Abstract
The expression of high levels of P-glycoprotein (Pgp) in circulating mononuclear cells allowed us to use an ex vivo assay as a surrogate measure of Pgp antagonism. Efflux of rhodamine from CD56+cells was measured before the start of PSC 833 and at varying times thereafter. Patients receiving PSC 833 had decreased rhodamine efflux from their circulating CD56+ cells. Time course studies showed that following a single oral dose of PSC 833, decreased rhodamine efflux was found in some patients within 15 minutes of treatment. Maximal inhibition was observed at times ranging from 45 minutes to 60 minutes. A dose-response relationship was shown between the concentration of PSC 833 in the blood and the inhibition of rhodamine efflux, with an apparent plateau of the inhibition of rhodamine efflux at approximately 1,000 ng/mL. The Ki, defined as the concentration required for half-maximal inhibition of Pgp-mediated rhodamine efflux, was determined to be in the range of 29 to 181 ng/mL; although results in two patients were distinctly different, with Ki values of 914 and 916 ng/mL. MRK-16 staining was similar among all patients. We conclude that measurement of rhodamine efflux from CD56+ cells provides a surrogate assay with the potential for monitoring Pgp antagonism in clinical trials.
EARLY STUDIES AIMED at reversal of P-glycoprotein (Pgp)-mediated resistance used compounds already available and in use in clinical medicine for indications unrelated to oncology. These studies, referred to as ‘first generation’ reversal studies, provided several insights into the obstacles that must be surmounted to prove that reversal of Pgp-mediated resistance could be of clinical benefit. A variety of agents, including verapamil, quinidine, quinine, cyclosporin A, tamoxifen, and megestrol acetate were investigated with response rates of 0% to 73%, depending on the tumor type studied, the reversal agent chosen, and the anticancer regimen used.1,2 The failure of these studies to convincingly show a role for Pgp antagonism in the clinic was likewise related to the use of antagonists in patients with tumors in whom resistance was not clearly Pgp-mediated, inadequate blood concentrations of reversal agents, and the use of drugs or schedules for which resistance was not clearly present.2 The observation that blood concentrations of the reversal agents were often below that needed in the in vitro setting for the reversal of resistance in cell lines expressing high levels of P-glycoprotein led to the development of ‘second generation’ antagonists. These second generation agents, including S9788, GF910128, dexniguldipine, PSC 833, and VX 710, reverse high levels of resistance at concentrations that are clinically attainable.3 In phase I studies, the maximum tolerated dose of PSC 833 has been shown to achieve concentrations of 3,000 to 4,000 ng/mL.4 This is 3- to 10-fold higher than that shown to be effective in vitro.5
In addition to the difficulty in achieving sufficient concentrations of the reversal agents, in vitro laboratory studies with a variety of antagonists raised concerns that protein binding in serum impaired the Pgp antagonism.6-9 PSC 833 was reported by Ludescher et al to be the antagonist least affected by performing the drug accumulation assay in serum.6 The extent to which these in vitro studies reflect decreased antagonist potency in patients has not yet been confirmed, because direct assays measuring intratumoral drug concentrations are rare. Although the first-generation antagonists are effectively used in the clinic for their primary indications, protein binding could be a greater problem for Pgp antagonism if the affinity of the antagonist was less for Pgp than for serum proteins or for the drugs’ principal target.
Thus, several attempts have been made to develop surrogate assays for Pgp antagonism. We used an approach based on the observation that hematopoietic cells, including circulating mononuclear cells, express Pgp and show Pgp-mediated efflux of rhodamine, which can be inhibited by systemic administration of a reversal agent before blood collection.10 Peripheral mononuclear cells including CD3+, CD4+, and CD8+ T cells, as well as the CD56+ natural killer (NK) subset, have been shown to overexpress P-glycoprotein, with highest levels observed in CD8+ and CD56+ cells.11-14 The role that Pgp plays in these cells is largely unexplored. Phytohemagglutinin (PHA) activation of mononuclear cells has been shown to increase Pgp expression.15 One report showed that Pgp antagonists were able to inhibit interleukin-2 (IL-2) transport in T cells.16 The Pgp present in the CD56+ population of cells appears functionally identical to that observed in multidrug resistant cells, with highest levels of RNA, protein, as well as drug efflux observed in the CD56+population. For this reason, we examined Pgp function and expression in the CD56+ population.
The results presented here show that rhodamine efflux in the CD56+ population provides a surrogate marker for Pgp antagonism and that inhibition of rhodamine efflux occurs at clinically achievable concentrations of PSC 833.
MATERIALS AND METHODS
Clinical studies.
Blood samples were obtained from patients enrolled on two separate multidrug resistance reversal trials at the Clinical Center, National Cancer Institute. The 20 patients examined for this study ranged in age from 20 to 76 years old (mean, 54 years). Diagnoses for these patients are presented in Table 1. In these trials, patients received PSC 833 formulated in a soft gelatin capsule, which has 30% to 50% bioavailability, in combination with either vinblastine or paclitaxel.17 Patients on the PSC 833 + vinblastine study were receiving PSC 833 at doses of 3, 4, and 5 mg/kg every 6 hours, whereas patients on the PSC 833 + paclitaxel study were receiving PSC 833 at 4 or 5 mg/kg every 6 hours. Samples were typically obtained as “pre,” before initial exposure to chemotherapy or to PSC 833; at indicated time points following the first dose of PSC 833 administered (0, 15, 30, 45, 60, and 120 minutes); as “peak,” 2 hours following ingestion of PSC 833; and as “trough,” 6 hours following PSC 833 administration. The peak and trough samples were obtained after at least 6 days of PSC 833 administration on the every-6-hour schedule. It should be noted that pharmacokinetic studies have suggested that peak PSC 833 concentration may occur from 2 to 4 hours following ingestion of PSC 833.17 18 The time course studies were obtained over the initial 2 hours following the first administration of PSC 833.
Materials.
PSC 833 was provided by Novartis Research Institute (Hanover, NJ).
Cell lines.
SW620 Ad2, Ad5, Ad10, Ad20, and Ad300 are multidrug resistant human colon carcinoma sublines, selected from parental SW620 cells and maintained in 2, 5, 10, 20, and 300 ng/mL of doxorubicin, respectively.19 Parental cells and sublines are carried in RPMI, 10% fetal calf serum (FCS), penicillin, streptomycin, and glutamine. SW620 Ad2, Ad5, Ad10, Ad20, and Ad300 cells possess a multidrug resistant phenotype and do not appear to have mechanisms of resistance other than overexpression of Pgp.19
Isolation of peripheral blood mononuclear cells (PBMCs).
Whole blood (15 to 30 mL) was drawn into a heparinized syringe from patients enrolled on the PSC 833 protocols. The blood was used directly in the rhodamine efflux assay, as described below, or was diluted 1:1 with Dulbecco’s phosphate-buffered saline (DPBS), layered onto Lymphocyte Separation Medium (Organon Teknika, Durham, NC) and centrifuged at 2,000 rpm for 30 minutes. The mononuclear layer was removed, washed with cold DPBS, and then frozen in 10% DMSO/90% FCS and stored at −80°C for later use.
Rhodamine 123 efflux.
Assays were performed as previously described in cultured cells20 and modified as described below for mononuclear cells. Whole blood was obtained from each patient in a heparinized syringe. Rhodamine 123 (Sigma, St Louis, MO) with or without PSC 833 was added to aliquots of whole blood to achieve a final rhodamine concentration of 0.5 μg/mL and PSC 833 concentration of 3 μg/mL. The blood was incubated for 30 minutes at 37°C in 5% CO2. After the accumulation period, the blood was layered onto Lymphocyte Separation Medium and centrifuged at 2,000 rpm for 5 minutes. The mononuclear cell layer from each tube was transferred to a separate tube, washed with cold DPBS, resuspended in 200 μL cold DPBS with 2% FCS (DPBS/FCS), and held at 4°C for later staining. Cells that were to be subjected to an efflux period were then resuspended in rhodamine-free complete media (phenol red-free improved modified Eagle’s medium [IMEM] with 10% FCS) with or without 3 μg/mL PSC 833 and incubated another 60 minutes at 37°C in 5% CO2. After the efflux period, cells were sedimented and washed twice with cold DPBS/FCS. After the washings, the cells were resuspended in 200 μL cold DPBS/FCS. The cells were then stained with phycoerythrin (PE)-labeled CD56 antibody (Becton Dickinson, San José, CA) or PE-labeled mouse IgG1 (Becton Dickinson) as a negative control. After staining, the cells were washed twice and then resuspended in DPBS and kept on ice in the dark until analyzed. A FACSort flow cytometer (Becton Dickinson) with a 488-nm argon laser was used to analyze the samples. Rhodamine fluorescence was collected after a 520 nm bandpass filter and PE fluorescence was collected after a 585 nm bandpass filter. A minimum of 5,000 events were collected per sample, and the samples were gated on forward scatter versus side scatter to exclude clumps and debris. Dead cells were excluded based on propidium iodide staining.
Definition of mean channel shift.
Rhodamine efflux experiments were performed on samples from patients before and following the clinical administration of PSC 833. Fluorescence histograms for CD56+ cells were generated and the mean channel numbers for the histograms were compared following rhodamine accumulation with and without exogenous PSC 833 (PSC − Control) and following the efflux period with and without exogenous PSC 833 (PSC/Efflux − Efflux). The PSC − Control value is calculated from the mean channel numbers after the 30-minute period of accumulation in which rhodamine (Control) or rhodamine and PSC 833 (PSC) are added directly to whole blood. The value is calculated from the mean channel numbers after the 30-minute accumulation period, lymphocyte separation, wash, and 60-minute efflux period occurring in rhodamine-free medium with (PSC/Efflux) or without (Efflux) added PSC 833. A small value or zero value for PSC − Control would indicate complete antagonism of Pgp in the cells by the PSC 833 present in the patient’s blood. Similarly, a low or zero value for PSC/Efflux − Efflux would indicate complete antagonism of Pgp in the cells by the PSC 833 present in the patient’s blood, whereas higher mean channel shifts would indicate less antagonism.
MRK-16 staining and quantitation.
Cultured cells were trypsinized, washed, and incubated in MRK-16 antibody (Kamiya, Seattle, WA) or the IgG2a negative isotype control (Becton Dickinson) for 30 minutes, washed twice with DPBS/FCS, and incubated for 30 minutes with fluorescein isothiocyanate (FITC)-conjugated horse anti-mouse antibody (Vector Laboratories, Burlingame, CA). In the case of blood samples, PBMCs were stained again with PE-conjugated CD56 antibody or PE-conjugated IgG1 isotype control, washed 2 times in DPBS/FCS, and analyzed. A minimum of 5,000 events was collected. MRK-16 staining was expressed as the difference of the mean channel value of the isotype control histogram from the mean channel value of the MRK-16 histogram or, in the case of the mononuclear cells, the histogram for cells stained with both MRK-16 and the desired T-cell marker.21
Determination of PSC 833 levels.
The assay is based on the competition of PSC 833 and [G-3H]Dihydro-PSC 833 tracer in whole blood and binding to a monoclonal antibody to Cyclosporine A. PSC 833 concentrations in human blood were determined using PSC 833 radioimmunoassay kits provided by ANAWA Laboratorien AG (Zurich, Switzerland). Briefly, appropriate volumes of blood standards, unknowns, and quality control samples were dispensed into glass tubes. To each sample, 1 mL methanol was dispensed, vortex mixed, and centrifuged at 3,000 rpm at approximately 4°C for 10 minutes. The supernatant from each tube was decanted into a clean tube and evaporated to dryness using a vortex evaporator at approximately 54°C. To each tube, appropriate volumes of buffer and human plasma were added and vortex mixed to redissolve all the sample residue. Subsequently, antibody (specific Cyclosporine A monoclonal antibody) and tracer (3H-Dihydro-PSC 833) were added, vortex mixed, and incubated overnight at approximately 4°C. The following morning, prechilled charcoal suspension was added to each sample. The sample was vortex mixed and incubated for 10 minutes at approximately 4°C and then centrifuged for 7 minutes at 4°C and 3,000 rpm. The supernatant was decanted into a scintillation vial containing 10 mL of Picofluor. Each vial was thoroughly mixed and counted for 5 minutes in a beta counter.
Estimations of the human blood PSC 833 concentrations in samples were obtained using the computer program RIAPROG (Mercer Computer Systems, New York, NY). The program converts the raw counts obtained for the standards to the response curve, (Y). The Y-response versus standard concentration (standard curve) is linearized by the logit-log method, and the unknown concentrations are interpolated form the linearized curve.22
Determination of kinetic parameters.
Of the data that we had collected for the 20 patients in the study, 12 were sufficiently complete as to allow a quantitative analysis of the effect of plasma PSC 833 levels on the reversal of P-glycoprotein. For these 12 data sets, we plotted the difference between rhodamine fluorescence in CD56+ cells in the presence of PSC 833 and in its absence against the plasma concentration of PSC 833. For each data set, we fitted the points to the equation:
where Δ is the difference between the rhodamine fluorescence signal in CD56+ cells obtained during the accumulation period with and without exogenously added PSC 833 (PSC − Control), S is the concentration of PSC 833 in the plasma of the patient, and Ki is a parameter that determines the concentration at which a half-maximal effect of the reverser is found. This equation assumes that the interaction between the reverser and P-glycoprotein obeys a simple ligand-binding isotherm.23 24We also fitted the data to the more complex equation:
RESULTS
Rhodamine efflux and MRK-16 staining in mononuclear cell populations.
Figure 1A presents the results of rhodamine efflux in three different subsets of peripheral mononuclear cells derived from a normal volunteer. MRK-16 staining of these subsets is shown in Fig 1B. As noted by other investigators, both MRK-16 cell surface staining and rhodamine efflux are detectable in the three subsets: the CD3+ cells, which encompass both the CD4+ and the CD8+ populations, have the lowest level of staining, and the least rhodamine efflux of the three; intermediate levels are observed in the CD8+ population; and highest levels are observed in CD56+ cells. The accumulation of rhodamine in the presence of PSC 833 without an efflux period (PSC) yields a histogram that is identical to that following efflux in the presence of PSC alone (PSC/Efflux) and thus is not depicted in the figure.
Rhodamine efflux and MRK-16 staining in T-cell subsets. (A) Rhodamine efflux in isolated mononuclear cells from a normal patient. Blank histogram (solid line), cells incubated 30 minutes in media alone. Control histogram (dotted line), cells incubated 30 minutes in media with 0.5 μg/mL rhodamine 123. PSC/Efflux histogram (dashed line), cells incubated 30 minutes in media with 0.5 μg/mL rhodamine 123 and 3 μg/mL PSC 833, washed, and incubated in rhodamine-free media with 3 μg/mL PSC 833 for 60 minutes. Efflux histogram (heavy solid line), cells incubated 30 minutes in media with 0.5 μg/mL rhodamine 123, washed, and incubated in rhodamine-free medium for 60 minutes. Staining with the appropriate T-cell marker was performed with each sample. (B) MRK-16 staining of normal mononuclear cells. Blank histogram (solid line), unstained mononuclear cells. IgG Control histogram (dotted line), cells incubated with mouse IgG1, stained with FITC-labeled horse anti-mouse antibody, then stained with the appropriate PE-labeled T-cell antibody. MRK-16 histogram (dashed line), cells were incubated in unlabeled MRK-16 antibody for 30 minutes, stained with FITC-labeled horse anti-mouse, and subsequently labeled with the desired PE-labeled T-cell marker.
Rhodamine efflux and MRK-16 staining in T-cell subsets. (A) Rhodamine efflux in isolated mononuclear cells from a normal patient. Blank histogram (solid line), cells incubated 30 minutes in media alone. Control histogram (dotted line), cells incubated 30 minutes in media with 0.5 μg/mL rhodamine 123. PSC/Efflux histogram (dashed line), cells incubated 30 minutes in media with 0.5 μg/mL rhodamine 123 and 3 μg/mL PSC 833, washed, and incubated in rhodamine-free media with 3 μg/mL PSC 833 for 60 minutes. Efflux histogram (heavy solid line), cells incubated 30 minutes in media with 0.5 μg/mL rhodamine 123, washed, and incubated in rhodamine-free medium for 60 minutes. Staining with the appropriate T-cell marker was performed with each sample. (B) MRK-16 staining of normal mononuclear cells. Blank histogram (solid line), unstained mononuclear cells. IgG Control histogram (dotted line), cells incubated with mouse IgG1, stained with FITC-labeled horse anti-mouse antibody, then stained with the appropriate PE-labeled T-cell antibody. MRK-16 histogram (dashed line), cells were incubated in unlabeled MRK-16 antibody for 30 minutes, stained with FITC-labeled horse anti-mouse, and subsequently labeled with the desired PE-labeled T-cell marker.
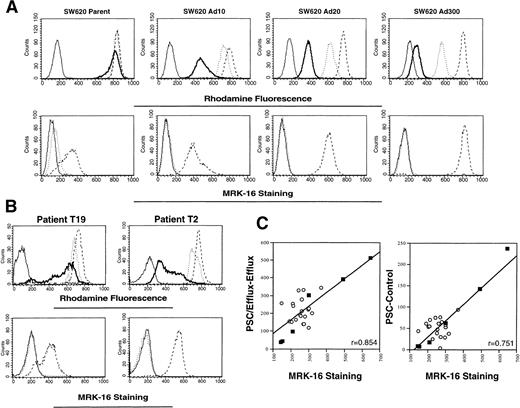
Rhodamine efflux and MRK-16 staining in multidrug resistant SW620 sublines and patient samples acquired before treatment.
Rhodamine efflux is readily observed in multidrug resistant sublines selected from SW 620 human colon carcinoma cells. In Fig 2A, increased rhodamine efflux (top row) and increased MRK-16 staining (bottom row) are observed in the SW620 Ad10, Ad20, and Ad300 sublines. By quantitative polymerase chain reaction (PCR), the Ad20 and Ad300 sublines have been shown to overexpress MDR-1 at levels 100- and 500-fold above the level found in parental SW620 cells.25 Adriamycin resistance in the SW620 Ad20 cells can be fully reversed by 10 μg/mL verapamil, whereas 25 μg/mL verapamil cannot fully reverse resistance in the Ad300 cells.19 Results from two patients (T19 and T2) representing the range seen in rhodamine efflux (upper row) and MRK-16 staining (lower row) are shown in Fig 2B. The MRK-16 staining in the CD56+ cells was highest for patient T2 and appears to be comparable to that found in the resistant SW620 Ad20 subline, whereas that of T19 was lowest and appears closer to that found in parental SW620 cells. Figure 2C summarizes the results in 20 patients (empty circles), and the SW620 parental and resistant cell lines (filled squares). MRK-16 staining was correlated with the difference in mean channel shift values obtained for rhodamine accumulation with and without added PSC 833, designated PSC − Control (right graph of Fig 2C, r = .751), and also with the value for PSC/Efflux − Efflux (left graph of panel C, r = .854). Both calculations show a significant correlation between rhodamine efflux and MRK measurement of Pgp.
Correlation of rhodamine efflux with MRK-16 staining. (A) Rhodamine efflux and MRK-16 staining was performed on SW 620 parental cells and cells selected in 10, 20, and 300 ng/mL Adriamycin, and designated as described in Fig 1, except that cells were not subjected to the CD56 labeling step. (B) Whole blood from each patient was obtained before initial treatment. Rhodamine efflux and MRK-16 staining were performed on mononuclear cells, and then stained with PE-labeled CD56 antibody. Designations as described in Fig 1. (C) The difference in rhodamine accumulation with and without exogenous PSC 833 (PSC − Control) and rhodamine efflux with and without exogenous PSC 833 (PSC/Efflux − Efflux) was plotted versus the difference between the MRK-16 histogram and the IgG1 negative control histogram for patients before receiving treatment (empty circles) and for the SW620 parental, Ad2, Ad5, Ad10, Ad20, and Ad300 cell lines (filled squares).
Correlation of rhodamine efflux with MRK-16 staining. (A) Rhodamine efflux and MRK-16 staining was performed on SW 620 parental cells and cells selected in 10, 20, and 300 ng/mL Adriamycin, and designated as described in Fig 1, except that cells were not subjected to the CD56 labeling step. (B) Whole blood from each patient was obtained before initial treatment. Rhodamine efflux and MRK-16 staining were performed on mononuclear cells, and then stained with PE-labeled CD56 antibody. Designations as described in Fig 1. (C) The difference in rhodamine accumulation with and without exogenous PSC 833 (PSC − Control) and rhodamine efflux with and without exogenous PSC 833 (PSC/Efflux − Efflux) was plotted versus the difference between the MRK-16 histogram and the IgG1 negative control histogram for patients before receiving treatment (empty circles) and for the SW620 parental, Ad2, Ad5, Ad10, Ad20, and Ad300 cell lines (filled squares).
Pgp antagonism following vinblastine, paclitaxel, and PSC 833 administration in patients on protocol.
Peripheral mononuclear cells were obtained from patients enrolled on phase I trials with PSC 833. These patients were treated with a first cycle of either infusional vinblastine or paclitaxel alone, and then PSC 833 was administered separately. In this way, the individual pharmacokinetic parameters of PSC 833 and the chemotherapeutic agent (taxol or vinblastine) could be obtained. To determine whether the initial infusion of vinblastine or paclitaxel would affect rhodamine efflux, peripheral mononuclear cells were obtained before and after the infusion. No significant difference could be observed in PSC/Efflux − Efflux values between the two samples, with the average of PSC/Efflux − Efflux values before chemotherapy being 319 ± 71 and after chemotherapy 338 ± 75. A Student’s t-test yielded a value of P > .5, indicating no difference between the samples as a result of the initial vinblastine or paclitaxel treatment.
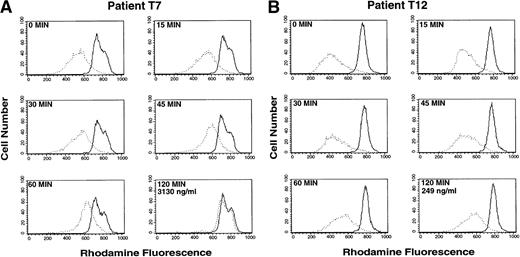
In contrast, treatment with a single oral dose of PSC 833 resulted in marked inhibition of rhodamine efflux from the CD56+ cells. Time course studies were performed on the CD56+ cells, and serum levels of PSC 833 were obtained. Figure 3 depicts two time course studies representing two extremes, one patient with antagonism of rhodamine efflux and one patient without. Patient T7 (Fig 3A) showed almost complete antagonism of rhodamine efflux from the CD56+ cells 2 hours following the first dose of PSC 833. In contrast, in Patient T12 (Fig 3B), significant reversal was not observed within the first 2 hours following ingestion of PSC 833. These differences, observed despite the administration of identical PSC 833 doses in the two patients, were correlated with markedly different PSC 833 levels, 3,130 ng/mL and 249 ng/mL for patients T7 and T12, respectively, 2 hours following PSC 833 administration. This may represent differences in drug bioavailability or metabolism.
Inhibition of rhodamine efflux from CD56+cells after initial exposure to PSC 833. Whole blood was obtained 0, 15, 30, 45, 60, and 120 minutes after an initial dose of PSC 833. Rhodamine 123 was added to whole blood and incubated for 30 minutes and the mononuclear cells were separated and incubated for an additional 60 minutes in rhodamine-free media before staining with PE-labeled CD56 antibody to generate the Efflux histogram (dotted line), or rhodamine 123 and PSC 833 were both added to the blood during the 30-minute incubation period and the mononuclear cells were separated and incubated in rhodamine-free media with PSC 833 before PE-labeled CD56 staining to yield the PSC/Efflux histogram (solid line). A small difference between the Efflux and PSC/Efflux peak signifies greater blocking of Pgp by PSC 833 in patient blood; a larger difference indicates poor reversal. (A) Patient T7 shows near complete reversal of Pgp-mediated rhodamine efflux in CD56+ cells. Reversal can be seen 45 minutes after a single oral dose of PSC 833. (B) Patient T12 shows incomplete reversal of Pgp in CD56+ cells. Virtually no effect is seen 2 hours after administration of PSC 833. The two patients shown received identical doses of PSC 833. The PSC 833 levels in the two patients after two hours are 3130 ng/mL (T7) and 249 ng/mL (T12).
Inhibition of rhodamine efflux from CD56+cells after initial exposure to PSC 833. Whole blood was obtained 0, 15, 30, 45, 60, and 120 minutes after an initial dose of PSC 833. Rhodamine 123 was added to whole blood and incubated for 30 minutes and the mononuclear cells were separated and incubated for an additional 60 minutes in rhodamine-free media before staining with PE-labeled CD56 antibody to generate the Efflux histogram (dotted line), or rhodamine 123 and PSC 833 were both added to the blood during the 30-minute incubation period and the mononuclear cells were separated and incubated in rhodamine-free media with PSC 833 before PE-labeled CD56 staining to yield the PSC/Efflux histogram (solid line). A small difference between the Efflux and PSC/Efflux peak signifies greater blocking of Pgp by PSC 833 in patient blood; a larger difference indicates poor reversal. (A) Patient T7 shows near complete reversal of Pgp-mediated rhodamine efflux in CD56+ cells. Reversal can be seen 45 minutes after a single oral dose of PSC 833. (B) Patient T12 shows incomplete reversal of Pgp in CD56+ cells. Virtually no effect is seen 2 hours after administration of PSC 833. The two patients shown received identical doses of PSC 833. The PSC 833 levels in the two patients after two hours are 3130 ng/mL (T7) and 249 ng/mL (T12).
Determination of mean channel shifts following PSC 833.
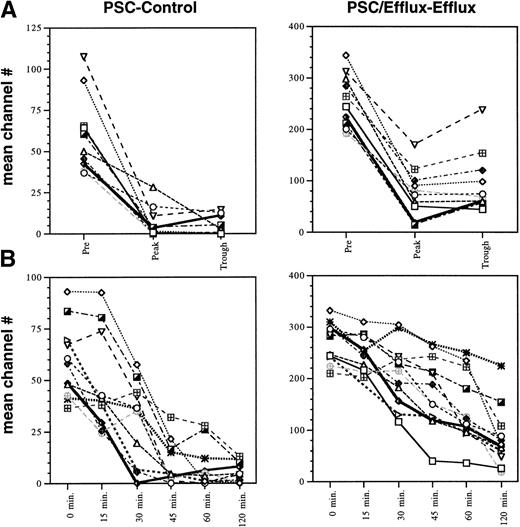
The fluorescence-activated cell sorter (FACS) results were quantitated by determining the difference in rhodamine fluorescence in CD56+ cells measured in mean channel numbers, termed the mean channel shift, as a result of adding PSC 833 in the laboratory. The two values quantitated, PSC − Control and PSC/Efflux − Efflux, have been previously shown to reflect the degree of inhibition of P-glycoprotein–mediated drug efflux.20 26 In this assay, the cells incubated with exogenous PSC 833 will provide a “maximum” fluorescence value, whereas the fluorescence measured in the Control and Efflux histograms will depend on the level of PSC 833 present in the patient’s plasma. The PSC − Control value compares the increased rhodamine fluorescence in CD56+cells due to PSC 833 in the patient’s plasma to the “maximum” value obtained by the addition of PSC 833 in the laboratory. The second value, PSC/Efflux − Efflux, compares the ability of PSC 833 in the patient’s plasma to increase rhodamine fluorescence in CD56+ cells during the accumulation period and prevent rhodamine efflux during the efflux phase to the “maximum” fluorescence value. This measurement is highest in cells obtained from patients before PSC 833 administration and has the widest range among the patients. Because the PBMCs must be allowed efflux in rhodamine-free media during the efflux assay, the cells are subjected to a washing step which effectively removes the PSC 833 as well as the rhodamine. The PSC/Efflux − Efflux value, therefore, measures the effective inhibition of Pgp-mediated rhodamine efflux by PSC 833 remaining inside the cell. Although PSC 833 is poorly transported by Pgp, it may diffuse from the cell or be effluxed at a low level in the PSC 833-free, rhodamine-free media used for the efflux assay. Thus, the channel shift observed in the patient samples following the efflux period could underestimate the extent of Pgp inhibition occurring in the patient during continued exposure to PSC 833. As the PSC − Control value is not affected by this washing step, the difference between the PSC and the Control histograms is smaller and provides a narrower range in which to evaluate differences among patients. As a result, the data were quantitated in both ways. As seen in Fig 4, a wide range in basal efflux levels was observed in patients before administration of PSC 833, at the pre- and 0-minute time points. Complete inhibition of efflux is seen in the samples obtained 2 hours following administration of oral PSC 833 as evidenced by the PSC − Control value, with the mean channel shifts decreasing to an insignificant difference of 0 to 12 channel numbers in the majority of samples. The differences following the efflux period, as represented by the PSC/Efflux − Efflux values are markedly reduced, but more variable than the PSC − Control samples. This is most likely due to the wash step mentioned previously.
Effect of PSC 833 in 12 patients studied. (A) Difference in rhodamine fluorescence in CD56+ cells with and without exogenous PSC 833 (PSC − Control) or following the efflux period with and without exogenous PSC 833 (PSC/Efflux − Efflux) were compared at the “pre” time point, before initial chemotherapy treatment; the “peak” time point, 2 hours following ingestion of PSC 833; and the “trough” time point, 6 hours following PSC 833 administration for 12 patients on study. The peak and trough samples were obtained after at least 6 days of PSC 833 administration. (B) PSC − Control and PSC/Efflux − Efflux values were compared at 0, 15, 30, 45, 60, and 120 minutes after a single dose of PSC 833.
Effect of PSC 833 in 12 patients studied. (A) Difference in rhodamine fluorescence in CD56+ cells with and without exogenous PSC 833 (PSC − Control) or following the efflux period with and without exogenous PSC 833 (PSC/Efflux − Efflux) were compared at the “pre” time point, before initial chemotherapy treatment; the “peak” time point, 2 hours following ingestion of PSC 833; and the “trough” time point, 6 hours following PSC 833 administration for 12 patients on study. The peak and trough samples were obtained after at least 6 days of PSC 833 administration. (B) PSC − Control and PSC/Efflux − Efflux values were compared at 0, 15, 30, 45, 60, and 120 minutes after a single dose of PSC 833.
Correlation of Pgp antagonism with PSC 833 blood level.
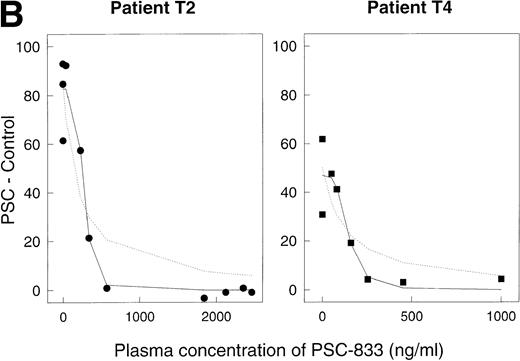
For each time point, PSC 833 levels in the blood were measured by radioimmunoassay. For 10 of the 12 data sets, complete inhibition of rhodamine efflux was observed (Fig 5A), with a Ki value in the range 29 to 181 ng/mL (mean 108 ± 16 SE, n = 10). For two sets (open squares [T5] and open diamonds [T6] in Fig 5A), higher Ki values, 914 and 916 ng/mL, were calculated, suggesting that in these two patients, complete inhibition of rhodamine efflux was not achieved at the PSC 833 levels attained. However, one aberrant value in patient T6 may have contributed to the higher Ki. For a number of data sets (in particular for those where numerous data points were available) the dose dependence of the reversal effect was steeper than a simple binding isotherm would predict. Figure 5B depicts two of these data sets, replotted from Fig5A on a larger scale. In this graph, the dashed lines represent the best-fit predictions modeled on a simple binding isotherm. The solid lines are the predictions of the more complex model in which a number of PSC 833 molecules cooperate together to block P-glycoprotein. The fact that the solid lines visually provide a better fit suggests a possible interaction between several PSC 833 molecules that results in greater inhibition of rhodamine efflux. Such a phenomenon has been documented for several Pgp antagonists in in vitro experiments.9,27 28
Reversal of Pgp activity by PSC 833 in plasma. Ordinates, (PSC − Control). Abscissas, concentration of PSC 833 in plasma in ng/mL. (A) Data for 12 different patients. Open squares aberrant patient T5, open diamonds aberrant patient T6. (B) Data for patients T2 (left panel) and T4 (right panel) replotted from (A). Dashed lines, best-fit predictions using the simple binding isotherm model; solid lines, best fits for model that includes cooperative interactions between molecules of PSC 833. The lines drawn are obtained by using the Marquardt-Levenberg algorithm.
Reversal of Pgp activity by PSC 833 in plasma. Ordinates, (PSC − Control). Abscissas, concentration of PSC 833 in plasma in ng/mL. (A) Data for 12 different patients. Open squares aberrant patient T5, open diamonds aberrant patient T6. (B) Data for patients T2 (left panel) and T4 (right panel) replotted from (A). Dashed lines, best-fit predictions using the simple binding isotherm model; solid lines, best fits for model that includes cooperative interactions between molecules of PSC 833. The lines drawn are obtained by using the Marquardt-Levenberg algorithm.
DISCUSSION
As our knowledge of the molecular mechanisms of drug action and resistance increases, measurement of a drug’s effects will enhance clinical trial designs. For studies designed to overcome Pgp-mediated resistance, measurement of the extent of in vivo inhibition of Pgp-mediated drug efflux is deemed essential. To that end, this manuscript presents the results of an ex vivo assay developed to detect reversal of multidrug resistance in patients receiving a Pgp antagonist. Blood samples were obtained from patients on either of two multidrug resistance reversal studies using PSC 833. Following incubation of cells with rhodamine 123, peripheral mononuclear cells were separated by centrifugation, and rhodamine fluorescence in CD56+ cells was determined by FACS analysis. Rhodamine efflux was measured in two ways: (1) by comparing rhodamine fluorescence in cells after an accumulation period with or without exogenous PSC 833 (PSC − Control) or (2) by comparing rhodamine fluorescence in cells following an efflux period during which exogenous PSC 833 was or was not present (PSC/Efflux − Efflux). Both measurements show inhibition of rhodamine efflux from CD56+ cells in patients treated with PSC 833.
Efflux of rhodamine from CD56+ cells as a surrogate marker requires the assumption that Pgp in CD56+ cells has an identical substrate and antagonist profile to Pgp in cancer cells. Support for this assumption includes several studies that describe increased uptake of either a chemotherapeutic agent or a drug surrogate in the normal liver and kidney, following the administration of Pgp antagonists in both humans and in animal models.29-34 These studies support the thesis that Pgp in normal tissues has the same substrate and antagonist specificities as cancer cells and provide the basis for the development of a surrogate assay to measure inhibition of Pgp-mediated drug efflux.
The need for a surrogate marker to establish the extent of inhibition of Pgp-mediated efflux is clear; the results of clinical trials cannot be interpreted without knowledge of the extent of Pgp inhibition. The ideal measurement would be actual drug accumulation in malignant cells. However, because this ideal is not possible, alternative approaches have been evaluated, usually with PSC 833, the first “second generation” antagonist to be evaluated in patients. One such approach is the inhibition of Tc-99m sestamibi efflux from tumors. Sestamibi is a radionuclide imaging agent that is a substrate for Pgp-mediated efflux.35 Imaging studies with sestamibi have shown efflux from Pgp-expressing tumors in both animals and man.36-39 Following PSC 833 administration, these studies showed marked increases in sestamibi uptake in the liver and kidneys as well as an increase in a subset of tumors.36,37,40 However, a dose-response relationship between sestamibi uptake and PSC 833 level was not shown.37 This may be related to interpatient variability in tumor or normal tissue uptake that may or may not be due to Pgp expression. Additional studies are needed to establish the sensitivity of sestamibi imaging to determine its adequacy as a surrogate marker and to evaluate how well the pharmacodynamics of sestamibi reflect the pharmacodynamics of the anticancer agents.
A second approach to evaluate the extent of Pgp inhibition has been to add serum or plasma from patients treated with Pgp modulators to an in vitro assay comprised of multidrug-resistant cells and a detectable Pgp substrate.9 Rhodamine or radiolabeled daunomycin or mitoxantrone have been used as substrates in this assay. Lehnert et al showed a threefold to eightfold increase in rhodamine retention in 8226/Dox6 human myeloma cells, using serum from patients treated with dexverapamil.41 Solary et al showed a 20% to 80% increase in mitoxantrone uptake in CEM/VBL cells exposed to serum from patients with quinine levels above 4 μg/mL.42 As with the results described herein, these methods do not provide information about antagonist levels or availability in tumor tissue itself.
One outcome of our study is the observation that a plateau in efflux antagonism occurs, suggesting that the levels of PSC 833 achievable in patients reach a maximum inhibition of Pgp-mediated efflux. The plateau begins at approximately 1,000 ng/mL, a concentration well below that achieved at maximally tolerated doses of PSC 833. Calculations using experimental models with high levels of P-glycoprotein suggest that a single modulator cannot completely inhibit Pgp-mediated efflux.43 The plateaus observed in the present study are consistent with the absence of complete reversal. However, they indicate substantial (near maximal) reversal, which may be sufficient to improve drug efficacy. Additionally, we found that the data most fit a model in which cooperativity between molecules of PSC 833 is assumed, as shown in Fig 5B. Cooperativity may be beneficial to the patient because it means that the reversal effect happens over a small concentration range.
Furthermore, this assay offers the ability to follow reversal of Pgp-mediated efflux clinically, to ensure that adequate levels are reached. It could also guide the addition of a second modulator, by indicating whether further inhibition of efflux can be achieved, and it could similarly guide the clinical use of Pgp antagonists by helping to establish the minimum dose of a modulator needed to inhibit drug efflux. This is particularly exemplified in Fig 3, in which the histograms of patient T7 suggest that PSC 833 provides complete antagonism, while little is observed in CD56+ cells of patient T12. The PSC 833 blood levels of the two patients, 3,130 ng/mL (T7) and 249 ng/mL (T12) at 2 hours are concordant with the reversal observed.
The possibility that the inhibition of rhodamine efflux from CD56+ lymphocytes may not accurately reflect the inhibition of anticancer drug efflux from a solid tumor must be considered. A number of factors may dictate results that are inferior to those obtained in the surrogate assay using circulating cells. These factors include (1) decreased drug and modulator uptake in tumor due to poor vascularity and perfusion, increased interstitial pressure, and binding to interstitial macromolecules; (2) increased affinity of the anticancer drug for intracellular targets, such that less compound is available for efflux, relative to rhodamine, which is compartmentalized in mitochondria and readily effluxed from the cell; and (3) differences in affinity of the anticancer drug for Pgp, because not all compounds are equally well transported. These factors preclude precise conclusions about Pgp reversal in solid tumors. This assay could also be used in hematopoietic malignancies to confirm effective inhibition of drug transport. Because the malignant cells are circulating, the degree of Pgp antagonism should be comparable to that observed in CD56+ cells.
Similarly, the potential for variability in Pgp expression in lymphocytes must also be considered. As shown in Fig 2, increased expression of Pgp as measured by MRK-16 staining correlates with a decreased PSC 833 effect, and a range in expression can be detected. Furthermore, in one or two patients, antagonism was inferior compared with that achieved in the other patients. An expanded study, with increased numbers of patients would provide information about how frequently this type of aberration would be observed. Very high levels of Pgp, or the presence of another transporter, could potentially explain the failure to antagonize the rhodamine efflux in patient T5. Several reports suggest interpatient variation in the expression of Pgp in circulating lymphocytes. An early study provided indirect evidence that prior chemotherapy (with Adriamycin) may increase Pgp level or function,44 whereas increased expression was noted in rheumatoid arthritis patients receiving prednisolone45 and in patients undergoing a kidney transplant following treatment with cyclosporin A.46 However, a subset analysis was not performed in these two studies, precluding a direct extrapolation of these findings to the CD56+ population. Furthermore, an increase in Pgp expression in CD4+ and CD8+lymphocytes obtained from older patients suggests a potential correlation between age and Pgp expression.47 This variability could impact on the clinical utility of the rhodamine reversal assay described here.
Clearly, the ideal assay would determine inhibition of drug efflux from tumor cells. However, with the exception of leukemia or multiple myeloma, where repetitive sample acquisition is possible, this ideal cannot be achieved in the clinical setting. Despite its limitations, we believe the rhodamine assay described here should provide valuable information. The ability to obtain serial specimens with minimal risk to patients, its relative simplicity, and its proven accuracy and reproducibility suggests it can serve as a surrogate marker for modulation of Pgp in patients.
ACKNOWLEDGMENT
The authors thank Anne Rutt for coordinating the collection of patient samples, the nurses of 12W and 13E for collection of patient samples, and Susan Mertins for advice on statistical calculations.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Susan Bates, MD, NIH, NCI, 9000 Rockville Pike, Bldg 10 Room 12N226, Bethesda, MD 20892; e-mail:sebates@helix.nih.gov.