Abstract
Thrombin generation in platelet-rich plasma (PRP) involves complex interactions between platelets and coagulation proteins. We previously reported that the addition of fibrin to PRP enhances tissue-factor initiated thrombin generation by ≈ 40%, and the current studies were designed to assess the mechanism(s) underlying thrombin generation in the absence and presence of fibrin. Blocking platelet GPIIb/IIIa + vβ3 receptors with a monoclonal antibody (MoAb) inhibited basal thrombin generation, but did not affect the enhancement produced by fibrin. In contrast, blocking GPIb with any of three different MoAbs had no effect on basal thrombin generation, but essentially eliminated fibrin enhancement of thrombin generation. When thrombin generation was tested in PRP deficient in von Willebrand factor (vWF), both basal and fibrin-enhanced thrombin generation were markedly reduced, and the addition of factor VIII did not normalize thrombin generation. Botrocetin, which induces the binding of vWF to GPIb, enhanced thrombin generation. In all studies, the ability of PRP to support thrombin generation correlated with the production of platelet-derived microparticles and serum platelet-derived procoagulant activity. Thus, two separate mechanisms, both of which depend on vWF, appear to contribute to platelet-derived procoagulant activity: one is independent of fibrin and relies primarily on GPIIb/IIIa, but with a minor contribution from vβ3; and the other is fibrin-dependent and relies on GPIb. These data may have implications for understanding the mechanisms of the abnormalities in serum prothrombin times reported in Bernard-Soulier syndrome, hemorrhage in von Willebrand disease (vWD), and the increased risk of thrombosis associated with elevated vWF levels.
THE FORMATION OF AN arterial thrombus involves platelet deposition, activation of coagulation, and fibrin formation. There is abundant evidence that platelets can facilitate thrombin generation by a number of different mechanisms.1We recently showed that in a fibrin-free system consisting of gel-filtered platelets and defibrinated plasma, inhibition of platelet GPIIb/IIIa + αvβ3 receptors with monoclonal antibody (MoAb), 7E3, inhibits ex vivo thrombin generation induced by tissue factor by ≈ 47%1; similar inhibition by 7E3 was observed using platelet-rich plasma (PRP),1 a system in which fibrin formation occurs late in the course of the experiment. The inhibition of thrombin generation correlated with decreased platelet microparticle formation, offering a possible mechanistic explanation, as platelet microparticle formation results in loss of the normal asymmetry of platelet membrane phospholipids leading to surface exposure of negatively-charged phospholipids, which are highly active in supporting thrombin generation.2-4
In separate experiments, we found that fibrin itself can enhance platelet membrane procoagulant activity (PMPA) when added to PRP, even when fibrin is formed in a way that it does not contain thrombin.5 6 Thus, the explosive generation of thrombin that occurs in recalcified PRP after a lag-phase (see eg, Fig 1) is probably the result of a composite resonance loop in which (1) the generation of small amounts of thrombin converts fibrinogen to fibrin, (2) both thrombin and fibrin activate platelet to produce PMPA, and (3) the enhanced PMPA facilitates the generation of more thrombin and fibrin. Thus, fibrin appears to play an active role in amplifying thrombin generation and further fibrin formation.
The current studies were designed to assess the relative contributions of thrombin and fibrin to PMPA production under different experimental conditions and to identify the platelet membrane receptors and adhesive ligands that are responsible for the fibrin-platelet interactions that result in enhanced thrombin generation. Thus, we investigated the roles of GPIIb/IIIa, αvβ3, GPIb, GPIa/IIa (α2β1), and von Willebrand factor (vWF).
MATERIALS AND METHODS
Reagents.
The chromogenic substrate used for measuring thrombin was S2238: H-D-Phe-Pip-Arg-pNA.2HCl. Buffer A: 20 mmol/L HEPES, 150 mmol/L NaCl, 0.5 g/L bovine serum albumin (BSA; Lot A-7030, Sigma, St Louis, MO), pH 7.35. Buffer B: same as buffer A with 20 mmol/L EDTA, pH = 7.9. Antibody binding buffer (FACScan): 10 mmol/L HEPES, 0.15 mol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 2.5 mmol/L CaCl2 (pH 7.4). Synthetic d-arginyl-L-glycyl-L-aspartyl-L-tryptophan (d-RGDW) (Mr 530) was obtained from Rhone-Poulenc Rorer Antony (Paris, France). Botrocetin was from Kordia (Leiden, The Netherlands). Phospholipid vesicles were prepared from a mixture of 20% brain phosphatidyl serine (PS) and 80% egg phosphatidyl choline (PC) sonicated into a buffer containing 50 mmol/L Tris HCl (pH 7.35 ) and 100 mmol/L NaCl. All other reagents were of the highest grade commercially available.
Proteins and antibodies.
Bovine factors Xa and Va and human prothrombin were kindly provided by Dr R Wagenvoord (Maastricht University, Maastricht, The Netherlands). Recombinant tissue factor was obtained from Dade (Düdingen, Switzerland). Agihal, purified fraction of Agkistrodon halys halys snake venom, which splits fibrinopeptide A from fibrinogen, was obtained from Prof L. Yukelson (Tashent, Uzbekistan). Staphylocoagulase was prepared as described.7 Recombinant factor VIII (rFVIII; Recombinate, Baxter, Deerfield, IL) contains only traces of vWF (<2 ng per unit of factor VIII). Fluorescein isothiocyanate (FITC)-labeled annexin V (Apoptest, Oregon Green) was from NeXius Research BV (Hoeven, The Netherlands).
Murine MoAbs, 7E3 (anti-GPIIb/IIIa + αvβ3)8, 6D1 (anti-GPIbα),9 and 6F1 (anti-GPIa/IIa)10 have been previously described in detail. MoAb CD42b (anti-GPIb) was from Immuntech (Marseille, France) and MoAb AP-1 (anti-GPIb) was a kind gift of Dr Thomas Kunicki (Scripps Institute, La Jolla, CA). R-phycoerythrin-conjugated MoAbs to human GPIIb/IIIa (5B12) and GPIb (AN51) were obtained from Dako (Glostrup, Denmark). An affinity-purified polyclonal antibody to vWF was obtained from the Central Laboratory of the Red Cross (CLB) in Amsterdam, The Netherlands.
Preparation of plasma.
PRP was obtained by centrifuging fresh citrated blood (9 parts of blood to one part of 0.13 mol/L trisodium citrate) at 250g, 15°C for 10 minutes. The platelet count was adjusted to 3 × 108/mL using autologous platelet-poor plasma (PPP).
The study included four patients with Glanzmann thrombasthenia of Iraqi-Jewish descent (GPIIIa defect leading to loss of both GPIIb/IIIa and αvβ3) and three of Arab descent (GPIIb defect leading to loss of GPIIb/IIIa, but normal or increased αvβ3) residing in Israel.11 By courtesy of Dr Karly Hamulyak (Academic Hospital, Maastricht), blood was obtained from one patient with Glanzmann thrombasthenia, and one with von Willebrand disease (vWD) type IIa. By courtesy of Dr J. Eikelboom12 (Academic Hospital Leiden), blood was obtained from a patient with type III vWD (described in Weiss et al13).
Preparation of clots.
Fibrin I clots (noncross-linked, des AA fibrin) were prepared as previously described using the snake venom protease Agihal. Addition of clots to PPP did not cause coagulation within 2 hours and did not influence either the clotting time or thrombin generation in recalcified PPP. Three clots were added to PRP in the thrombin generation experiments, representing ≈ 3 times the potential fibrin content of the PRP.
Measurement of thrombin generation.
Thrombin generation in plasma was performed as described previously.14 15 In short, for thrombin generation in PPP, 20 μL of a kaolin suspension and 20 μL of Buffer A were added to 240 μL of PPP. At 4 minutes, 20 μL of PS/PC (20 μmol/L) was added and at 5 minutes, coagulation was triggered by adding 60 μL of 0.1 mol/L CaCl2. For thrombin generation in PRP, 240 μL of PRP was incubated with 60 μL of Buffer A or buffer containing the antibody or other additions to be tested for 10 minutes at 37°C. Fibrin clots were added just before coagulation was initiated by adding 60 μL of 0.1 mol/L CaCl2, 1.8 fmol/L tissue factor.
One minute after initiating coagulation, 10 μL-samples of the reaction mixture were taken at 1-minute intervals and added to prewarmed (37°C) cuvettes containing 490 μL of 200 μmol/L S2238 in buffer B. The reaction was stopped after 2 minutes by adding 300 μL of 1 mol/L citric acid, and the optical density (OD) was measured at 405 nm. Thrombin amidolytic activity was calculated by comparing the OD/minute value of the test sample to a thrombin standard calibration curve. Free thrombin was calculated from thrombin amidolytic activity using our previously described computer program that takes into account the contributions of free thrombin and α2-macroglobulin–bound thrombin.15
The lag time of thrombin formation is defined as the time from addition of the triggering solution to the time at which the thrombin concentration increases above 10 nmol/L and the endogenous thrombin potential (ETP) is defined as the area under the thrombin generation curve.16 17 In normal PRP, the ETP is 411 ± 13 nmol/L × min (mean ± standard error of mean [SEM], n = 28).
Measurement of platelet-derived procoagulant activity and microparticles in serum.
The platelet-membrane derived procoagulant phospholipid activity (PMPA) was determined by diluting serum prepared from PRP after thrombin generation experiments 3:20 in buffer A and adding a 50-μL aliquot of the diluted serum to 100 μL of an assay mixture containing 0.45 nmol/L factor Xa, 10.5 nmol/L factor Va, 3 μmol/L prothrombin and 12 mmol/L Ca2+ in buffer A. At 4 minutes, a 10 μL subsample was added to cuvettes containing 465 μL of buffer B. Thrombin concentrations were then assayed with S2238 by determining the change in absorbance over time. Normal serum gives values of 115 ± 5.5 nmol/L/minute (mean ± SEM, n = 22), equivalent to the effect of 300 nmol/L PS/PC (20%/80%) vesicles. Normal PPP or the serum left after a thrombin generation experiment in PPP gives values < 12 nmol/L/minute.
To detect platelet-derived microparticles, 15 μL of serum was incubated with R-phytoerythrin-conjugated mouse MoAbs against GPIIb/IIIa or GPIb. To assess the presence of anionic phospholipids on the microparticles, 250 μL of 2 μg/mL FITC-labeled annexin V was added. The analysis was performed in a flow cytometer (EPICS XL-MCL; Becton Dickinson & Co, San Jose, CA). A total of 10,000 events was recorded and the data were analyzed using the CellQuest software program, version 1.2 (Becton Dickinson & Co). There was a high correlation between annexin-V–positive and platelet glycoprotein-positive particles, indicating that the particles that exposed PS were derived from platelets.
Measurement of residual prothrombin in serum.
RESULTS
The effects of adding fibrin clots to normal PRP and of blocking GPIIb/IIIa + αvβ3, GPIb, and GPIa/IIa (α2β1) receptors.
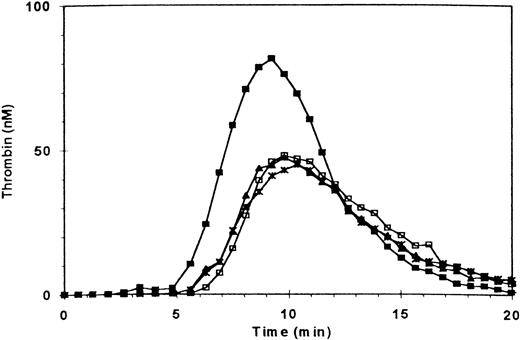
Consistent with our earlier observations, adding fibrin clots to normal PRP enhanced the ETP by ≈42%, peak thrombin generation by ≈64%, platelet-derived microparticles (PDMP) by ≈44%, and PMPA by ≈78%5(Fig 1 and Table 1). It also decreased residual prothrombin by ≈50% (Table 1). Also consistent with our earlier observations, blockade of GPIIb/IIIa + αvβ3 receptors by antibody 7E3 or the peptide d-RGDW (60 μmol/L) decreased ETP of PRP to 42% and 60% of normal, respectively1 (Fig 1 and Table1). The other parameters of thrombin generation were affected in a manner consistent with the inhibitory effects of these agents on the ETP (Table 1). What was most remarkable, however, was the ability of fibrin clots to enhance thrombin generation in PRP in which GPIIb/IIIa + αvβ3 receptors had been blocked by 7E3 or d-RGDW (Fig 1 and Table1).
Effect of blocking GPIIb/IIIa + vβ3 receptors on thrombin generation in the absence and presence of fibrin clots. Thrombin generation was triggered at t = 0 in PRP (adjusted to 3 × 108/mL) by recalcification and addition of tissue factor. (□) Control; (▪) three fibrin clots added at t = 0; (∗) PRP preincubated with antibody 7E3 (anti-GPIIb/IIIa + vβ3; 20 μg/mL); (▴) preincubation with 7E3 and fibrin clots added.
Effect of blocking GPIIb/IIIa + vβ3 receptors on thrombin generation in the absence and presence of fibrin clots. Thrombin generation was triggered at t = 0 in PRP (adjusted to 3 × 108/mL) by recalcification and addition of tissue factor. (□) Control; (▪) three fibrin clots added at t = 0; (∗) PRP preincubated with antibody 7E3 (anti-GPIIb/IIIa + vβ3; 20 μg/mL); (▴) preincubation with 7E3 and fibrin clots added.
Blocking the vWF binding domain of GPIb with antibody 6D1 had the mirror image effect of GPIIb/IIIa + αvβ3 blockade; thus, it had no effect on thrombin generation in the absence of added fibrin, but prevented the procoagulant-enhancing effect of fibrin (Fig 2 and Table 1). Two other antibodies against GPIb (AP-1 and CD 426) gave similar results (Table 1). When antibodies 7E3 and 6D1 were used in combination, the results were additive, with both a reduction in thrombin generation and near elimination of the enhancement of thrombin generation by adding fibrin (Table 1). The MoAb 6F1, which blocks GPIa/IIa, affected neither normal thrombin generation nor the enhanced thrombin generation in the presence of fibrin, and thus served as a control (Table 1). Ionomycin-treated PRP supported thrombin generation to the same extent as did normal PRP with added fibrin (Table 1). None of the antibodies inhibited thrombin generation supported by ionomycin-treated platelets (data not shown).
Effect of blocking GPIb receptors on fibrin-enhanced platelet procoagulant activity. Thrombin generation was measured as in Fig 1. (□) Control; (▪) three fibrin clots added at t = 0; (∗) PRP preincubated with antibody 6D1 (anti-GPIb, 20 μg/mL); (▴) preincubation with 6D1 and fibrin clots added.
Effect of blocking GPIb receptors on fibrin-enhanced platelet procoagulant activity. Thrombin generation was measured as in Fig 1. (□) Control; (▪) three fibrin clots added at t = 0; (∗) PRP preincubated with antibody 6D1 (anti-GPIb, 20 μg/mL); (▴) preincubation with 6D1 and fibrin clots added.
Studies using PRP from patients with Glanzmann thrombasthenia.
Because antibody 7E3 blocks both platelet GPIIb/IIIa and αvβ3, we tried to assess the contributions of each of these receptors by comparing the results using PRP from different patients with Glanzmann thrombasthenia. Iraqi-Jewish patients have no detectable GPIIb/IIIa or αvβ3, whereas Israeli-Arab patients have virtually no GPIIb/IIIa, but approximately twice the normal level of platelet αvβ3.11 19 In both patient groups, thrombin generation in PRP is decreased to about 60% of normal (Table 2). Antibody 7E3 decreased thrombin generation in PRP of two Arab patients tested (from 68% to 59% and from 35% to 25%), but had virtually no effect on thrombin generation in the PRP of Iraqi-Jewish patients (from 71% to 70% and from 63% to 65%) (Table 2). Addition of fibrin clots to the PRP of patients in either group resulted in increased thrombin generation (increases of ≈18%, 44%, and 103% for Arab patients B, C, and D; and ≈28% for Iraqi-Jewish patient A), supporting the conclusions derived from the antibody studies, namely that the effect of fibrin does not require either GPIIb/IIIa or αvβ3 receptors.
Experiments with vWF.
Because vWF has been reported to bind to fibrin and support an interaction with platelet GPIb20, we investigated the effect of vWF on thrombin generation. Decreasing vWF activity of normal PRP with a neutralizing antibody not only prevented the enhancement of thrombin generation produced by fibrin, but, unexpectedly it also diminished baseline thrombin generation (Fig 3). In contrast, thrombin generation in PPP was unaffected by vWF neutralization (Fig 3, inset), indicating that there was sufficient factor VIII coagulant activity in the antibody-treated plasma to support thrombin generation. Addition of ionomycin (Fig 3) or a frozen and thawed platelet lysate (not shown) restored normal thrombin generation, indicating that the defect caused by vWF could be overcome by activated platelets.
Effect on thrombin generation of reducing vWF activity in plasma. (□) Control (normal PRP with 10 μg/mL rabbit IgG); (•) PRP preincubated with vWF antibody (10 μg/mL); (X) PRP preincubated with anti-vWF (10 μg/mL), three fibrin clots added at t = 90 s; (▴) preincubated with vWF antibody and 10 μmol/L ionomycin added at t = 10 s. (Inset) Thrombin generation in PPP. The reaction was triggered with PS/PC and Ca2+. (□) Control PPP with 10 μg/mL rabbit IgG; (•) PPP preincubated with anti-vWF (10 μg/mL).
Effect on thrombin generation of reducing vWF activity in plasma. (□) Control (normal PRP with 10 μg/mL rabbit IgG); (•) PRP preincubated with vWF antibody (10 μg/mL); (X) PRP preincubated with anti-vWF (10 μg/mL), three fibrin clots added at t = 90 s; (▴) preincubated with vWF antibody and 10 μmol/L ionomycin added at t = 10 s. (Inset) Thrombin generation in PPP. The reaction was triggered with PS/PC and Ca2+. (□) Control PPP with 10 μg/mL rabbit IgG; (•) PPP preincubated with anti-vWF (10 μg/mL).
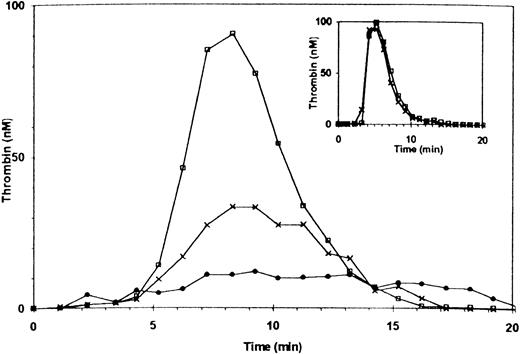
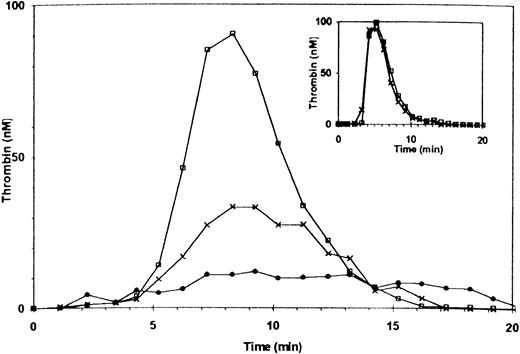
Additional experiments were performed with PRP of patients with mild and severe vWD. Thrombin generation in the PRP of a patient with mild vWD (type IIa, ≈30% factor VIII and 4% vWF antigen) was ≈60% of normal. Adding anti-vWF antibody reduced thrombin generation to ≈12% of normal (Fig 4). The patient’s factor VIII coagulant activity was sufficient to support thrombin generation as demonstrated by normal thrombin generation using the patient’s PPP (Fig 4, inset), with or without the addition of the anti-vWF antibody.
Thrombin generation in PRP of a patient with mild type IIa vWF deficiency. The patient’s plasma contained 30% factor VIII and ≈ 4% of vWF antigen. (□) Control PRP (with 10 μg/mL rabbit IgG); X, patient’s PRP (rabbit IgG added); (•) patient’s PRP preincubated with anti-vWF antibody (10 μg/mL). Inset: thrombin generation in PPP. (□) Normal control; (X) patient.
Thrombin generation in PRP of a patient with mild type IIa vWF deficiency. The patient’s plasma contained 30% factor VIII and ≈ 4% of vWF antigen. (□) Control PRP (with 10 μg/mL rabbit IgG); X, patient’s PRP (rabbit IgG added); (•) patient’s PRP preincubated with anti-vWF antibody (10 μg/mL). Inset: thrombin generation in PPP. (□) Normal control; (X) patient.
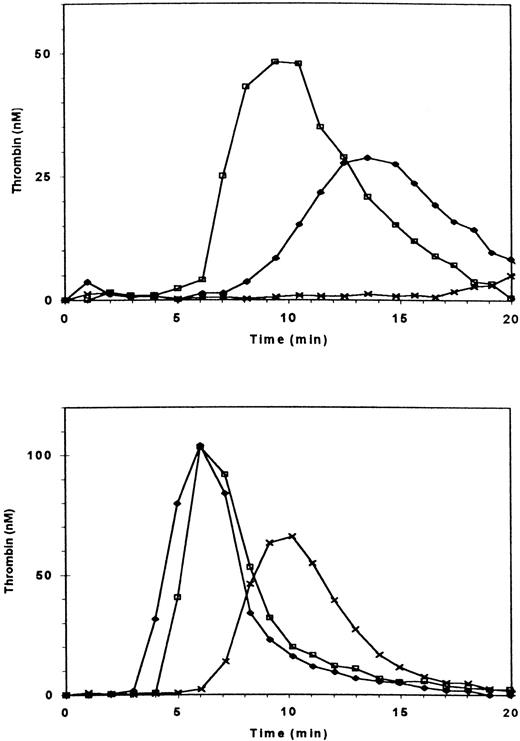
Thrombin generation in PRP from a patient with type III, severe vWD (< 1% of vWF antigen and factor VIII) was more severely diminished than in the PRP of a patient with mild vWD (Fig 5, upper frame). Addition of sufficient rFVIII to increase the factor VIII coagulant activity to 100% normalized thrombin generation in PPP (Fig 5, lower frame) yet only partially restored thrombin generation in PRP to 40% of normal (Fig 5, upper frame). The addition of 2.5% of normal rFVIII was without effect on thrombin generation in PRP, but the addition of 2.5% of normal PPP, which provides both vWF and essentially the same amount of factor VIII, restored thrombin generation (Fig 6). Adding ionomycin or either normal or patient platelet lysate (not shown) restored thrombin generation, indicating that procoagulant phospholipids were indeed rate-limiting. To further assess whether the interaction of vWF and GPIb enhanced thrombin generation, we added botrocetin to PRP and found that it did, indeed, increase thrombin generation (Fig7). Ristocetin could not be tested because in control experiments it inhibited thrombin generation in PPP.
Thrombin generation in plasma of patient with type III severe vWD. The patient has less than 1% of factor VIII and vWF antigen and ristocetin cofactor activity. (Upper frame) (□) Normal PRP; (X) patient’s PRP; (⧫) patient’s PRP with 100% recombinant factor VIII added. (Lower frame) Thrombin generation in intrinsically triggered nondefibrinated PPP. (□) Normal PPP; (X) patient’s PPP; (⧫) patient’s PPP with 100% recombinant FVIII added.
Thrombin generation in plasma of patient with type III severe vWD. The patient has less than 1% of factor VIII and vWF antigen and ristocetin cofactor activity. (Upper frame) (□) Normal PRP; (X) patient’s PRP; (⧫) patient’s PRP with 100% recombinant factor VIII added. (Lower frame) Thrombin generation in intrinsically triggered nondefibrinated PPP. (□) Normal PPP; (X) patient’s PPP; (⧫) patient’s PPP with 100% recombinant FVIII added.
Thrombin generation in PRP of a patient with severe (type III) deficiency of vWF. (▴) patient’s PRP with 2.5% recombinant FVIII added; (▵) patient’s PRP with 2.5% normal PPP added; (+) patient’s PRP with 2.5% normal PPP and with a frozen and thawed suspension of normal platelets added at t = 90 s; (•) patient’s PRP with 2.5% normal PPP and with a frozen and thawed suspension of the patient’s platelets. The final concentration of platelet material was equivalent to 2 × 107 platelets/mL.
Thrombin generation in PRP of a patient with severe (type III) deficiency of vWF. (▴) patient’s PRP with 2.5% recombinant FVIII added; (▵) patient’s PRP with 2.5% normal PPP added; (+) patient’s PRP with 2.5% normal PPP and with a frozen and thawed suspension of normal platelets added at t = 90 s; (•) patient’s PRP with 2.5% normal PPP and with a frozen and thawed suspension of the patient’s platelets. The final concentration of platelet material was equivalent to 2 × 107 platelets/mL.
Effect of botrocetin on thrombin generation in PRP. (□) Normal PRP; (▪) 5 μg/mL botrocetin added; (X) 25 μg/mL; (▴) 50 μg/mL.
Effect of botrocetin on thrombin generation in PRP. (□) Normal PRP; (▪) 5 μg/mL botrocetin added; (X) 25 μg/mL; (▴) 50 μg/mL.
DISCUSSION
We previously observed two different phenomena related to platelets and thrombin generation: (1) in a fibrin-free system or in a system in which fibrin is generated late in the reaction, blockade of GPIIb/IIIa and to a lesser extent αvβ3 decreases thrombin generation, and (2) adding fibrin to PRP enhances thrombin generation. The present studies were designed to obtain data on the receptors, ligand, and mechanisms responsible for these phenomena. Despite the ability of GPIIb/IIIa to bind fibrinogen, polymerizing fibrin, and clotted fibrin under static or flow conditions,21-27 our current data indicate that the enhancing effect of fibrin on thrombin generation cannot be attributed to a GPIIb/IIIa-mediated mechanism because fibrin retains its stimulating effect in the presence of a GPIIb/IIIa + αvβ3 blocking antibody or peptide, as well as when added to the PRP of Glanzmann patients.
We next studied the interaction between GPIb and vWF because platelets have been shown to bind to fibrin through the interaction between fibrin-bound vWF and platelet GPIb.20 Although anti-GPIb MoAbs had no effect on thrombin generation in the absence of added fibrin clots, they essentially abolished the enhancing effect of the added fibrin. Thus, the fibrin effect seems to be mediated via GPIb. This observation provides a possible explanation for the abnormal prothrombin consumption previously reported in patients with Bernard-Soulier syndrome, whose platelets lack GPIb28-32and the abnormal prothrombin consumption that we previously reported when antibody 6D1 was added to normal blood.9
In view of the important role of GPIb in the fibrin-dependent enhancement of thrombin generation in PRP, it is perhaps surprising that in normal, recalcified PRP, thrombin generation initiated by tissue factor is minimally inhibited by blocking GPIb. The most likely explanation is that in these experiments, fibrin begins to form late in the process, at the very beginning of the thrombin burst, and thus there is insufficient time for it to affect the process. When fibrin in the form of preformed clots is added before the thrombin burst occurs, it enhances thrombin generation and shortens the lag-phase in a GPIb-dependent mechanism. Taken together, our previous and current data indicate that these are two different pathways for augmenting platelet coagulant activity: (1) a GPIIb/IIIa- and perhaps αvβ3dependent pathway that operates independently of fibrin, and (2) a fibrin-and GPIb-dependent pathway. Of note, based on data using neutralizing antibody to vWF and plasmas of patients with vWD, as well as our data using botrocetin, both pathways appear to depend on vWF, suggesting that vWF binding to GPIIb/IIIa (and perhaps αvβ3) is important in the development of platelet coagulant activity.
The generation of PMPA and platelet-derived microparticles in serum and the consumption of prothrombin followed the same pattern of inhibition as did thrombin generation. This suggests that microparticle formation is responsible for the PMPA and that GPIIb/IIIa (and perhaps αvβ3), GPIb, and vWF are all required for maximal microparticle formation in a fibrin-containing system. The ability of ionomycin-treated platelets and platelet lysates to overcome the abnormalities produced by the antibodies further suggests that the defects result in decreased microparticle formation.
From our results it appears that the fibrin in a clot or thrombus is not merely an inert, mechanical component.33 It also is clear that vWF, apart from its established function in platelet adherence and as a carrier of factor VIII, may also play an important role in the generation of thrombin through its effect on the generation of platelet microparticles and platelet coagulant activity. Because it has been proposed that the thrombosis associated with heparin-induced thrombocytopenia is linked to platelet microparticle formation,34,35 it is interesting to speculate that variations in vWF levels may account for the interindividual differences in thrombotic risk. Recently, platelet microparticles were found to support transcellular metabolism of eicosanoids,36leading to activation of platelets and endothelial cells, as well as modulation of monocyte-endothelial interactions,37 and so it is possible that the mechanisms we are studying have implications for these phenomena as well. Finally, our observations have potential implications for understanding better the pathophysiology of the bleeding in vWD, as well as the association between elevated plasma vWF activity and acute myocardial infarction,38,39 as well as death after stroke.40
ACKNOWLEDGMENT
We are grateful to the patients who volunteered to give their blood and to their doctors, Dr Karly Hamulyak and Dr J. Eikenboom, for arranging the opportunity to study their plasma. Dr Ariella Zivelin has been a great support in the experiments with the PRP from the Iraqi-Jewish and Arab Glanzmann patients. Our thanks are due to Dr J.A. van Mourik for providing us the vWF antibodies. We also thank Dr J. Heemskerk for performing the Ca2+-influx experiments and Dr Hu Kai for his method of measurement of microparticle procoagulant activity.
Supported in part by Program Grant No. 900-526-192 from the Dutch Organization for Scientific Research (N.W.O.) and in part by Grants No. 19278 and 54469 from the National Heart, Lung and Blood Institute, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to B. S. Coller, MD, Box 1118, Mount Sinai School of Medicine, One Gustave L. Levy Place, New York, NY 10029; e-mail: bcoller@smtplink.mssm.edu.