Abstract
The tumor necrosis factor (TNF) receptor-associated factor 1 (TRAF1) is a member of the recently defined TRAF family. It takes part in the signal transduction of the TNF receptor 2 (TNFR2), the lymphotoxin-β receptor (LT-βR), CD40, CD30, and LMP1; is induced by LMP1 in vitro; and protects lymphoid cells from apoptosis. To identify the cells in which TRAF1 is active in vivo, we studied TRAF1 transcripts in normal lymphoid tissue, in Epstein-Barr virus (EBV)-induced lymphoproliferations, and in malignant lymphomas with special reference to those that overexpress the cytokine receptor CD30 and CD40 of the TNF receptor family at the single-cell level using a radioactive in situ hybridization. In normal lymphoid tissue, TRAF1 message proved to be absent from all resting B and T cells as well as from macrophages and accessory cells (follicular dendritic cells and interdigitating cells) and present in few perifollicular and intrafollicular lymphoid blasts. In contrast, there was a high and consistent TRAF1 overexpression in EBV-induced lymphoproliferations and Hodgkin’s disease. Nearly all non-Hodgkin’s lymphoma show low or no TRAF1 expression. Only some cases of diffuse large B-cell lymphoma showed a moderate to high TRAF1 signal. Several of the latter cases were EBV+. These data confirm that TRAF1 is an inducible molecule and indicates its deregulation in the mentioned disorders with the potential of a blockage of the apoptotic pathway.
THE TUMOR NECROSIS factor (TNF) receptor-associated factor (TRAF) family is a recently established group of molecules that are involved in the intracellular signal transduction of several members of the tumor necrosis factor receptor (TNFR) family, eg, the TNF receptor 2 (TNFR2), the lymphotoxin-β receptor (LT-βR), the Epstein-Barr virus (EBV)-encoded latent membrane protein 1 (LMP1), CD40, and CD30.1-8 The TRAF family is defined by a C-terminal homology region, the TRAF domain,1-3 which is further divided into two subdomains. The N-terminal TRAF domain sequences are less conserved than the C-terminal part and appear to contribute to the oligomerization of TRAF proteins.1 The highly conserved C-terminal portion of the TRAF domain is capable of binding to the intracellular portion of TNFR2, CD40, CD30, LT-βR, and LMP1.1 These members of the TNFR family, like TNFR1 and Fas (APO-1/CD95), are known to transduce signals regulating cell death and proliferation9 but lack the intracellular death domain present in TNFR1 and Fas. Recently, it has been shown that TNFR2, CD40, CD30, LT-βR, and LMP1 exert their function by interacting with TRAF1 (EBI6), TRAF2 (TRAP), TRAF3 (CD40bp/CRAF1/LAP1), TRAF5,7,8 or TRAF6,10whereas the Fas and TNFR1 receptors bind to another group of molecules, including FADD (MORT1),11,12 RIP,13 and TRADD,14 by their death domain.
Several members of the TNFR family mediate pleiotropic signals in distinct lymphoid cells and there are examples that members of this receptor group transduce contrary effects.9,15,16 These different effects may be related in part to the differential expression of receptor-associated molecules resulting in different signaling pathways in these cells. To understand the mechanisms in the signal transduction of lymphoid cells, one prerequisite is the knowledge of the expression pattern of its components under normal, inflammatory, and dysregulated neoplastic conditions. Although considerable data have been accumulated about the function and molecular structure of the members of the TRAF family, little is known about their expression in vivo. Most data are derived from Northern blotting of RNA prepared from murine tissues, demonstrating that TRAF2, TRAF3, TRAF5, and TRAF6 are ubiquitously expressed, with the highest levels of TRAF2 and TRAF5 messages being observed in the spleen8 and in the lung and spleen, respectively.10 In contrast, the expression of TRAF1 and TRAF4 (CART1) appears to be more restricted, with selective expression of TRAF4 in breast carcinoma17 and TRAF1 in tonsils, spleen, and lung.4 TRAF1 expression was shown to be induced in B-lymphoblast cell line BL41/B95-8 by the EBV-encoded LMP1.4 These data suggest that, among the members of the TNFR family, TRAF1 is the main candidate for being predominantly and differentially expressed in the lymphoid system. Our interest in the TRAF1 molecule was further raised by the observation that TRAF1 can exert an apoptosis protective effect on CD8+ T cells,18 pointing to the possibility that this molecule might be involved in the regulation of apoptosis in certain reactive lymphoid cells and their neoplastic counterparts. To identify the lymphoid populations in which TRAF1 is active, we investigated reactive lymphoid tissues, EBV-induced lymphoproliferations, and a variety of malignant lymphomas for the expression of TRAF1 transcripts.
MATERIALS AND METHODS
Material.
Paraffin-embedded specimens were drawn from the files of the Institute of Pathology, Universitätsklinikum Benjamin Franklin, Freie Universität Berlin (Berlin, Germany). The present series consisted of 23 cases of classical Hodgkin’s disease (HD; 12 cases of mixed cellularity and 11 cases of nodular sclerosis), 5 cases of lymphocyte-predominant HD (LPHD), 13 cases of anaplastic large-cell lymphoma (ALCL; 6 cases of B-ALCL, 5 cases of T-ALCL, and 2 cases of 0-ALCL), 5 cases of B-cell chronic lymphocytic leukemia/lymphoma (B-CLL), 15 cases of diffuse large B-cell lymphoma (DLBCL), 5 cases of plasmoblastic lymphoma (PLBLL), 2 cases of Burkitt’s lymphoma, 6 cases of atypical EBV-associated lymphoproliferative disorders (ALP), 7 tonsils with the diagnosis of infectious mononucleosis, and further 5 tonsils with the diagnosis of follicular hyperplasia.
The lymphomas were classified according to the REAL classification.19
TRAF1 and EBV-encoded small RNA (EBER) probes.
RNA was prepared from the large anaplastic lymphoma cell line Karpas 299.20 The cDNA was generated with the Moloney murine reverse transcriptase. The complete open reading frame of TRAF1 cDNA from nucleotide position 76 to 1326 of the published sequence4 was amplified with the following primers: 5′-ATGGCTGCAGCTAGCGT-3′ and 5′-TTAGAGCCCTGTCAGGTCC-3′ by polymerase chain reaction (PCR; 40 cycles with 55°C annealing temperature, 72°C elongation step, and 94°C denaturation). The EBER probe was a generous gift from Dr Niedobitek (Birmingham, UK).21
In situ hybridization.
After cloning of the PCR product into the pAMP1 vector (GIBCO-BRL, Eggenstein, Germany) and linearization, antisense and sense probes were generated by SP6 and T7 polymerases (GIBCO-BRL, Eggenstein, Germany), respectively. In situ hybridization was performed as described previously.22 Two sections of each case were incubated with the TRAF1 antisense probe and a further section of each case was hybridized with the TRAF1 sense probe as negative control. Autoradiography was performed after coating the slides with radiographic emulsion (Amersham, Braunschweig, Germany) exposed at 4°C for 3 to 6 weeks, developed in Kodak D19 developer (Kodak, Hemel Hempstead, UK), and counterstained with hematoxylin and eosin. The detection of EBER was performed according to Herbst et al.23
Evaluation.
The number of positive and negative cells was counted. The intensity of the TRAF1 signal was estimated by counting the grains over 20 cells of each case after an exposure time of 6 weeks. An average of grains was calculated for each case. The hybridizations with the TRAF1 sense probe showed only weak background staining of about 5 to 8 grains per cell. Cells that showed 10 or less than 10 grains after hybridization with the TRAF1 antisense probe were considered to be TRAF1−. Low intensity was defined as less than 30 grains but more than 10 grains, medium intensity was defined as more than 30 grains but less than 150 grains, and strong intensity was defined as more than 150 grains above one cell.
Immunohistochemistry.
Paraffin sections of 5 to 6 μm were mounted, dried, and boiled for 2 minutes in 10 mmol/L citrate buffer, pH 6.0. Subsequently, immunohistochemistry and immunocytochemistry were performed using the alkaline phosphatase anti-alkaline phosphatase (APAAP) technique.24 EBV infection was analyzed by immunostaining for LMP1 with the mixture of monoclonal antibodies (MoAbs; CS1-4; DAKO, Hamburg, Germany) in every case. In LMP1+ cases, the EBV latency infection type was further characterized by immunostainings for EBNA2 (MoAb PE2; DAKO) and ZEBRA (MoAb BZ1; DAKO).
The data were statistically analyzed using the χ2 test.
RESULTS
TRAF1 expression in reactive lymphoid tissue.
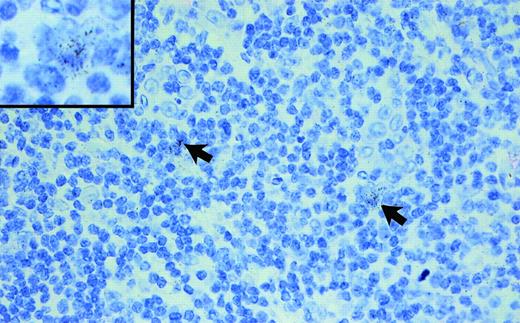
In all 5 hyperplastic tonsils investigated, a weak to intermediate TRAF1 message was detected in a few extrafollicular and intrafollicular blasts (Fig 1). Resting lymphoid cells, including mantle cells and small paracortical lymphoid cells as well as nearly all cells of the follicular center, were TRAF1−. Macrophages and accessory cells, such as follicular dendritic cells and interdigitating cells, also did not show any TRAF1 message.
Hyperplastic tonsil hybridized with a TRAF1 antisense probe (exposure, 6 weeks; original magnification × 250; original magnification of the insert × 900). Note the few faintly TRAF1+ lymphoid blasts (arrows and insert).
Hyperplastic tonsil hybridized with a TRAF1 antisense probe (exposure, 6 weeks; original magnification × 250; original magnification of the insert × 900). Note the few faintly TRAF1+ lymphoid blasts (arrows and insert).
TRAF1 expression in EBV-associated disorders (infectious mononucleosis and atypical lymphoproliferation [ALP]).
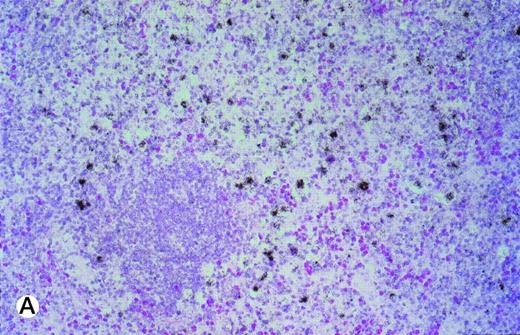
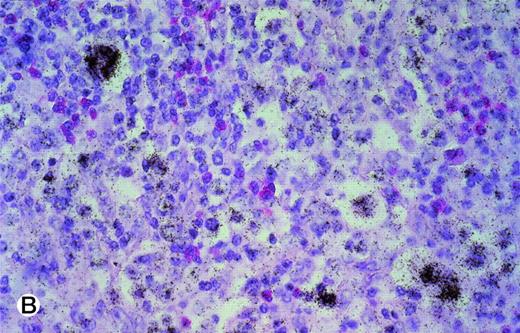
In 6 of 7 tonsils affected by infectious mononucleosis, moderate to strong TRAF1 hybridization signals were detectable in about half of the interfollicular lymphoid blasts (Table 1). Simultaneous hybridization with an EBER and TRAF1 antisense probe showed in 5 of 6 cases that more than 50% of EBV+ lymphoid blasts coexpressed a medium to great amount of TRAF1 mRNA (Fig 2A and B). In ALP, the TRAF1 expression was moderate or high (Table 2). In 2 cases, nearly all lymphoid blasts harbored an intermediate or high amount of TRAF1 mRNA. In the remaining 4 cases, 30% to 40% of the lymphoid blasts showed medium to high amounts of TRAF1 transcripts.
(A and B) Double-labeling of tonsillar tissue from patients with infectious mononucleosis with the TRAF1 (black) and EBER (red) antisense probes (exposure of autoradiography, 6 weeks; original magnifications × 350 [A] and × 150 [B]). The TRAF1 probe was labeled by 35S-UTP, whereas the EBER probe was labeled by UTP-digoxigenin and detected by antidigoxin alkaline phosphatase conjugates.
(A and B) Double-labeling of tonsillar tissue from patients with infectious mononucleosis with the TRAF1 (black) and EBER (red) antisense probes (exposure of autoradiography, 6 weeks; original magnifications × 350 [A] and × 150 [B]). The TRAF1 probe was labeled by 35S-UTP, whereas the EBER probe was labeled by UTP-digoxigenin and detected by antidigoxin alkaline phosphatase conjugates.
TRAF1 expression in lymphomas.
The Hodgkin and Reed-Sternberg (HRS) cells of 23 cases of classic HD studied contained in 16 cases a high amount and in 5 cases an intermediate amount of TRAF1 transcripts (Fig 3A and B; Table 3). Seven of the 23 cases showed a few TRAF1+ bystander cells, with low amount of TRAF1 mRNA in 6 and large amounts in 1 case. In LPHD, the level of TRAF1 message seemed to be lower than in classical HD.
(A and B) TRAF1 in situ hybridization of two cases of classical HD. (A) represents a case of the nodular sclerosis subtype (exposure, 6 weeks; original magnification × 175), whereas a case of the mixed cellularity subtype is shown in (B) (exposure, 6 weeks; original magnification × 350). Note the distinct label of the tumor cells in both cases.
(A and B) TRAF1 in situ hybridization of two cases of classical HD. (A) represents a case of the nodular sclerosis subtype (exposure, 6 weeks; original magnification × 175), whereas a case of the mixed cellularity subtype is shown in (B) (exposure, 6 weeks; original magnification × 350). Note the distinct label of the tumor cells in both cases.
ALCLs were found to express lower amounts of TRAF1 when compared with HD (P < .001). Among ALCL, the TRAF1 expression was lowest in T-ALCL. In general, a minority of tumor cells showed a low to moderate TRAF1 expression in cases of DLBCL. Only in 2 cases was more than 50% of tumor cells moderately to strongly TRAF1+, whereas 1 case of DLBCL was completely TRAF1−. In PLBLL, TRAF1 expression was detectable in only 1 of 5 cases. The low-grade B-cell lymphoma of B-CLL type was TRAF1−, except for some paraimmunoblasts.
A correlation between TRAF1 expression and EBV infection was also found in lymphomas. A high TRAF1 expression was found more frequently in the lymphomas with EBV+ tumor cells than in those ones with EBV− tumor cells (P < .001). This association was confirmed by double-labeling for TRAF1 and EBER transcripts in several cases of EBV+ classical HD, showing that most of the HRS cells were TRAF1+/EBV+.
DISCUSSION
This study presents the first data about TRAF1 expression in human reactive and neoplastic lymphoid tissues at the single-cell level. We focussed our work on TRAF1 because Northern blot data4 had shown that, in comparison with other members of the TRAF family, TRAF1 appears to be most restricted in its expression to the lymphoid tissue. To identify the lymphoid cells that express TRAF1, we applied a highly sensitive in situ hybridization technique using radioactive probes specific for TRAF1. These studies showed that TRAF1 message is absent from resting lymphoid cells, including mantle cells and small paracortical T cells. Among the proliferating cells of the normal lymphoid tissue, only a few extrafollicular and intrafollicular lymphoid blasts contained significant amounts of TRAF1 message. This distribution resembles that of CD30+ blasts,25suggesting that these blasts coexpress TRAF1 and CD30. A moderate to strong expression of TRAF1 was found in the EBV-infected blasts present in infectious mononucleosis and EBV-induced atypical lymphoproliferations. In both lesions, many of the infected cells also express the EBV-encoded LMP1. These findings are of interest in respect to observations showing that LMP1 induces TRAF1 expression in EBV-transformed B-lymphoblastic cell lines and that LMP1 binds most of the TRAF1 protein in these cells.4 26 Our findings demonstrate that this mechanism might also work in vivo.
The TRAF1 expression pattern found in normal and reactively diseased lymphoid tissue fits in with the TRAF1 expression encountered in malignant lymphomas. Among these, the most constant and highest TRAF1 expression was found in HRS cells of HD. The TRAF1 transcript signals were strongest in the EBV+ cases. Moderate to low amounts of TRAF1 message were seen in EBV-infected DLBCL, whereas the non-Hodgkin’s lymphoma (NHL) of B-CLL type that closely corresponds to normal resting B cells was TRAF1− except for a few paraimmunoblasts. TRAF1 expression in other NHLs was low and/or heterogeneous, with the number of cases being too small to be representative.
Recently, Liebowitz27 demonstrated that TRAF1 colocalizes with LMP1 by double-immunofluorescence microscopy and that both proteins could be coimmunoprecipitated in all LMP1+ cases of acquired immunodeficiency syndrome (AIDS)-associated NHL and posttransplantational lymphoproliferative disease. He found functional consequences of the TRAF1-LMP1 cooperation in all of these LMP1+ cases by the demonstration of activated NF-κB in electrophoretic mobility shift assays. Our data extend the expression pattern of TRAF1 reported, particularly in the cases of EBV-associated HD and infectious mononucleosis, and suggest that similar mechanisms might lead to NF-κB induction in the HRS cells of EBV-associated HD.28
There is compelling evidence that B cells that are unable to express Igs are eliminated by apoptosis.29 HRS cells of most cases of HD have recently been shown to contain clonally rearranged Ig genes30,31 but consistently lack Ig expression.32 Hence, it follows that HRS cells represent B cells that should die of apoptosis. The fact that this usually does not happen points towards a blockage of the apoptotic pathway in HRS cells. Recent studies provided evidence that NF-κB is involved in the protection of HRS cells from apoptosis.28 Devergne et al26 showed that NF-κB activation is likely mediated by TRAF1/TRAF2 heteroaggregates. In view of the findings by Speiser et al18 that TRAF1 overexpression in transgenic mice inhibits antigen-induced apoptosis in CD8+ T lymphocytes, it is tempting to speculate that TRAF1 is involved in the blockage of apoptosis in HRS cells.
TRAF1 takes part in the signal transduction of TNFR2, LT-βR, CD40, CD30, and LMP1.1,4,33 In HD and EBV-induced lymphoproliferations, CD40 and CD30 are most consistently expressed.34-36 Therefore, CD40 and CD30 are the most likely cooperation partners of TRAF1 in these disorders apart from LMP1 in the EBV+ cases. Further studies are needed to clarify how these receptors interact with TRAF1 to transduce their signals.
Messineo et al37 published that TRAF1 expression is highly heterogenous in the HRS cells of 6 cases of HD. They used a single-cell PCR technique on suspended lymphoid tissues. These results are partially in contradiction with our data demonstrating that TRAF1 is highly overexpressed in the majority of HRS cells. This discrepancy may be due to the difficulty in identifying HRS cells in cellular suspensions.38
Taken together, the data presented here confirm that TRAF1 is an inducible molecule and show that TRAF1 in normal lymphoid tissue is expressed rarely and weakly, but in EBV-induced lymphoproliferations and HD is consistently and strongly expressed. The differential expression of TRAF1 demonstrated here might partially explain that one and the same member of the TNFR family can induce cell death after its stimulation in one cell type and proliferation in another type.9,15 16 Further investigations are necessary to clarify the role of TRAF1 in EBV-associated lymphoproliferations and in HD.
ACKNOWLEDGMENT
The authors are indebted to E. Berg for the excellent technical assistance.
Supported by Deutsche Forschungsgemeinschaft (SFB 366) and the Deutsche Krebshilfe (Grants No. W76/93/Dü1 and Ste318/5-2).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Horst Dürkop, MD, Institut für Pathologie, UK Benjamin Franklin, Hindenburgdamm 30, D-12200 Berlin, Germany.

![Fig. 2. (A and B) Double-labeling of tonsillar tissue from patients with infectious mononucleosis with the TRAF1 (black) and EBER (red) antisense probes (exposure of autoradiography, 6 weeks; original magnifications × 350 [A] and × 150 [B]). The TRAF1 probe was labeled by 35S-UTP, whereas the EBER probe was labeled by UTP-digoxigenin and detected by antidigoxin alkaline phosphatase conjugates.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.617/4/m_blod402dur02z.jpeg?Expires=1768350837&Signature=QSUM7eQwpQZ~WI9rNPatKIcfs5VjwicgfyMyR9-P4gEY4SVrTnt-l1o2I5IhWeQkCLuIUJAtr~7qQPTd1ukhJe-A9P~3grmDY~G0bHnUeAr5AEHzVhkjsB-wcddyEapQcDM~fj1HfJhBTT5PbwMJVtLxh97efyTtu6m-ktKGzFjRCSHhsO1Xv3Hd2dpJU2-wRoCYSjQdOW9-8j-hoaLU5U1tuBkvxjSKeCMKae1FMfKbo2QCkGW8BjJ175XSCWSY7DFtomd8MapwxmBoDkfOVyLG61Nrl0F48mrGzexvGnSmz0ypZy2Z5wNwuyg9Y2yhDn2Zt9X6iViN7UZM6lkAVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)