Abstract
Theophylline, a drug known to inhibit several classes of adenosine 3′5′ cyclic monophosphate (cAMP) phosphodiesterases (PDEs), induces apoptosis in chronic lymphocytic leukemia (CLL) cells. Because the PDE target for theophylline in CLL remains unknown, we examined the ability of isoform-specific PDE inhibitors to increase cAMP levels and induce apoptosis in primary CLL cells. Reverse transcriptase-polymerase chain reaction of purified CLL cDNA amplified transcripts for PDE1B, 4A and 4B. The type 4 PDE inhibitor rolipram but not the type 1 inhibitor vinpocetine increased CLL cAMP levels. Rolipram-inhibitable (type 4) but not calcium-calmodulin augmented (type 1) PDE enzyme activity was detected in CLL samples. In samples from 13 of 14 CLL patients, rolipram induced apoptosis in a dose-dependent fashion over a 48-hour period. Interleukin-2 (IL-2)–cultured whole mononuclear cells (WMC) and anti-Ig stimulated CD19+ B cells were resistant to the induction of apoptosis by rolipram while unstimulated CD19+ B cells, which had a high basal apoptotic rate, were more sensitive. Rolipram stimulated elevations in cAMP levels in all four of these cell populations, suggesting that they differed in sensitivity to cAMP-induced apoptosis. Consistent with this hypothesis, incubation with the cell permeable cAMP analog dibutyryl-cAMP induced apoptosis in CLL cells and unstimulated B cells but not in IL-2–cultured WMC or anti-Ig stimulated B cells. These data identify PDE4 as a family of enzymes whose inhibition induces apoptosis in CLL cells.
B-CELL CHRONIC lymphocytic leukemia (CLL) is a CD5+, CD19+, CD23+ malignancy that has not been associated with a single unifying molecular defect but in which abnormalities in the induction of apoptosis appear to play a role. Although CLL patients may have initial clinical responses to alkylating agents such as chlorambucil or adenosine analogs such as fludarabine, many ultimately become refractory to therapy and there is a pressing need for the identification of novel approaches to this disease. Mentz et al1 have reported that CLL cells undergo apoptosis when exposed to theophylline, a methylxanthine known to nonspecifically inhibit 3′:5′ cyclic adenosine monophosphate (cAMP) phosphodiesterases (IC50 = 200 μmol/L). Theophylline synergizes with chlorambucil in inducing CLL apoptosis in vitro and a phase 2 trial suggests that this observation may be clinically relevant.2 3
The first observation that some lymphoid cells die after exposure to agents that increase intracellular cAMP levels was made by Daniel et al,4 who found that the murine lymphoma cell line S49.1 underwent cytolysis after 48 hours of exposure to the combination of theophylline and a cell permeable 3′:5′ cAMP analog, dibutyryl cAMP (dbcAMP). When mutant S49.1 clones resistant to the cytolytic effects of dbcAMP were isolated in soft agar, they were shown to be defective in the regulatory subunit of protein kinase A, confirming that cytolysis occurred as a direct result of PKA-mediated phosphorylation of unknown lymphoid target proteins.5Subsequent work has shown that cAMP-induced cytolysis occurs by apoptosis.6 Certain normal T- and B-lymphoid subsets express the same marked sensitivity to cAMP-induced toxicity as tumor cell lines. Within the T lineage, CD4+CD8+thymocytes appear to be more sensitive to the induction of apoptosis by cAMP than mature T cells.6 Apoptosis in resting human B lymphocytes, which occurs spontaneously at a high rate in culture, can be augmented by the addition of stimuli which elevate intracellular cAMP levels, such as the diterpene adenylate cyclase activator forskolin.7
Cyclic AMP is catabolized within cells to 5′-AMP by 3′:5′ cAMP phosphodiesterases (PDE), a diverse group of enzymes encompassing 15 gene products and 7 classes of enzymes which have proven to be the target of successful pharmaceutical agents for neurologic, cardiovascular, and inflammatory disorders.8Despite this large array of cyclic nucleotide PDEs, only a subset of these enzymes have been reported in human lymphoid cells. Among them, the most commonly reported enzymes in human T cells are types 1, 3, and 4. Calcium-calmodulin–dependent type 1 PDE activity has been detected in phytohemagglutinin-stimulated, but not resting, peripheral blood lymphocytes.9 One isoform from this family, PDE1B1, was recently detected in acute lymphocytic leukemia cells; inhibition of this enzyme was reported to induce apoptosis.10 PDE1 enzymes, which can catalyze the degradation of both cAMP and cyclic guanosine monophosphate (cGMP), are specifically inhibited by vinpocetine (IC50 = 21 μmol/L).11 Two groups have reported both type 3 and type 4 PDE in human T lymphocytes; lectin-mediated proliferation was completely suppressed only by treating cells with specific inhibitors of both classes of enzymes.12,13 Although four human PDE4 genes have been cloned, only two of the isoforms (PDE4A and B) have been identified in lymphocytes. Type 4 enzymes are specifically inhibited by rolipram[4-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone] (IC50 = 1 μmol/L) and the structurally related compound RO20-1724 (IC50 = 2 μmol/L).14 15
Theophylline induces apoptosis in CLL cells, as noted above, but both the clinical and research applications of theophylline are complicated by its activity as an adenosine receptor antagonist.16 As an alternate approach, we used transcript-specific probes, enzyme assays, and isoform-specific nonmethylxanthine cAMP PDE inhibitors to identify PDE targets in CLL cells and confirm that inhibition of specific PDE enzymatic activities induces CLL apoptosis.
MATERIALS AND METHODS
Reagents.
The following reagents were obtained from commercial sources: alkaline phosphatase, cAMP, dibutyryl cAMP, calmodulin, forskolin (Sigma Chemical Co, St Louis, MO); vinpocetine (Alexis Biochemicals, San Diego, CA); recombinant human interleukin-2 (rhIL-2; Genzyme, Boston, MA); F(ab′)2 fragment goat anti-human IgG and IgM (Jackson Immunoresearch Laboratories, West Grove, PA); Hoechst 33342 (Molecular Probes, Eugene, OR). Rolipram (racemate of 4-[3′-cyclopentyloxy-4′-methoxyphenyl]-2-pyrrolidone) was a gift from Dr Ronald Wohl (Berlex Laboratories, Wayne, NJ).
Patient selection.
After Institutional Review Board (IRB)-approved informed consent, blood was drawn in heparinized tubes from patients with flow cytometry–verified CLL who were either untreated or at least 1 month postchemotherapy. Patients with active infections or other serious medical conditions were not included in this study. Charts were reviewed to establish patients' sensitivity to chemotherapy and the stage of CLL. Resistance to a chemotherapeutic agent was defined as an increase in peripheral leukemic cell count or progression of adenopathy or splenomegaly before the initiation of the next scheduled cycle of chemotherapy.
Cell purification and culture.
Mononuclear cells were obtained by density gradient centrifugation over Histopaque 1077 (Sigma). As flow cytometry showed that CLL cells made up more than 95% of the mononuclear cells so purified, both apoptosis and cAMP assays were performed with these patient cell preparations. For polymerase chain reaction (PCR) experiments on CLL cells or for all experiments on normal circulating B cells, the whole mononuclear cells were further purified by incubation with Dynal anti-CD19 magnetic beads at a 1:1 bead:cell ratio, extensive washing with a magnetic particle concentrater, and elution with CD19 Detachabead reagent (Dynal, Lake Success, NY). Leukemic cells from two CLL patients showed sensitivity to rolipram-mediated apoptosis whether or not they were further purified by anti-CD19 magnetic beads. Cells were cultured in RPMI 1640 media (Biowhittaker, Walkersville, MD) supplemented with 10% fetal calf serum, 50 μmol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, 10 mmol/L HEPES pH 7.4, 100 U/mL penicillin, and 100 U/mL streptomycin (Sigma) at 37°C and 5% CO2 in air.
Reverse transcriptase (RT)-PCR and Northern analysis.
RNA was isolated from CLL or whole mononuclear cells using Ultraspec reagent (Biotecx, Houston, TX). cDNA was synthesized from 10 μg of total RNA using oligo d(T) primers and Maloney murine leukemia virus reverse transcriptase in a final volume of 40 μL (Stratagene, La Jolla, CA). One microliter of the first-strand cDNA product was then used as template for PCR amplification with AmpliTaq DNA polymerase (Roche Molecular Systems, Branchburg, NJ) by 40 thermocycles of 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute. The PDE PCR assay products were as follows with oligonucleotide sequences given 5′ → 3′: Human PDE1B1 (Genbank accession no. U56976) was 430 bp (first base 1660; sense = GTC TTC ATT GAG TCC AAA GTG , antisense = GAC CTG CCA GCT AAG ATC TGG).10 Human PDE3A (cGIP1, HSPDE3B) (X95520) was 340 bp (first base 2999, sense = GTA ACT CCT ATG ATG CTG CTG G, antisense = CTA TTC CTC TTC ATC TGC CTC).17,18 Of note, these PDE3 PCR oligonucleotides are selective for the human cGIP1 PDE, homologous to rat PDE3A, as the amplified sequence has only 50% nucleotide homology to the cardiac/platelet form of human PDE3 (cGIP2).19 Human PDE4A (M37744) was 461 bp (first bp 1819, sense = GGA GGA AGA AAT ATC AAT GGC CC, antisense = GAT GTG TCC TCC CCA AAT GTC).20Human PDE4B (L20966) was 479 bp (first bp 2213, sense = ATT CTG AAG GAC CTG AGA AGG, antisense = CAG TGA GTT CAG TCA CTG TCG).21For hybridization to Northern blots, these PCR products were subcloned into a plasmid vector (pCRII; Invitrogen, Carlsbad, CA) and subsequently used for PCR-based amplification of α32P dATP-labeled probes.
cAMP assay.
One million cells in 1 mL were treated for 2 hours with or without drugs. 0.8 mL of cells were pelleted by spinning at 4,000 rpm (relative centrifugal force [RCF] = 1,310) in a microcentrifuge tube. After discarding 0.7 mL, 400 μL of ethanol was added, vortexed, and left on ice for five minutes. Particulate cell debris was removed by centrifugation at 14,000 rpm (RCF = 16,000). The supernatant was stored at −20°C until the day of assay, at which time it was dried in a Speedivac (Savant, Farmingdale, NY) to a volume of 50 μL. After 10-fold dilution in 10 mmol/L Tris pH 8.0, 1 mmol/L EDTA, the cAMP sample was analyzed for cAMP concentration using a cAMP radioimmunoassay (RIA) kit (New England Nuclear [NEN], Boston, MA) according to the manufacturer's instructions using the nonacetylated protocol.
cAMP PDE assay.
The technique of Robicsek et al, itself adapted from an assay described by Thompson and Appleman, was used in modified form.12 22One hundred fifty million purified CLL cells were pelleted and sonicated (Branson 350 Sonifier with microtip probe; Branson Ultrasonics, Danburg, CT; output = 2, 50% duty cycle) on ice in 1.0 mL of a buffer which contained 20 mmol/L Tris (pH 6.8), 1 mmol/L EDTA, aprotinin (50 U/mL), pepstatin (1 mg/mL), phenylmethyl sulfonyl fluoride (PMSF) (1 mmol/L), and 3.75 mmol/L β-mercaptoethanol (β-ME). Assay buffer contained 100 mmol/L Tris (pH 8.0), 20 mmol/l MgCl2, 0.2% bovine serum albumin (BSA), and 7.5 mmol/L β-ME. [3H]-cAMP (NEN) was incubated with PDE for 10 minutes at 30°C in 20-μL volumes (10 μL of sonication buffer and 10 μL of assay buffer) which contained 0.22 U of alkaline phosphatase. Rolipram, 10 μmol/L, or 0.2 mmol/L calcium/20 nmol/L calmodulin were added to the assay buffer as appropriate. Reactions were halted by the addition of 0.5 mL of a 1:3 slurry wt/vol slurry of AG1-X8 anion exchange resin and a mixture of equal volumes of water and isopropanol. The resin bound the unreacted nucleotide but not the dephosphorylated nucleoside. Microcentrifuge tubes were spun at 3,000 rpm (RCF = 735) for 15 minutes. The radiolabeled nucleosides in the supernatant were counted using Ecoscint scintillation fluid (National Diagnostics, Atlanta, GA). Three to five enzyme dilutions were assayed to determine each velocity. Linearity of velocity with respect to enzyme concentration and time were verified.
DNA ladder gel assay.
Ten million purified CLL cells were obtained by centrifugation after exposure to drugs during a 72-hour tissue culture incubation. Cells were lysed in 0.5 mL of 20 mmol/L Tris (pH 7.4), 0.4 mmol/L EDTA, 0.25% Triton X 100 (American Bioanalytical, Natick, MA). After 15 minutes of incubation at room temperature, nuclei were removed by centrifugation at 14,000 rpm (RCF = 16,000). The supernatant was transferred to a new tube and soluble DNA precipitated overnight at −20°C following the addition of 55 μL of 5 mol/L NaCl and 550 μL of isopropanol. After centrifugation at 14,000 rpm for 10 minutes, followed by a 70% ethanol wash, the pellet was resuspended in 20 μL of 10 mmol/L Tris (pH 8.0), 1 mmol/L EDTA, 0.1 mg/mL RNase, and incubated at 37°C for 30 minutes before electrophoresis on 1.6% Tris borate EDTA agarose gels. DNA fragments were visualized by UV light after staining the gels with ethidium bromide.
Hoechst 33342 apoptosis assay.
Hoechst 33342 was dissolved in water and frozen at 33 mg/mL at −20°C. One million cells per well were incubated in duplicate or triplicate in 48-well tissue culture plates (Costar, Cambridge, MA) with or without drug treatment for 48 hours in 1 mL of culture media. Cells were transferred to 12 × 75-mm polystyrene Falcon 2054 FACS tubes (Becton Dickinson Labware, Lincoln Park, NJ) and incubated for 10 minutes at 37°C with Hoechst 33342 at a final concentration of 0.25 μg/mL.23 Cells were stored on ice until analysis on a FACS Vantage flow cytometer (Becton Dickinson, San Jose, CA). Hoechst 33342 dye fluorescence was excited with a UV laser and detected using a 450 bandpass filter. Data were analyzed using Cellquest software (Becton Dickinson).
RESULTS
Identification of PDE targets in CLL cells.
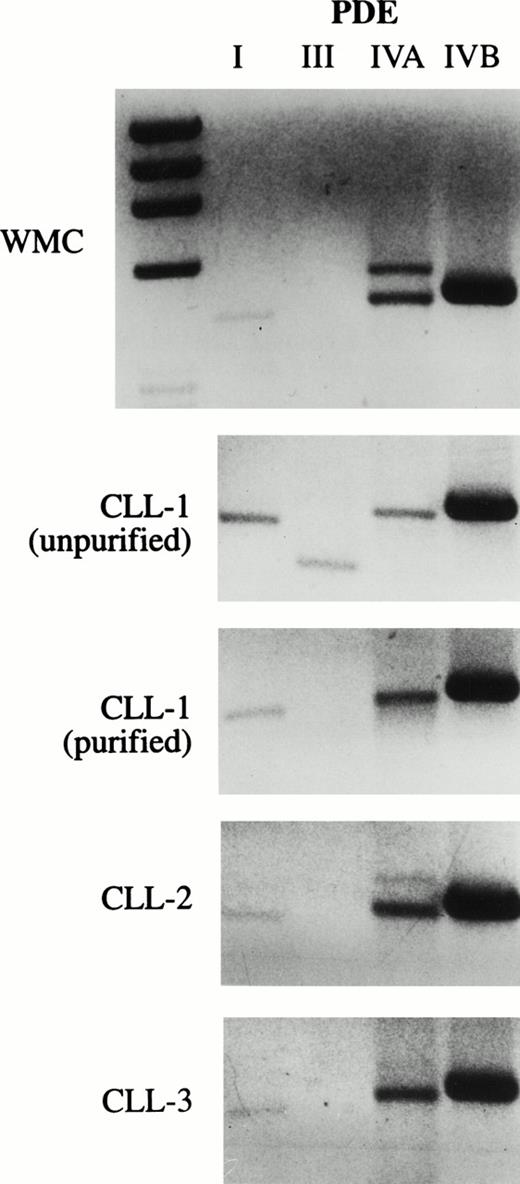
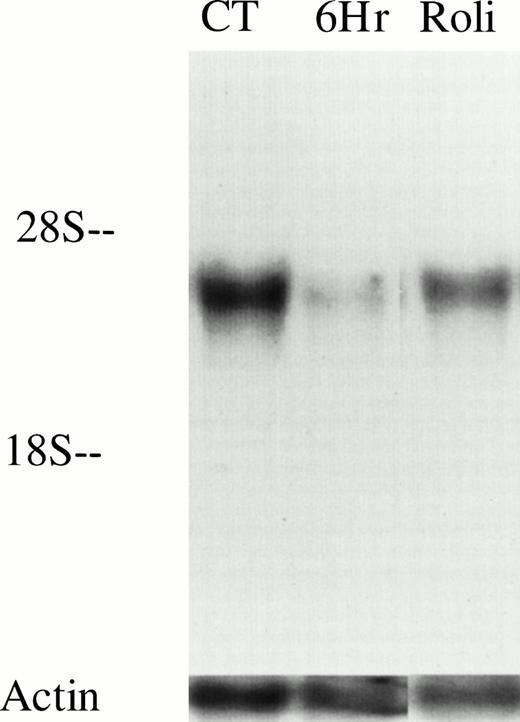
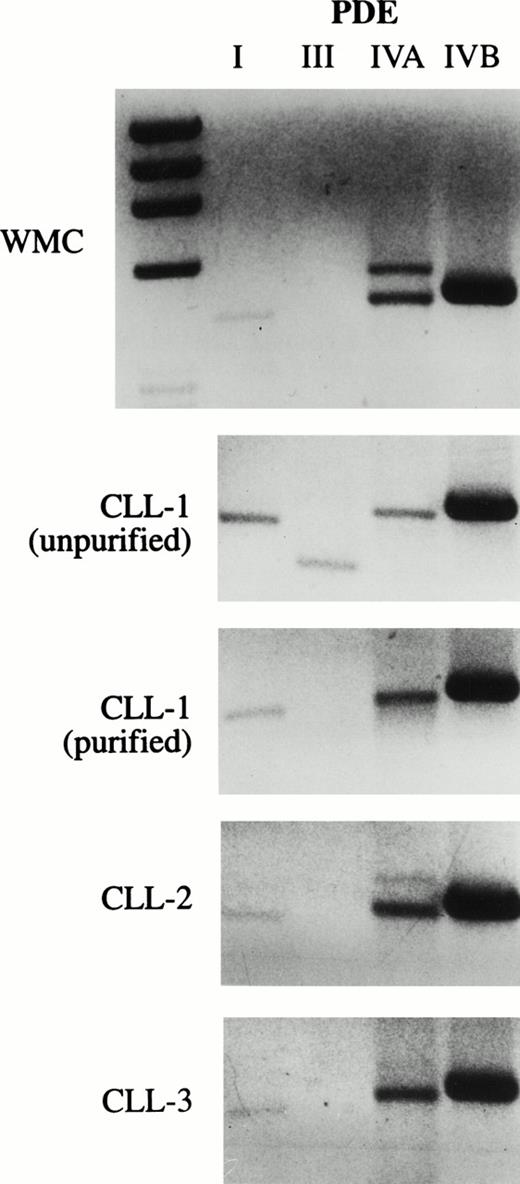
As an initial approach to identify potential PDE targets in CLL, we performed PCR on cDNA derived from unpurified mononuclear cells of a CLL patient with oligonucleotides specific for human PDE1-1B, PDE3A, PDE-4A, and PDE-4B, all of which have been previously identified in various normal and malignant primary lymphoid cells. We detected appropriate-sized PCR products from all four transcripts using this template. To reduce the likelihood of amplifying nonleukemic cell transcripts, we purified CD19+ cells from this and two other CLL patients before synthesizing cDNA. PDE1-1B, PDE-4A, and PDE-4B were still detected in these three templates, as they were in cDNA derived from normal whole mononuclear cells (Fig 1). Using the same four PCR products as probes on Northern blots, only PDE-4B transcript was detectable in 10 μg of loaded RNA (Fig 2 and data not shown). PDE-4B transcript levels were significantly higher in freshly isolated CLL cells than in CLL cells which had been cultured for 6 hours. Addition of 10 μmol/L rolipram, a type 4 PDE-specific phosphodiesterase inhibitor, significantly reduced this decrease in PDE-4B transcript levels during the 6-hour culture period.
CLL cells contain transcripts for PDE 1B1, PDE4A, and PDE4B. PCR was performed on cDNA derived from WMC (purified from a normal donor) or CLL cells for PDE1, PDE3A, PDE4A, and PDE4B. The cDNA used in the PCR in the bottom three panels was derived from leukemic cells from three different patients purified by positive selection for CD19 expression. The lowest band in the MWM lane on the left in the upper panel is 603 bp. Expected PCR product sizes for PDE1, 3A, 4A, and 4B are 430, 340, 461, and 470 bp, respectively.
CLL cells contain transcripts for PDE 1B1, PDE4A, and PDE4B. PCR was performed on cDNA derived from WMC (purified from a normal donor) or CLL cells for PDE1, PDE3A, PDE4A, and PDE4B. The cDNA used in the PCR in the bottom three panels was derived from leukemic cells from three different patients purified by positive selection for CD19 expression. The lowest band in the MWM lane on the left in the upper panel is 603 bp. Expected PCR product sizes for PDE1, 3A, 4A, and 4B are 430, 340, 461, and 470 bp, respectively.
PDE4B levels decrease in CLL cells after culture but are partially maintained by treatment with 10 μmol/L rolipram. RNA was isolated from 20 million CLL cells (derived from patient no. 12) immediately after cell purification (CT), or after 6 hours culture in media alone (6Hr) or with addition of 10 μmol/L rolipram (Roli). Equal loading and transfer of RNA was confirmed by hybridization with an actin probe as shown. These results are representative of Northern analysis performed on leukemic cells from two patients.
PDE4B levels decrease in CLL cells after culture but are partially maintained by treatment with 10 μmol/L rolipram. RNA was isolated from 20 million CLL cells (derived from patient no. 12) immediately after cell purification (CT), or after 6 hours culture in media alone (6Hr) or with addition of 10 μmol/L rolipram (Roli). Equal loading and transfer of RNA was confirmed by hybridization with an actin probe as shown. These results are representative of Northern analysis performed on leukemic cells from two patients.
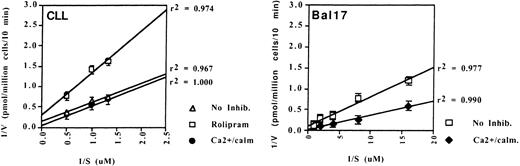
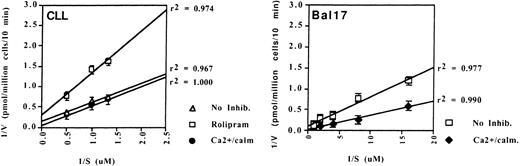
To determine whether these PDE transcripts were translated into protein with constitutive activity, we performed PDE enzyme assays on CLL cell lysates. We found no evidence of type 1 PDE activity in CLL cells because the basal PDE activity was not augmented by the addition of calcium and calmodulin (Fig 3, left). However, we were able to identify substantial constitutive type 1 PDE activity in the murine B-lymphoma cell line Bal-17, as shown by an increase in PDE activity with the addition of calcium and calmodulin (Fig 3, right). In contrast, CLL PDE activity was markedly inhibited by the type 4 specific inhibitor, rolipram in each of the three patients tested (Fig 3, left and data not shown). Rolipram altered both Km and the Vmax, consistent with its known activity as a noncompetitive antagonist.14
Type 1 and 4 PDE activity differs between a murine B lymphoma cell line (Bal-17) and CLL cells. Lineweaver-Burk analysis of PDE enzymatic activity in lysates of CLL cells and Bal-17 cells. (Left) PDE activity in CLL cells was inhibited by 10 μmol/L rolipram but was not augmented by the addition of 0.2 mmol/L calcium and 20 nmol/L calmodulin. (Right) Bal-17 PDE activity is augmented by the addition of calcium and calmodulin. The CLL data are representative of enzymatic assays on three patients.
Type 1 and 4 PDE activity differs between a murine B lymphoma cell line (Bal-17) and CLL cells. Lineweaver-Burk analysis of PDE enzymatic activity in lysates of CLL cells and Bal-17 cells. (Left) PDE activity in CLL cells was inhibited by 10 μmol/L rolipram but was not augmented by the addition of 0.2 mmol/L calcium and 20 nmol/L calmodulin. (Right) Bal-17 PDE activity is augmented by the addition of calcium and calmodulin. The CLL data are representative of enzymatic assays on three patients.
To determine what role type 1 and type 4 PDE activity might play in the catabolism of cyclic nucleotides in CLL cells, we measured cAMP levels after culturing leukemic cells with varying concentrations of PDE-isoform specific inhibitors, either alone or in conjunction with an activator of adenylate cyclase, the diterpene forskolin. When incubated with forskolin and the PDE-1 inhibitor vinpocetine, CLL cell cAMP was not augmented above levels induced by forskolin alone (Fig 4D). Incubation of Bal-17 cells with vinpocetine reduced both basal and forskolin-stimulated cAMP levels, a result in keeping with the reported primary effect of this drug on cGMP rather than cAMP levels (data not shown).11 In contrast, addition of the type 4 PDE inhibitor rolipram augmented CLL cAMP levels, both when used alone and more dramatically when combined with forskolin (Fig 4A through D). CLL cells were not unique with respect to their response to these isoform-specific inhibitors. cAMP levels in both a predominantly T-cell population (IL-2 supplemented normal whole mononuclear cells; >90% CD3+ T cells by flow cytometry) and magnetic-bead purified CD19+ B cells increased after inhibition of type 4 but not type 1 PDE activity (Fig 4E and F, and data not shown).
Rolipram raises cAMP levels in CLL cells, WMC, and resting B cells. One million freshly isolated cells were incubated with the indicated drugs for 2 hours before analysis of cAMP in cell lysates using a radioimmunoassay. Data are the mean of three individually assayed culture wells. In (D), vinpocetine was added at 30 μmol/L. The experimental conditions indicated with an asterisk had a greater [cAMP] than, as appropriate, the untreated or forskolin-treated control cells (t-test: one-tailed significance level <.05).
Rolipram raises cAMP levels in CLL cells, WMC, and resting B cells. One million freshly isolated cells were incubated with the indicated drugs for 2 hours before analysis of cAMP in cell lysates using a radioimmunoassay. Data are the mean of three individually assayed culture wells. In (D), vinpocetine was added at 30 μmol/L. The experimental conditions indicated with an asterisk had a greater [cAMP] than, as appropriate, the untreated or forskolin-treated control cells (t-test: one-tailed significance level <.05).
Type 4 PDE inhibition induces CLL apoptosis.
Having identified the type 4 cAMP phosphodiesterase family as an important regulator of cAMP levels in CLL cells, we next turned to a study of the activity of type 4–specific PDE inhibitors as inducers of cAMP-mediated apoptosis in leukemic cells from CLL patients. CLL cells were incubated for 72 hours either alone or with 10 μmol/L rolipram, 40 μmol/L forskolin, or both agents. We tested whether rolipram induces internucleosomal DNA fragmentation characteristic of apoptosis by isolating soluble DNA from the leukemic cells with detergent treatment, then removing DNA from intact nonapoptotic nuclei by centrifugation. As shown in Fig 5, while culture of CLL cells in media alone resulted in only a faint DNA “ladder,” treatment with rolipram and/or forskolin resulted in pronounced internucleosomal DNA fragmentation.
Rolipram and forskolin induce DNA fragmentation in CLL cells. Soluble DNA was isolated from 10 million CLL cells cultured for 72 hours in media (CT), 10 μmol/L rolipram (Roli), 40 μmol/L forskolin (Fsk) or a combination of the latter two agents (Ro/Fs). DNA fragments were resolved by electrophoresis on a 1.5% agarose gel and visualized with ethidium bromide. These data are representative of the four leukemic cell samples tested.
Rolipram and forskolin induce DNA fragmentation in CLL cells. Soluble DNA was isolated from 10 million CLL cells cultured for 72 hours in media (CT), 10 μmol/L rolipram (Roli), 40 μmol/L forskolin (Fsk) or a combination of the latter two agents (Ro/Fs). DNA fragments were resolved by electrophoresis on a 1.5% agarose gel and visualized with ethidium bromide. These data are representative of the four leukemic cell samples tested.
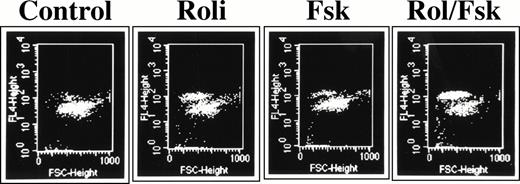
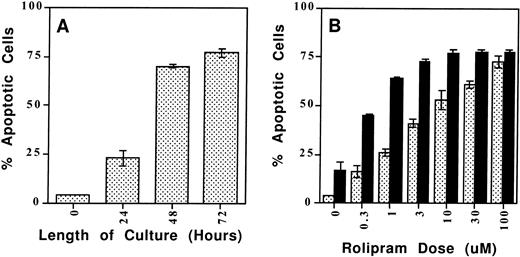
As a more quantitative analysis of CLL apoptosis, we used a flow cytometry method in which apoptotic cells are distinguished both by their reduced size (FSC) and their increased uptake of the lipophilic UV fluorescent dye Hoechst 33342 (FL-4) when the intact, heterogeneous cell population is incubated with a low concentration of the dye (0.25 μg/mL) for 10 minutes at 37°C (Fig6).23 Previous reports of cAMP-induced lymphoid apoptosis have noted that this form of programmed cell death may take 48 to 72 hours to develop maximally.6 Using the Hoeschst 33342 assay in a time-course experiment, we found that the combination of 10 μmol/L rolipram and 40 μmol/L forskolin induced significant CLL apoptosis which plateaued 48 to 72 hours after the addition of these drugs (Fig 7, left panel). Using the 72-hour culture period, we found a dose-dependent increase in CLL cell apoptosis when leukemic cells were incubated with rolipram (Fig 7, right panel). Treatment of CLL cells with forskolin alone induced moderate apoptosis, but the combination of forskolin with even low doses of rolipram resulted in a supra-additive effect on induction of CLL apoptosis (Fig 7, right panel). We obtained similar results with a structurally distinct PDE4 inhibitor, RO20-1724, or when isoproterenol or prostaglandin E2 were used to activate CLL adenylate cyclase rather than forskolin (data not shown).
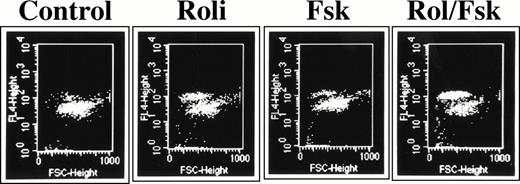
Rolipram-induced apoptosis in CLL cells is detectable by Hoechst 33342 flow cytometry. Cells were cultured for 72 hours in media (1), 1 μmol/L rolipram (2), 40 μmol/L forskolin (3), or a combination of the two drugs. The abcissa reflects forward light scatter and the ordinate Hoechst 33342 fluorescence. Apoptotic cells are characterized by reduced forward light scatter and increased Hoechst 33342 fluorescence.
Rolipram-induced apoptosis in CLL cells is detectable by Hoechst 33342 flow cytometry. Cells were cultured for 72 hours in media (1), 1 μmol/L rolipram (2), 40 μmol/L forskolin (3), or a combination of the two drugs. The abcissa reflects forward light scatter and the ordinate Hoechst 33342 fluorescence. Apoptotic cells are characterized by reduced forward light scatter and increased Hoechst 33342 fluorescence.
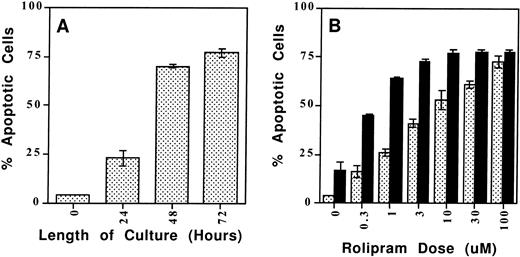
The type 4–specific cAMP phosphodiesterase inhibitor rolipram induces apoptosis in CLL cells. (A) Time course: Rolipram (10 μmol/L) and forskolin (40 μmol/L) were added to cultures of 1 million CLL cells for the indicated time period before determination of apoptosis by Hoechst 33342 flow cytometry. (B) Dose titration: CLL cells were cultured for 72 hours in 1 mL of media with the indicated concentration of rolipram with (▪) or without (▧) the addition of 40 μmol/L forskolin. The SEMs of triplicate cultures are indicated. All of the rolipram or rolipram/forskolin-treated cultures had a significantly greater percentage of apoptotic cells than the media or forskolin-only–treated cultures, respectively (t-test: one-tailed significance level <.05).
The type 4–specific cAMP phosphodiesterase inhibitor rolipram induces apoptosis in CLL cells. (A) Time course: Rolipram (10 μmol/L) and forskolin (40 μmol/L) were added to cultures of 1 million CLL cells for the indicated time period before determination of apoptosis by Hoechst 33342 flow cytometry. (B) Dose titration: CLL cells were cultured for 72 hours in 1 mL of media with the indicated concentration of rolipram with (▪) or without (▧) the addition of 40 μmol/L forskolin. The SEMs of triplicate cultures are indicated. All of the rolipram or rolipram/forskolin-treated cultures had a significantly greater percentage of apoptotic cells than the media or forskolin-only–treated cultures, respectively (t-test: one-tailed significance level <.05).
When CLL cells were incubated with the type 1 PDE inhibitor vinpocetine, they underwent apoptosis at dosages of 10 or 30 μmol/L but not at 2 μmol/L. Given that vinpocetine failed to augment cAMP accumulation and that we were unable to detect type 1 PDE activity in CLL cells, we suspect that this drug may induce apoptosis by a mechanism unrelated to cAMP. Consistent with this hypothesis, the kinetics of CLL apoptosis were different when vinpocetine was used with peak apoptosis by 24 rather than 48 hours (data not shown). Nonetheless, we cannot rule out either a temporally restricted or a topologically compartmentalized cAMP-mediated apoptotic effect from this drug.
Given that CLL is a clinically heterogeneous disease, we tested a total of 14 CLL patients of varying clinical stage, treatment history, and known resistance to chemotherapeutic agents for the sensitivity of their cells to phosphodiesterase inhibitor-mediated apoptosis. Patients were assessed for the apoptotic sensitivity of their leukemic cells to rolipram, forskolin, or both drugs. In samples from 10 “rolipram-sensitive patients,” treatment with 10 μmol/L rolipram induced apoptosis in more than a third of the leukemic cells, with overall apoptosis ranging from 44% to 80% (see Table 1 for tabulated results). Among the seven rolipram-sensitive patients whose cells were treated with both conditions, 40 μmol/L forskolin as a single agent induced less apoptosis than rolipram alone, suggesting that blockade of cAMP catabolism induced a more potent apoptotic signal than further augmentation of adenylate cyclase activity (P < .08, Wilcoxon signed-ranks test for matched pairs). Among four relatively rolipram-resistant patient samples (patient nos. 11 through 14; Table1), the absolute increase in apoptotic cells was less than 33%, with overall apoptosis ranging from 14% to 40%. Nonetheless, addition of forskolin to rolipram augmented apoptosis (68% and 69%) in two of the three patient samples examined within this group.
Effect of PDE4 inhibition on normal B and T cells.
Given that cAMP has been reported to be cytocidal to specific normal lymphocyte subsets, we wished to determine if rolipram also induced apoptosis in normal circulating human B- and T-cell populations. We found that IL-2–cultured whole mononuclear cells (WMC) (>90% CD3+ T cells by flow cytometry) were resistant to even high doses of rolipram and forskolin (Fig 8, upper panel). In contrast, magnetic bead purified CD19+ B cells were sensitive to rolipram, although the increment in apoptosis observed was superimposed on a high basal apoptosis rate that has previously been reported in cultured human B cells.7 Given that crosslinking of cell-surface Ig on resting B cells has been reported to reduce basal and forskolin-induced apoptosis in culture, we also stimulated CD19+ cells with a polyclonal Fab′2 anti-IgM/IgG reagent 30 minutes before the addition of the phosphodiesterase inhibitor.7 Prior stimulation through surface Ig markedly reduced both basal apoptosis and the sensitivity of the B cells to rolipram (Fig 8, middle panel). In contrast, anti-Ig stimulation of CLL cells derived from two patients failed to protect these cells from rolipram-induced apoptosis, a result that is consistent with reported defects in CLL cells in either surface μ heavy-chain expression or mutations in the B29 (CD79b) B-cell receptor accessory protein (Fig 8, bottom panel).24
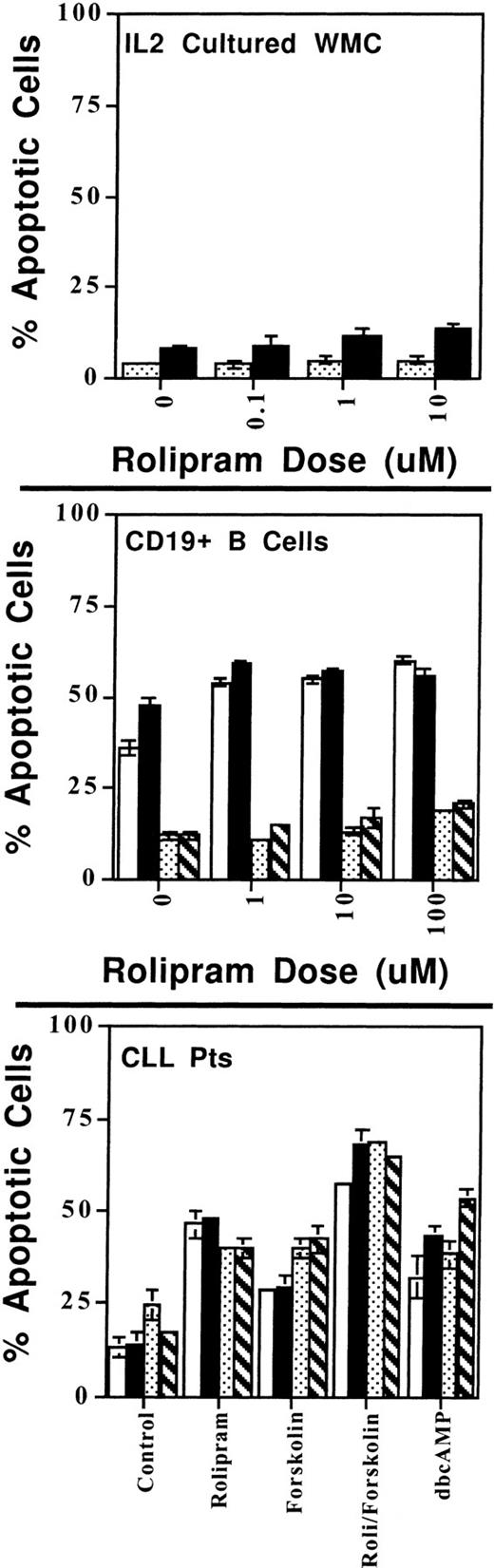
CLL cells, WMC cultured with IL-2, and resting and activated CD19+ B cells vary in their sensitivity to rolipram-induced apoptosis. (Top) One million WMC were cultured with the indicated concentration of rolipram with (▪) or without (▧) the addition of 40 μmol/L forskolin for 72 hours in the presence of 2 U/mL IL-2. Apoptosis was determined by Hoechst 33342 flow cytometry. (Middle) CD19+ peripheral B cells were purified by adherence to anti-CD19 magnetic beads. These cells were then cultured with the indicated concentration of rolipram with (▪) or without (□) 40 μmol/L forskolin. In addition, an identical set of cells were stimulated through their surface Ig by the addition of 10 μg/mL of Fab′2 goat anti-human IgG/M 30 minutes before addition of rolipram with (▧) or without (▧) forskolin. (Bottom) CLL cells from two patients were cultured using an experimental design similar to that described for the middle panel. Rolipram was used at 10 μmol/L and forskolin at 40 μmol/L. (□), Media patient no. 1; (▪), anti-Ig patient no. 1; (▧), media patient no. 2; (▧), anti-Ig patient no. 2. The SEMs of triplicate cultures are indicated.
CLL cells, WMC cultured with IL-2, and resting and activated CD19+ B cells vary in their sensitivity to rolipram-induced apoptosis. (Top) One million WMC were cultured with the indicated concentration of rolipram with (▪) or without (▧) the addition of 40 μmol/L forskolin for 72 hours in the presence of 2 U/mL IL-2. Apoptosis was determined by Hoechst 33342 flow cytometry. (Middle) CD19+ peripheral B cells were purified by adherence to anti-CD19 magnetic beads. These cells were then cultured with the indicated concentration of rolipram with (▪) or without (□) 40 μmol/L forskolin. In addition, an identical set of cells were stimulated through their surface Ig by the addition of 10 μg/mL of Fab′2 goat anti-human IgG/M 30 minutes before addition of rolipram with (▧) or without (▧) forskolin. (Bottom) CLL cells from two patients were cultured using an experimental design similar to that described for the middle panel. Rolipram was used at 10 μmol/L and forskolin at 40 μmol/L. (□), Media patient no. 1; (▪), anti-Ig patient no. 1; (▧), media patient no. 2; (▧), anti-Ig patient no. 2. The SEMs of triplicate cultures are indicated.
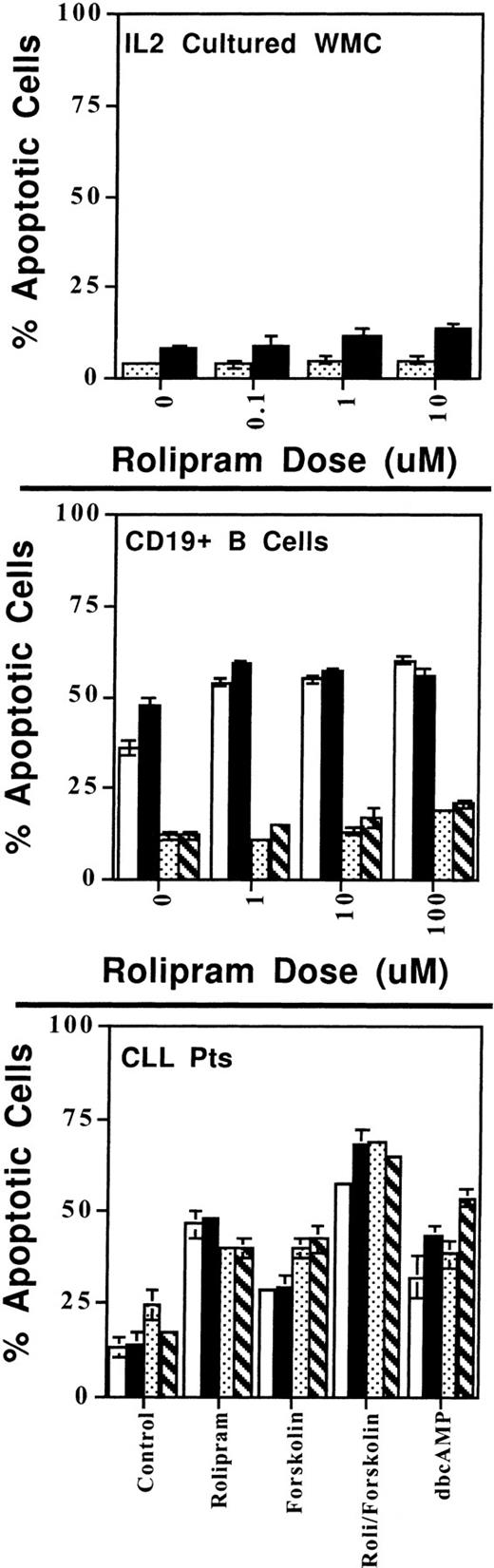
The alteration in rolipram sensitivity in anti-Ig–stimulated B cells was not caused by a change in the ability of this drug to augment cAMP levels at 2 hours in these cells, as rolipram increased cAMP levels equivalently in unstimulated or stimulated CD19+ B cells (Fig 9). To determine whether the rolipram-sensitive cell populations had a more prolonged elevation of cAMP than the insensitive cell populations following drug treatment, we measured cAMP levels 6 or 24 hours after addition of rolipram, times at which levels of apoptosis were still low even in sensitive populations. For each of the four cell populations, at 2 or 6 hours after drug treatment, cAMP levels were higher for rolipram/forskolin-treated cells than for forskolin only treated cells (t-test: one-tailed significance level <.05) (Fig 9). By 24 hours, there was no longer significant rolipram-induced augmentation of forskolin-stimulated cAMP accumulation in any of the four cell populations (Fig 9). Thus, the degree of cAMP augmentation by rolipram did not predict the sensitivity of cell populations to induction of apoptosis by this drug at any time point tested.
Rolipram blocks cAMP catabolism in both sensitive and resistant lymphoid populations. One million cells from the leukemic cells of two CLL patients (top; [▧], patient no. 1; [▪], patient no. 2), magnetic bead-purified CD19+ B cells (middle; [▧], resting; [▪], stimulated), or IL-2 supplemented WMC (bottom panel) were cultured with media, 10 μmol/L rolipram, 40 μmol/L forskolin, or both agents for 2, 6, or 24 hours, as indicated. cAMP content was determined by radioimmunoassay. In the case of the CD19+ cells, the cells were first cultured in media or Fab′2 anti-IgG/M for 30 minutes before treatment with rolipram or forskolin. Data are the mean of duplicate wells for each condition and are representative of two experiments with similar results.
Rolipram blocks cAMP catabolism in both sensitive and resistant lymphoid populations. One million cells from the leukemic cells of two CLL patients (top; [▧], patient no. 1; [▪], patient no. 2), magnetic bead-purified CD19+ B cells (middle; [▧], resting; [▪], stimulated), or IL-2 supplemented WMC (bottom panel) were cultured with media, 10 μmol/L rolipram, 40 μmol/L forskolin, or both agents for 2, 6, or 24 hours, as indicated. cAMP content was determined by radioimmunoassay. In the case of the CD19+ cells, the cells were first cultured in media or Fab′2 anti-IgG/M for 30 minutes before treatment with rolipram or forskolin. Data are the mean of duplicate wells for each condition and are representative of two experiments with similar results.
A potential weakness of inhibitor studies is that, although experiments may correlate drug effects (in this case, apoptosis) with inhibition of a known target enzyme (in this case, reflected by increased cAMP levels), the observed effect may still be caused by other, unrelated activities of the inhibitor. In the experiments described above, we have found that rolipram augments cAMP in both sensitive (CLL, resting B cells) and insensitive (WMC, activated B cells) cell populations, raising the concern that the observed cell death is the result of non–cAMP-related rolipram activity. Therefore, to address the variability of rolipram-induced apoptosis directly, without using the inhibitor itself, we examined the sensitivity of these four populations to the cell permeable cAMP analog, dibutyryl cAMP. As shown in Fig 10, we found a strong correlation between rolipram- and dbcAMP-induced apoptosis. For CLL cells and resting B cells, the percentage of apoptotic cells increased significantly relative to control cells after treatment with rolipram and forskolin or concentrations of dbcAMP ≥30 μmol/L (t-test: one-tailed significance level <.05). For anti-Ig–stimulated B cells or IL-2–cultured WMC, comparable treatments did not significantly increase the percentage of apoptotic cells. Consistent with this results, in the seven CLL patients studied thus far, there has also been a correlation between sensitivity to rolipram and sensitivity to dbcAMP (see Table 1). These data indicate that type 4 PDE is the relevant target for rolipram in its induction of apoptosis in CLL cells.
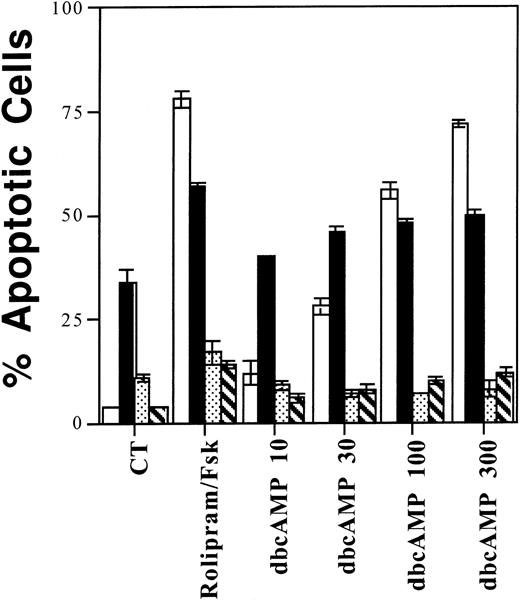
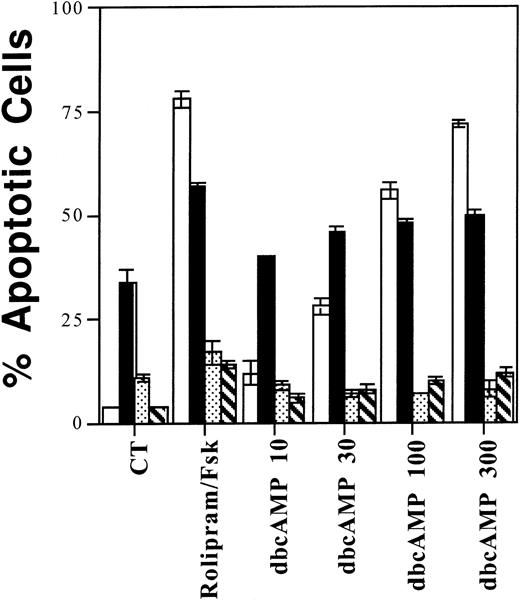
Among four different lymphoid populations, sensitivity to rolipram mirrors sensitivity to dbcAMP. One million CLL cells (□), resting (▪) or anti-Ig activated (▧) CD19+ cells, or IL-2–supplemented WMC (▧) were cultured for 72 hours with 10 μmol/L rolipram, 40 μmol/L forskolin, or the indicated dbcAMP concentration (in μmol/L) before measurement of apoptosis by Hoechst 33342 flow cytometry. The SEMs of triplicate cultures are indicated.
Among four different lymphoid populations, sensitivity to rolipram mirrors sensitivity to dbcAMP. One million CLL cells (□), resting (▪) or anti-Ig activated (▧) CD19+ cells, or IL-2–supplemented WMC (▧) were cultured for 72 hours with 10 μmol/L rolipram, 40 μmol/L forskolin, or the indicated dbcAMP concentration (in μmol/L) before measurement of apoptosis by Hoechst 33342 flow cytometry. The SEMs of triplicate cultures are indicated.
DISCUSSION
We have identified inhibition of type 4 cAMP phosphodiesterase activity as a novel means by which to trigger apoptosis in CLL cells in vitro. After finding type 4A and 4B transcripts as well as rolipram-inhibitable PDE activity in CLL cells, we determined that treatment of CLL cells with the PDE4 family-specific inhibitor rolipram increased cAMP levels and induced apoptosis in a dose- and time-dependent manner, an effect which correlated with apoptosis induced by dbcAMP.
Examination of treatment history and therapy resistance showed no clear relationship between these parameters and the sensitivity of the leukemic cells to rolipram-induced apoptosis. Five of the patients whose cells were sensitive to rolipram had shown clinical resistance to chlorambucil, cyclophosphamide, and/or fludarabine (see Table1). In contrast, several chemotherapy naive patients were largely resistant to rolipram as a single agent. Most patients' leukemic cells had not undergone cytogenetic analysis to allow correlation with such prognostically useful markers as 11q deletions, trisomy 12, or abnormalities at 13q12 or 13q14.25 Of note, however, the most rolipram-resistant patient had an elevated (20%) proportion of cells with prolymphocytic morphology on peripheral smear as well as multiple chromosomal abnormalities involving chromosomes 1, 2, 8, 9, 14-16, 18, and 22.
If PDEs are to be a useful pharmacologic target in the therapy of CLL, drug dosages that trigger apoptosis in leukemic cells in vivo must have clinically tolerable effects on other tissues. Type 4 PDEs are widely distributed enzymes; transcripts for both PDE4A and PDE4B have been detected by RT-PCR in all cell types tested, with the exception of the PDE4B within the T lineage.26 PDE4C and PDE4D are also widely distributed, although within the hematopoietic system PDE4C is absent and PDE4D appears to be restricted to the myelomonocytic lineage.26 Despite their widespread tissue distribution, type 4 inhibitors have been used effectively as anti-inflammatory drugs in animal models of asthma, inhibiting pulmonary eosinophil accumulation after allergen challenge of sensitized animals.27,28 Rolipram has been studied extensively as an antidepressant in humans and is well tolerated, although higher dosages are emetogenic. The clinical utility of type 4–specific PDE inhibitors related to rolipram is likely to improve further with the introduction of compounds, now identified by several groups, which maintain potency as PDE inhibitors yet have reduced ability to displace high-affinity [3H]rolipram binding to rat cortex. The high-affinity binding of rolipram to brain is a characteristic of this drug which appears to be separable from its activity as a PDE inhibitor, although central nervous system binding may be to an allosteric site on PDE4 enzymes.29
Although the feasability of type 4 PDE inhibition as a therapeutic approach for lymphoid malignancies will require further preclinical studies, we have made an effort in this work to determine whether rolipram's ability to induce apoptosis in the leukemic cells of some CLL patients is unique to this malignant population or shared by normal circulating lymphocytes. We observed that IL-2 cultured WMC and sIg-triggered B cells were largely insensitive to both rolipram- and dbcAMP-induced apoptosis, whereas treatment of nonstimulated B cells with rolipram or dbcAMP induced a moderate increase in apoptosis, albeit superimposed on considerable basal apoptosis. All our studies on B-cell apoptosis and cAMP metabolism were performed on cells positively selected by adherence to anti-CD19 antibody-coated magnetic beads, a technique that could alter the selected cells' subsequent responses. This acknowledged, our findings of elevated basal levels of apoptosis in nonstimulated human B cells and modest sensitivity to cAMP-mediated apoptosis are in keeping with studies reported by Lomo et al7 examining forskolin-induced apoptosis. In contrast, Mentz et al2 reported low levels of both basal and theophylline-induced apoptosis in their normal human B-cell controls. Although formal reports on the sensitivity of human circulating T cells to cAMP are scarce, murine peripheral T cells have been shown to be insensitive to cAMP-mediated apoptosis, in contrast to their thymocyte precursors.6 Whether the variable in vitro sensitivity of these lymphoid populations to cAMP-induced apoptosis accurately predicts the sensitivity of these cells in vivo will have to be addressed in preclinical, animal studies.
Given the complexity of cell-cell interactions and the hormonal and cytokine milieu of recirculating lymphocytes in vivo, cAMP metabolism in leukemic cells within a patient may prove to be different from that of cells maintained in tissue culture. Among those patients whose CLL cells were sensitive to rolipram, at low dosages of the inhibitor, the addition of the adenylate cyclase activator forskolin augmented apoptosis, suggesting that the basal leukemic cell adenylate cyclase activity in vitro was insufficient to adequately elevate cAMP levels. The finding that transcript levels for PDE4B are significantly higher in freshly isolated CLL cells than following culture is consistent with the hypothesis that cAMP production is higher in vivo than in vitro, as PDE4B transcript levels are known to be upregulated by elevated cAMP levels. However, given the length of time necessary for purification of lymphoid cells, it is difficult to prove or disprove this possibility experimentally. Although it is possible that the “flux-mediated” sensitivity of leukemic cells to treatment with rolipram as a single agent may be higher in vivo than in vitro, the efficacy of PDE inhibitors in CLL treatment might also be augmented by hormonal agents that enhance adenylate cyclase activity in circulating lymphocytes.
The resistance to cAMP-mediated apoptosis observed in Ig-stimulated B cells and IL-2–cultured WMC, as well as that observed in several CLL patients, could result either from alterations in cAMP metabolism or changes in the ability of PKA signaling to activate apoptotic signal transduction. Our studies indicate that, at least with regard to normal cell populations, insensitivity to cAMP-mediated apoptosis is not likely to result from altered cAMP metabolism. We found that at early time points, cAMP levels increased just as dramatically in the normal cell populations that were insensitive to cAMP as in those that were sensitive. Whether resistant CLL patients resemble sensitive ones with regard to cAMP metabolism will require further study; thus far, rolipram treatment has elevated cAMP levels in all CLL samples examined.
If lymphoid populations sensitive or insensitive to cAMP-mediated apoptosis differ with respect to apoptotic signaling by downstream targets for PKA, the identity of the relevant apoptotic target remains unknown. Transfection studies in a glucocorticoid receptor defective line derived from the human T-ALL cell line, CEM.C7, have suggested that functional glucocorticoid receptor signaling is required for cAMP-mediated apoptosis.30 Although steroid resistance is often noted clinically in CLL patients, deletions or alterations in the gene for glucocorticoid receptor are uncommon in CLL. Nonetheless, the glucocorticoid receptor remains a potential target for cAMP's cytolytic activity in CLL and nondeletional alterations in this receptor's signaling properties might underlie cAMP resistance.
BCL-2 and related proteins are likely to regulate sensitivity to apoptosis in CLL and are also potential targets for cAMP-mediated signal transduction. Although less than 10% of CLL patients have chromosomal translocations involving BCL-2, hypomethylation and high-level BCL-2 transcription is common.31 In their examination of theophylline's ability to synergize with chlorambucil in the induction of apoptosis by CLL cells, Mentz et al2 report that theophylline treatment reduced BCL-2. These observations are now complicated by the fact that McConkey et al32 have reported that incubation of CLL cells in vitro leads per se to BCL-2 downregulation. In the same report, downregulation of BAX with prolonged culture of CLL cells was found to be predictive of resistance to glucocorticoid or fludarabine/mitoxantrone-induced apoptosis.32 Whether such reduced BAX levels are also predictive of insensitivity to cAMP-mediated apoptosis has not been addressed.
ACKNOWLEDGMENT
We thank Drs Rita Blanchard, Mark Brauer, Evan Vosburgh, and Lewis Weintraub for their help in obtaining blood samples from CLL patients; Dr Keith Tornheim for advice regarding the PDE enzyme assay; Maris Handley for technical assistance during the Hoechst dye apoptosis assay; Kim Lui for excellent technical assistance; Dr Ronald Wohl for providing rolipram (Berlex Laboratories, Wayne, NJ); and Drs Thomas Rothstein, David Seldin, and Daniel Wright for helpful discussions throughout this project.
Supported by a Leukemia Society of America Translational Research Grant Award and a grant from the American Cancer Society (IN97U).
Address correspondence to Adam Lerner, MD, Department of Medicine, Section of Hematology and Oncology, Evans 539, Boston Medical Center, 88 E Newton St, Boston, MA 02118.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. Rolipram raises cAMP levels in CLL cells, WMC, and resting B cells. One million freshly isolated cells were incubated with the indicated drugs for 2 hours before analysis of cAMP in cell lysates using a radioimmunoassay. Data are the mean of three individually assayed culture wells. In (D), vinpocetine was added at 30 μmol/L. The experimental conditions indicated with an asterisk had a greater [cAMP] than, as appropriate, the untreated or forskolin-treated control cells (t-test: one-tailed significance level <.05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2484/3/m_blod41921004x.jpeg?Expires=1769816659&Signature=VTih8RwnxGfSbLprkXTdV1-hnVpWpaRqqeGwteLcHVraNzgA1ZlKKGeVOOVpCrr2bW~NH8JF3i5BwG1asmAfUl2STXJLwXnZCMPl~qXnuCIoE~n9OIfKBue9s0e7lZlVs3KLPCyeVNsGimJrbIeBqZiR07GOI8RHob2ers26otG7tex8vHHslsl0eg7jj50yD35kDJs~IXjHG1mXn5FIhcerJ2fZRSkDBvFedS1xJ8Klg8zBeudW1sGNuBz2eZMUZvCergw6BdUzO5he5hKy7yALoWWcAqvBbfn4oG0xy0nK4gjVMmSNiRSAfQTV29YHCxgzjC0Vf3LZESFqd4k4mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 9. Rolipram blocks cAMP catabolism in both sensitive and resistant lymphoid populations. One million cells from the leukemic cells of two CLL patients (top; [▧], patient no. 1; [▪], patient no. 2), magnetic bead-purified CD19+ B cells (middle; [▧], resting; [▪], stimulated), or IL-2 supplemented WMC (bottom panel) were cultured with media, 10 μmol/L rolipram, 40 μmol/L forskolin, or both agents for 2, 6, or 24 hours, as indicated. cAMP content was determined by radioimmunoassay. In the case of the CD19+ cells, the cells were first cultured in media or Fab′2 anti-IgG/M for 30 minutes before treatment with rolipram or forskolin. Data are the mean of duplicate wells for each condition and are representative of two experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2484/3/m_blod41921009x.jpeg?Expires=1769816659&Signature=4zYfdbT3eqQwlKSCjgPXeHEOGohYCJErjYm~ckJ3ytF-v8qWz5Zdoqf9UjZGSzCJ-75WaKBh-v7sreMDUBRoElbuQ7po2H8ysrNW6tV1khe2SkRfb9TS66DCrTUz78TJ0T5G~wGl1Dq-cB1vFFjScPpC1A5gCHizQjEMg8TERT5sWLRznwMSUucm1kuiaT0NXETNh8zC0KEi3U2gIGfTHvDtWimTSSyPXc5tahD64apVpTu9HxWAh3dwVrA5aMLhVrRdHhIUcz4ltmGCcz4bUgM9WhuuUd1V1SnEgbeAPwG5NY5euSZ8aKeAUpHHeLG5BgvSdOQxr71Cevd6EZYvnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Rolipram raises cAMP levels in CLL cells, WMC, and resting B cells. One million freshly isolated cells were incubated with the indicated drugs for 2 hours before analysis of cAMP in cell lysates using a radioimmunoassay. Data are the mean of three individually assayed culture wells. In (D), vinpocetine was added at 30 μmol/L. The experimental conditions indicated with an asterisk had a greater [cAMP] than, as appropriate, the untreated or forskolin-treated control cells (t-test: one-tailed significance level <.05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2484/3/m_blod41921004x.jpeg?Expires=1769816660&Signature=lh0XmrMSjUvUBjkO2ApMpABSOO2-iASwuQrk5KqV0C7ET8XI1DUKpPySahaVs2glpLisL5A2QTCCGN9g88SPz3cy92-CZShavtdB48HQZs3FzOmgceVoSsZrfMVF63E2vHVdQzbZKcbUDdjzdh6KIqg~Nh5zCma1cRNp7-r08Gxr6cQBcuw2fVyro2tX4v7m6Ct2aTnOo9V663xcdngpVTFbGGr0x7ezx3l8mdtmddDc8ZbtCBGrGedC5TxAU94VNoLB9D64R86LUXt3cBg2cOr8TPUsk8kn~DtKK-Zx0cr00YEy9~oJZAas2q6NFGo3ynAJawNLVQt6o~vFVR~ffQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 9. Rolipram blocks cAMP catabolism in both sensitive and resistant lymphoid populations. One million cells from the leukemic cells of two CLL patients (top; [▧], patient no. 1; [▪], patient no. 2), magnetic bead-purified CD19+ B cells (middle; [▧], resting; [▪], stimulated), or IL-2 supplemented WMC (bottom panel) were cultured with media, 10 μmol/L rolipram, 40 μmol/L forskolin, or both agents for 2, 6, or 24 hours, as indicated. cAMP content was determined by radioimmunoassay. In the case of the CD19+ cells, the cells were first cultured in media or Fab′2 anti-IgG/M for 30 minutes before treatment with rolipram or forskolin. Data are the mean of duplicate wells for each condition and are representative of two experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2484/3/m_blod41921009x.jpeg?Expires=1769816660&Signature=LcU2sgnZv5keMrvuek3uotuzwz7GFV07NeFXSdCcC31iFyGyQP8E2VJGmTtJHN6uQi1xAu7ZwR8ad-vfZt94ypxPyHjtfVgLDZOfMm4F-RcPCWxFGe5E5k0mUrMD8IFoAM2aolmLpewXk3dRctaaaQVbK7g9alpUxs0cO7Szk45JIcXkIlc57kV6UWNMK4gX8KpdRm2wrPHYBsGPa4fTKUyeHXYWu-csryhWnqVROdZVJ8xt2NRdFJZea-wZosNQ6qED2azW0XcN3V2RAEIA8gqQ3LgAiI7E0WXCxl~5U3S5rJi0AH5zlV4TGyar8aCimmlJ0ZBekNQgYLb0QOB4QQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)