE-cadherin− α4-integrin−fraction). Thus, it is possible that the overall expression of theGata1 and Lmo2 genes is attributed to the contaminated primitive erythrocytes. Nevertheless, the highest frequency observed in the VE-cadherin− α4-integrin−fraction also indicates that these mature primitive erythrocytes contributed to neither the expression of the Gata2,Tal1, and Myb genes detected by RT-PCR nor the formation of hematopoietic cell colonies on stromal cells.
Hematopoietic potential of CD45−VE-cadherin+α4-integrin+ cells in the yolk sac and embryonic body of 9.5 dpc.
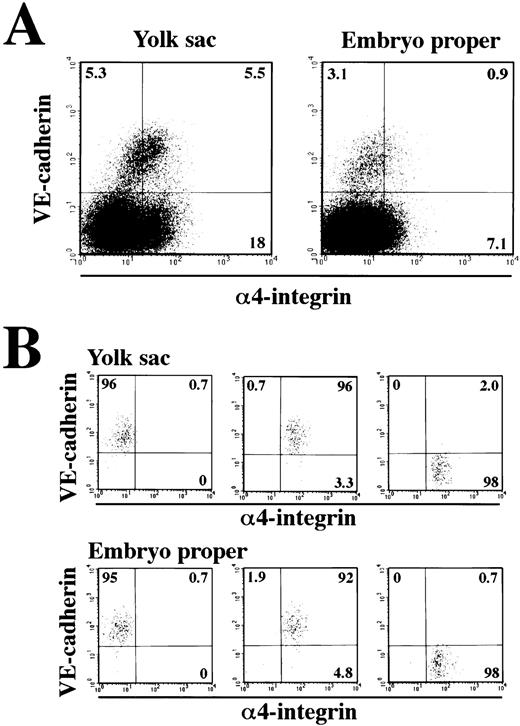
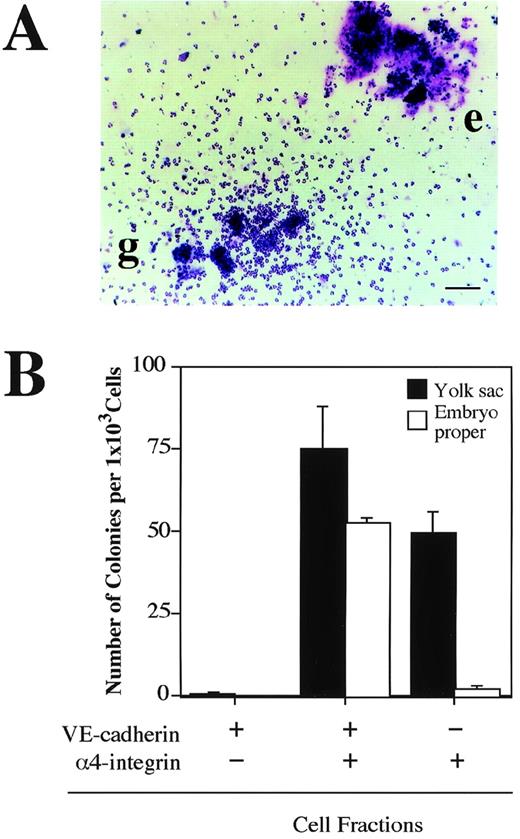
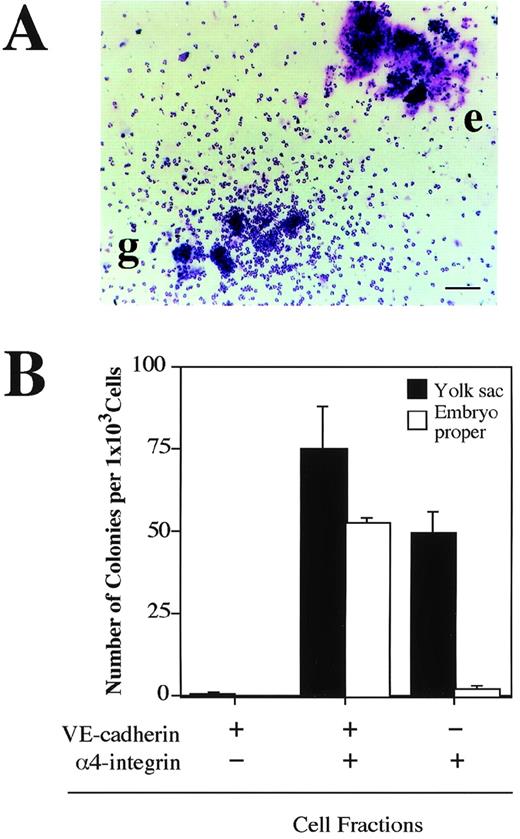
The next question we addressed was whether the VE-cadherin+α4-integrin+ and VE-cadherin−α4-integrin+ cells that were induced in the ES cell culture represent intermediate stages of the developmental pathway of hematopoietic lineage in the mouse embryo. Cells were dissociated from the yolk sac and lower trunk of embryo proper of 9.5 dpc mouse embryos (see Materials and Methods) and analyzed by flow cytometry. The VE-cadherin+ α4-integrin−, VE-cadherin+ α4-integrin+, and VE-cadherin− α4-integrin+ cells were all detected in the CD45− TER119−cell fraction dissociated from both the yolk sac and embryo proper (Fig 7A). To examine the hematopoietic potential of these cell populations, the cells were sorted by FACS as shown in Fig 7B and cultured in a type I collagen gel in the presence of recombinant IL-3, Epo, G-CSF, and MGF. The VE-cadherin+α4-integrin+ fraction but not the VE-cadherin+ α4-integrin− fraction sorted from both the yolk sac and embryo proper gave rise to hematopoietic cell colonies (Fig 8A and B). This result indicates that most of the precursors of hematopoietic cell lineage in the CD45− VE-cadherin+ cells in the embryos express α4-integrin on the surface. Although the VE-cadherin− α4-integrin+ cells were able to be dissociated from both the yolk sac and embryo proper, only the cells derived from the yolk sac gave rise to hematopoietic cell colonies (Fig 8B). May-Gruenwald Giemsa staining showed no morphological difference on the hematopoietic cell colonies derived from the VE-cadherin+ α4-integrin+ and VE-cadherin− α4-integrin+ fractions in the yolk sac (data not shown).
Expression of VE-cadherin and 4-integrin on the cells dissociated from 9.5 dpc mouse embryos. The yolk sacs and embryonic bodies (lower trunk) were separated from 9.5 dpc mouse embryos and a single-cell suspension was prepared by using dispase and cell dissociation buffer. Cells were analyzed for expression of VE-cadherin and 4-integrin by flow cytometry (A). CD45+ cells and TER119+ cells were excluded from the analyses. The VE-cadherin+ 4-integrin−, VE-cadherin+ 4-integrin+, and VE-cadherin− 4-integrin+ cells were sorted and reanalyzed (B). The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is a representative of three independent experiments.
Expression of VE-cadherin and 4-integrin on the cells dissociated from 9.5 dpc mouse embryos. The yolk sacs and embryonic bodies (lower trunk) were separated from 9.5 dpc mouse embryos and a single-cell suspension was prepared by using dispase and cell dissociation buffer. Cells were analyzed for expression of VE-cadherin and 4-integrin by flow cytometry (A). CD45+ cells and TER119+ cells were excluded from the analyses. The VE-cadherin+ 4-integrin−, VE-cadherin+ 4-integrin+, and VE-cadherin− 4-integrin+ cells were sorted and reanalyzed (B). The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is a representative of three independent experiments.
Hematopoietic potential of VE-cadherin+4-integrin+ cells sorted from yolk sac and embryonic body proper of 9.5 dpc mouse embryo. The VE-cadherin+4-integrin−, VE-cadherin+4-integrin+, and VE-cadherin−4-integrin+ cells were sorted from yolk sac and embryo proper of 9.5 dpc mouse embryos as shown in Fig 7. CD45+and TER119+ cells were excluded from the sorting gates. Sorted cells were cultured for 7 days in a matrix gel containing type I collagen in the presence of IL-3, Epo, G-CSF, and MGF. Morphology of hematopoietic cell colonies were examined by May-Gruenwald Giemsa staining (A). Colonies consisted of granulocytes (g) and erythrocytes (e) are observed. The bar represents 100 μm. (B) Frequency of hematopoietic colony-forming cells in the indicated cell fractions derived from yolk sac and embryonic body proper. Error bars indicate standard deviations for three independent determinations.
Hematopoietic potential of VE-cadherin+4-integrin+ cells sorted from yolk sac and embryonic body proper of 9.5 dpc mouse embryo. The VE-cadherin+4-integrin−, VE-cadherin+4-integrin+, and VE-cadherin−4-integrin+ cells were sorted from yolk sac and embryo proper of 9.5 dpc mouse embryos as shown in Fig 7. CD45+and TER119+ cells were excluded from the sorting gates. Sorted cells were cultured for 7 days in a matrix gel containing type I collagen in the presence of IL-3, Epo, G-CSF, and MGF. Morphology of hematopoietic cell colonies were examined by May-Gruenwald Giemsa staining (A). Colonies consisted of granulocytes (g) and erythrocytes (e) are observed. The bar represents 100 μm. (B) Frequency of hematopoietic colony-forming cells in the indicated cell fractions derived from yolk sac and embryonic body proper. Error bars indicate standard deviations for three independent determinations.
DISCUSSION
By using an in vitro differentiation system of ES cells, VE-cadherin+ endothelial cells that have a hematopoietic potential can be induced from FLK1+ proximal lateral mesodermal cells.26 Among the surface markers that have been used to label endothelial cells (including FLK1, CD31, and CD34), only VE-cadherin is exclusive for endothelial cells.35,41,42 VE-cadherin+ cells generated in vitro from ES cells give rise to VE-cadherin+FLK1+ CD31+ sheet-like structures on stromal cells, and these sheets incorporate Ac-LDL (Hirashima et al, manuscript submitted). Thus, the VE-cadherin+ cells differentiated from ES cells are most likely to represent the endothelial cell lineage. In this report, we showed that a subset of this VE-cadherin+ endothelial cell population expresses α4-integrin on the surface (Figs 1 and 2). The integrins that have been detected on resting endothelial cells in vivo include α6β1, α5β1, α3β1, α2β1, αvβ3, and α6β4, whereas the distribution of α4-integrin is restricted to the capillary of placenta.30 However, α4-integrin has been shown to be present on the endothelium of newly forming vessels during angiogenesis in the lung of mouse embryos.43 We also detected VE-cadherin+ α4-integrin+ cells in the yolk sac and embryo proper of 9.5 dpc mouse embryos (Fig 7). All the VE-cadherin+ cells isolated from 9.5 dpc embryos possess the capacity to incorporate Ac-LDL,41 indicating that they are endothelial cells. Thus, it is possible that α4-integrin is expressed transiently by a subset of developing endothelium in the mouse embryos. Our analyses demonstrated that the endothelial cells that have hematopoietic potential were found exclusively in the α4-integrin+ subset (Figs 3 and 8). However, because the VE-cadherin+ α4-integrin+ fraction also contains cells that give rise to endothelial cell colonies (Fig 5), expression of α4-integrin on an endothelial cell does not necessarily indicate that the cell is fully committed to hematopoietic cell lineage. A part of the α4-integrin+ endothelial cells may retain a capacity to form an endothelial cell sheet when cultured in an appropriate condition in vitro. However, it is still unknown whether a single VE-cadherin+ α4-integrin+ progenitor produces both hematopoietic cells and mature endothelium in vivo. In vivo labeling of single progenitors in the endothelial lining by an appropriate marker (ie, retroviral vector or green fluorescent protein) should be required to solve this question. Although α4-integrin can be used as a marker of the hematopoitic endothelium, lack of this molecule did not affect early development of hematopoietic cell lineage,44 45 which indicates that expression of α4-integrin is not essential for cell specification to hematopoietic lineage.
It is generally thought that different sets of transcription factors should be found in cell populations that have the same origin but different potential.46 Gene disruption studies have shown that several transcription factors play essential roles on the development of hematopoietic cells in mouse embryos. Loss of eitherGata2, Lmo2, or Tal1 profoundly affects development of all hematopoietic cell lineages.47-50 It has been proposed that the products of these three genes physically interact to establish a transcriptional transactivating complex.51 Although Gata1 gene product also can be integrated into the transactivating complex, loss of Gata1specifically affected erythroid lineage.52,53 Our RT-PCR analyses on the expression of these genes in the CD45− TER119−VE-cadherin+ fractions indicated that expression level of the Gata2 gene was higher in the VE-cadherin+α4-integrin+ fraction that has hematopoietic potential than in the nonhematopoietic VE-cadherin+α4-integrin− fraction (Fig 6). Although expression levels of the Lmo2 and Tal1 genes were the same in the two fractions, this result might be consistent with the hypothesis that the molecular complex formed by GATA2, Lmo2, and TAL1 is involved in early hematopoiesis.51 Furthermore, the expression of theTal1 gene in the VE-cadherin+α4-integrin− fraction that has a capacity to form endothelial cell colonies agrees with the previous report showing that TAL1 is present in endothelial cells in the developing mouse embryo54 (Figs 5 and 6). The Myb gene is essential for definitive erythropoiesis in the fetal liver but not for primitive erythropoiesis in the yolk sac.55 Expression of α4-integrin in the fetal liver was abrogated in theMyb-deficient mice, suggesting an important role forMyb in the regulation of the Itga4 (α4-integrin) gene in, at least, the hematopoietic lineage.56 We detected higher expression of the Myb gene in the VE-cadherin+ α4-integrin+ fraction than in the VE-cadherin+ α4-integrin− fraction (Fig 6), which is again consistent with the previous observations. Consequently, our results suggest that differential expression of some of the transcription factors, such as Gata2 andMyb, correlates with the potential of VE-cadherin+α4-integrin+ cells to give rise to hematopoietic cell differentiation. However, it should be noted that our analyses on the expression of transcription factors were not based on single cells. Investigations at the single-cell level on a fate of a cell that expresses a certain set of transcription factors should be required to clarify the relationship between expression of transcription factors and cell specification.
In addition to the CD45− TER119−VE-cadherin+ α4-integrin+ fraction, we detected another fraction, CD45−TER119− VE-cadherin−α4-integrin+, that also showed a potential to give rise to hematopoietic cells (Fig 3). Because we excluded CD45+cells from the analyses, it is unlikely that the hematopoietic potential of the VE-cadherin−α4-integrin+ fraction is attributed to hematopoietic stem cells that are known to be CD45+.57 We detected no significant difference between the hematopoietic cells derived from the VE-cadherin+ α4-integrin+ and VE-cadherin− α4-integrin+ fractions in cell morphology (Fig 4), in surface marker expression (Fig 4), and in transcription of the β-hemoglobin genes (data not shown). We also failed to detect any difference between two cell fractions in the expression levels of transcription factors that are involved in hematopoietic cell development (Fig 6). It is still unknown whether VE-cadherin− α4-integrin+ cells are immediate progeny of VE-cadherin+α4-integrin+ cells. Because hematopoietic stem cells express α4-integrin and mature hematopoietic cells do not express VE-cadherin on the surface, expression of VE-cadherin should be shut off and expression of α4-integrin should be turned on upon differentiation of the hematopoietic endothelial cells to hematopoietic cells. However, our results demonstrate that the VE-cadherin+ α4-integrin+ and VE-cadherin− α4-integrin+ fractions appeared simultaneously in the culture of ES-derived FLK1+cells (Fig 2). Thus, although some of the VE-cadherin− α4-integrin+ cells that have hematopoietic potential might differentiate from the VE-cadherin+ α4-integrin+ cells, it is equally possible that the VE-cadherin−α4-integrin+ cells are also derived directly from FLK1+ VE-cadherin− mesodermal cells.
Both CD45− TER119−VE-cadherin+ α4-integrin+ and CD45− TER119−VE-cadherin− α4-integrin+ fractions directly sorted from 9.5 dpc yolk sac had hematopoietic potential, whereas only the CD45− TER119−VE-cadherin+ α4-integrin+ fraction in the embryo proper at the same embryonic stage had the same potential (Fig8). This indicates that the VE-cadherin−α4-integrin+ fraction is not homogeneous and is likely to contain other lineages, such as smooth muscle cells.43 It is possible that our procedure simply failed to dissociate the VE-cadherin− α4-integrin+ cells that have hematopoietic potential from embryonic body proper. Alternatively, there may be two alternative pathways in early development of hematopoietic cell lineage: one path goes through the VE-cadherin+ stage, whereas the other does not. Both pathways might be taken by the progenitors present in the yolk sac, whereas the progenitors in the embryo proper follow only the VE-cadherin+ pathway. This hypothesis may be consistent with the previous model that the primitive hematopoiesis is generated in the yolk sac from hemangioblasts, whereas the definitive hematopoiesis is generated from endothelial cells in the embryo proper.58 Moreover, it should be remembered that hematopoietic endothelial cells are generated not only in the embryo proper, but also in the yolk sac.41 We previously reported that VE-cadherin+ endothelial cells derived from embryonic tissues have a potential to differentiate to lymphoid cell lineages.41 Thus, we currently hypothesize that the VE-cadherin+ α4-integrin+ progenitors represent precursors of definitive hematopoietic lineage that give rise to multiple types of blood cells.
In conclusion, the α4-Integrin+ subset of endothelial cells encompasses cells that are in the earliest step, so far identified, of diversification of the vascular endothelium toward the hematopoietic cell lineage. α4-integrin may provide a valuable tool that should allow the elucidation of the cellular and molecular basis underlying the developmental process of the hematopoietic cell lineage.
ACKNOWLEDGMENT
The authors are grateful to Drs M. Evans for the CCE cell line and A. Nagafuchi for the ECCD2 MoAb. We thank to Dr S. Fraser for critical reading of this manuscript.
Supported by grant from the Ministry of Education, Science and Culture of Japan (Grant No. 10770139).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Minetaro Ogawa, PhD, Department of Molecular Genetics, Faculty of Medicine, Kyoto University, Shogoin-Kawaharacho 53, Sakyo-ku, Kyoto 606-8507, Japan; e-mail:mogawa@virus.kyoto-u.ac.jp.