Abstract
In this study a flow cytometric technique for detecting cytoplasmic perforin (P) has been used to quantify age-related changes in perforin expression in human peripheral blood lymphocytes (PBL). Proportions of P+ lymphocytes increased after birth, but declined rapidly after the age of 70 years. This was true for both T cells and CD16+ and CD56+ natural killer (NK) cells. Children showed in addition to high levels of perforin positive CD8+ cells a much higher proportion of CD4+P+ cells than the other age groups. In elderly individuals there was also a highly significant reduction in mean levels of perforin per cell as compared with all other groups (P < .05 to .001). Adult women had consistently higher mean levels of perforin per cell than adult men for all P+cell phenotypes. Functional tests clearly showed the deficiency in early spontaneous cytotoxic potential of PBL from elderly persons due to relative P deficiency, which can be corrected by stimulation of cytolytic cells with target cells and interleukin-2 (IL-2). The deficiency in cytolytic activity on the contact with target cells may have implications for antiviral and antitumor immunity in elderly persons.
CYTOTOXIC LYMPHOCYTES or killer cells are the mediators in cell-mediated cytotoxicity (CMC) reactions. They comprise cytotoxic T lymphocytes (CTL) and natural killer (NK) cells. CTL are a part of the adaptive immune system and NK cells of the innate or natural immune system. Both CTL and NK cells are endowed with a potent cytolytic machinery located in the cytoplasmic granules containing the cytolytic molecule perforin (perforating protein) and associated granule proteases (granzymes).1,2 Perforin “perforates” target cell membranes by forming transmembrane pores, which lead to the equilibration of ionic gradients, normally maintained by cell membrane, osmotic lysis, and nuclear disintegration.3 The formation of membrane pores by perforin is also a prerequisite for apoptosis of target cells, in the case of successful membrane repair, stimulated by calcium ion influx. Granzymes, which are also located in the secretory granules of cytotoxic lymphocytes, gain entry into the target cell through perforin pores and mediate target cell DNA degradation through the activation of caspase 3, which is the protease responsible for cleavage of poly (adenine diphosphate [ADP] ribose) polymerase.4-7 The pathophysiologic significance of perforin-mediated cytotoxicity was confirmed in experiments with perforin-deficient animals, showing its role in protection against some viral infections, intracellular parasites, tumors, and allotransplants (for review, see Kagi8).
During postnatal life, from soon after birth until old age, the immune system in humans passes through many changes both in the numbers of immunocompetent cells and their phenotypic characteristics, as well as functional capabilities. Absolute numbers of leukocytes decline progressively, as do total lymphocytes, T, B, and NK cells.9,10 These changes are accompanied by a marked increase in the numbers of activated T cells (CD3+HLA DR+), as well as cells with potential to mediate non-major histocompatibility complex (MHC)-restricted cytotoxicity.11 NK activity assessed by cytotoxicity against the K-562 cell line is extremely low in the neonatal period, but increases rapidly to almost reach adult levels between 1 and 5 months of age and remains at that level throughout childhood.12
The age-related decline in the functional activity of CTL and NK cells has been recognized for many years. It has been demonstrated in mice that deterioration at the molecular level (perforin and granzymes) in the lytic mechanism may at least in part be responsible for the decline in CTL activity.13-16 However, there are almost no data on perforin protein expression in human peripheral blood cytolytic cells and their subsets during various periods of the postnatal life. The results obtained in this investigation point to both the serious deficit of perforin expression in old and the deficiency in spontaneous NK cytotoxicity. The significance of this finding for the functional reactivity of cytolytic cells is discussed.
MATERIALS AND METHODS
Subjects.
The study was approved by the Ethics Committee of the Medical Faculty University of Rijeka. For flow cytometric analyses, five groups were formed. Cord blood (CB) was obtained within an hour of normal vaginal delivery (n = 5). Children were normal healthy persons (n = 7; four females, three males; age = 5 years) attending regular health control clinic, and informed consent of their parents was obtained. Adults comprised normal laboratory donors; males were aged between 20 and 31 years (n = 9) and females were aged between 20 and 34 years (n = 12). Elderly people were normal apparently healthy individuals living in an old people's home, were aged between 73 and 77 years (n = 7; four females, three males), and informed consent was obtained. Proliferation and cytotoxicity assays were performed in adults and elderly persons only. For mixed lymphocyte reaction (MLR), six adults and six elderly persons were included and for cytotoxicity assays, five elderly persons and six adults.
Lymphocyte preparation.
Between 3 mL (children) and 10 mL of heparinized venous peripheral blood was layered onto Ficoll/Hypaque density gradient (Nycomed Pharma AS, Oslo, Norway) and centrifuged for 20 minutes at 800g. Cells accumulating at the interface were washed twice in RPMI 1640 and resuspended at a final concentration of 1 × 106 peripheral blood lymphocyte (PBL)/per sample in buffer for flow cytometry. Cell viability was checked by trypan blue.
Monoclonal antibodies (MoAbs).
A murine antihuman perforin MoAb δG9 (MoAb; IgG2b) was purified from Balb/c ascites.17 The following MoAbs were obtained conjugated to phycoerythrin from Becton Dickinson (Mountain View, CA): Leu-4 (anti-CD3), Leu-3a (anti-CD4), Leu-2a (anti-CD8), Leu-11b (anti-CD16), and Leu-19 (anti-CD56).
Simultaneous detection of cell surface and intracellular antigens by flow cytometry.
Our procedure for the simultaneous detection of intracellular perforin and cell surface membrane antigens has been described elsewhere.18 Briefly, PBL were cultured overnight at 37°C in complete RPMI 1640 medium containing 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin and 5 × 10−5 mol/L 2-mercaptoethanol. Nonadherent cells were aliquoted (106per aliquot) and washed in FACS buffer (2% FCS, 1 mmol/L EDTA, 0.1% NaN3 in phosphate-buffered saline [PBS], pH 7.4). They were then fixed in 100 μL PBS containing 4% paraformaldehyde, pH 7.4, for 10 minutes at room temperature. After two washes in FACS buffer, the cells were permeabilized with saponin buffer (0.1% saponin [Sigma, Poole, Dorset]), 2% goat serum and 1 mmol/L EGTA in PBS for 20 minutes at room temperature. Antiperforin MoAb in saponin buffer (3 μg/100 μL) was added to the cell suspension at a final concentration of 8 to 10 μg per sample and incubated for 30 minutes at +4°C. Second antibody (fluorescein-conjugated goat antimouse IgG; Becton Dickinson) was added for a further 30 minutes at +4°C, with this and subsequent rinsing steps being performed in saponin buffer. Cell surface antigens were then labeled by incubating for 30 minutes at +4°C with phycoerythrin-conjugated MoAbs after the integrity of the membranes was restored by a 10-minute incubation in FACS buffer. In some experiments, only cell surface labeling was performed. An irrelevant isotype-matched murine MoAb was used as a negative control.
Lymphocytes were gated on the basis of forward and side scatter (FSC and SSC). A minimum of 104 cells were analyzed on a FACScan (Becton Dickinson), and results are shown as contour graphs. Thresholds for positive staining were set at less than 2% using the negative control and percentages of positive cells were obtained by subtracting the value of the negative control.
Cell proliferation assays.
PBL from adults and elderly people (responding cells) were separated by Ficoll-Hypaque, as described. Allogeneic stimulating cells were buffy coat cells obtained from blood volunteer donors from the Transfusion Center. After the washing procedure, stimulating cells were placed in 50-mL plastic culture flasks (Grainer, Frickenhausen, Germany) in RPMI 1640 supplemented with 5% FCS (GIBCO, Gaithersburg, MD), L-glutamine (175 μg/L), tiamulin (100 mg/L), penicillin (500 IU/L), gentamycin (2 mg/L), and streptomycin (60 mg/L). The cells were cultured overnight at 37°C in a humidified atmosphere with 5% CO2 to allow adherence. The adherent cells (perforin negative) were removed by short-term (3 minutes) trypsin treatment. The process of trypsinization was stopped by adding 10% RPMI 1640. The population of cells obtained was mitomycin-C–treated and served as stimulating cells in one-way MLR. The MLR was performed by adding 1 × 106 PBL to 1 × 106 mitomycin-C–treated adherent cells and incubated either for 24 or 72 hours, when both the percentage of perforin positive cells and the content of perforin/cell (mean fluorescence intensity [MFI]) were measured. PBL from adults and elderly persons were cultured in high interleukin-2 (IL-2) (100 U/mL) medium (human rIL-2) for 6 and 24 hours, respectively, and perforin content was measured.
Cytotoxicity assay.
Functional NK cell assays were performed with PKH-26 (orange)-labeled K562 target cells following the manufacturer's instructions (Sigma Biosciences, St Louis, MO; PKH-26 Red Fluorescent Cell Linker Kit). PBL from adults or elderly persons were incubated for 2 hours with K-562 target cells prestained with PKH-26, a lipophilic dye that binds to the cell membrane and fluoresces orange. Briefly, 1 × 105/mL-labeled K562 cells were incubated with PBL effector cells at different killer to target ratios in a final volume of 200 μL, for 2 hours in short-term assays and 18 hours for long-term assays at 37°C in a 5% CO2 atmosphere. After washing in FACS medium, 200 μL of propidium iodide (concentration 10 μg/mL) was added and the percentage of killed cells was measured by flow cytometry. Killed cells were enumerated by detecting cells that have both orange (PKH-26) and red (propidium iodide) fluorescence.
Immunocytochemistry.
Perforin was detected in cytospins of PBL from adults and elderly persons. Briefly, lymphocytes, washed twice, were centrifuged onto glass microscope slides. After drying at room temperature, the cells were fixed in cold acetone for 10 minutes and washed once in Tris buffered saline (TBS). All incubations were performed at room temperature in a humidified atmosphere. Endogenous peroxidase activity was blocked by H2O2, and nonspecific binding was stopped by preincubation with 1% bovine serum albumin (BSA). The primary antibody (mouse antihuman perforin) was applied at a concentration of 30 μg/mL for 1 hour. Peroxidase-labeled antimouse IgG (Boehringer, Mannheim, Germany) was used at a dilution of 1:100 for 45 minutes. After two washing steps in TBS, the reaction was developed by 3-amino-9-ethyl carbazole (Sigma). Nuclei were counterstained with hematoxylin, and slides were mounted in gelatine-glycerol.
Statistical analysis.
Results were analyzed using the Sigma Plot for Windows, version 1.02 (Jandel Scientific, Chicago, IL). Statistical analyses were performed using a Student's t-test one-way analysis for comparison of means.
RESULTS
Total perforin positive cells in CB lymphocytes and PBL.
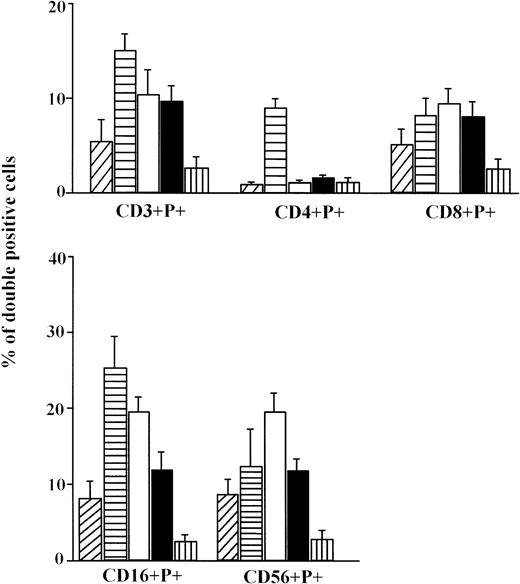
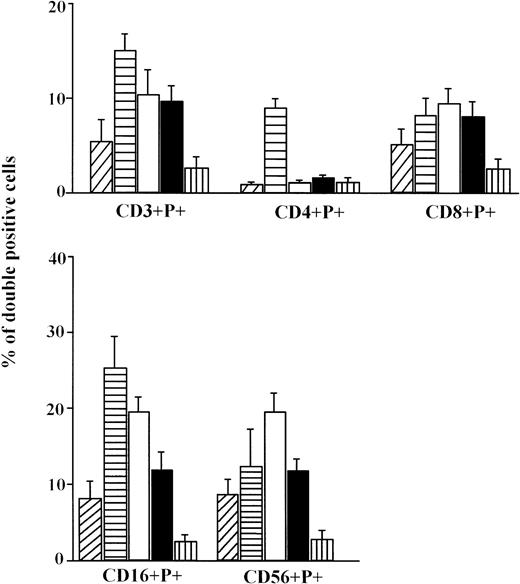
We have investigated the percentage of isolated lymphocytes containing the cytolytic protein perforin in their cytoplasmic granules. Approximately 12.2% of umbilical cord lymphocytes were perforin positive (Fig 1) suggesting that fetal PBL are endowed with cytolytic molecules, at least by the end of fetal life. Significantly higher percentages of PBL are perforin positive in children, not only when compared with CB (P < .001), but with other groups (P < .05), and particularly to the aged group (P < .001). Levels of perforin expression in PBL were substantially lower in aged persons than in all other groups. There was no difference between men and women.
The percentage of total perforin positive cells (mean ± standard error [SE]) in CB lymphocytes (A, ▨;) and PBL from children (B, ▤), men (C, □), women (D, ▪), and elderly persons (E, ▥). Levels of significance: (B, C, D) versus (A, E) P < .001; (A) versus (E) P < .05; (B) versus (C, D) P < .05.
The percentage of total perforin positive cells (mean ± standard error [SE]) in CB lymphocytes (A, ▨;) and PBL from children (B, ▤), men (C, □), women (D, ▪), and elderly persons (E, ▥). Levels of significance: (B, C, D) versus (A, E) P < .001; (A) versus (E) P < .05; (B) versus (C, D) P < .05.
As explained in Materials and Methods, in all groups, cells were double-labeled simultaneously for perforin (intracellular antigen) and for a panel of cell surface antigens characteristic for T lymphocytes (CD3, CD4, CD8) and NK cells (CD56, CD16).
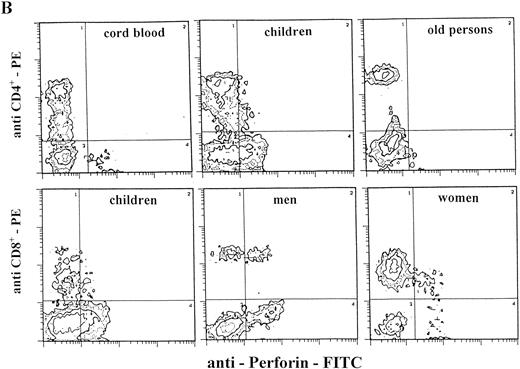
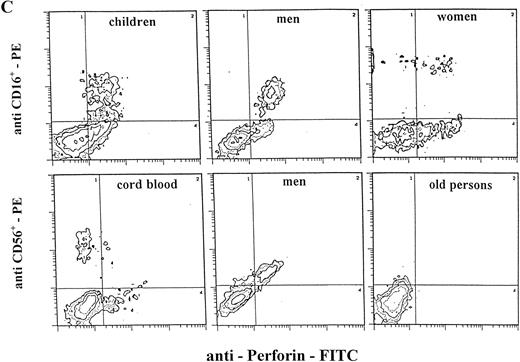
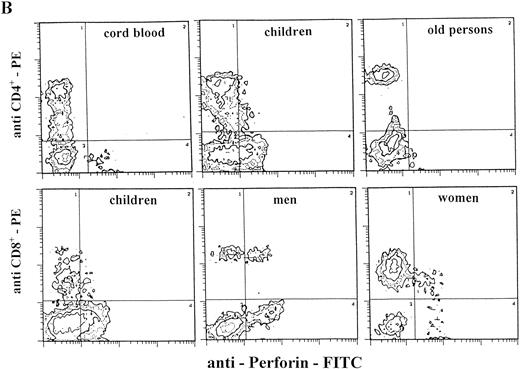
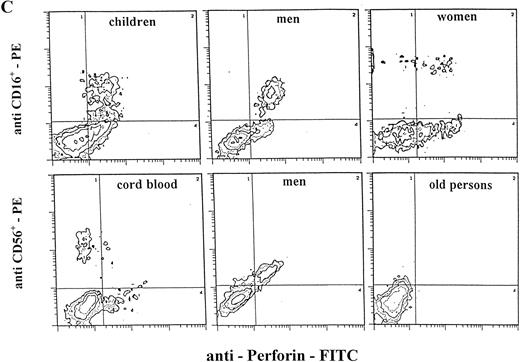
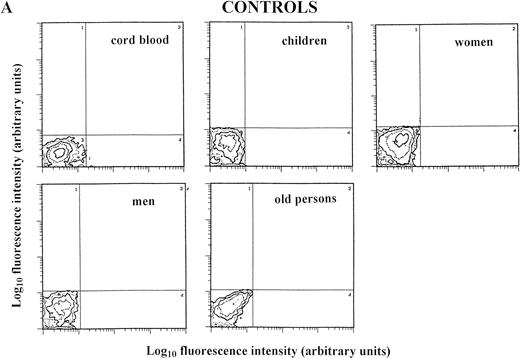
Staining for perforin expression in double-labeled cells from various groups as determined by two-color analysis is shown in Fig 2. The gates were set by using labeled isotype antibody controls for each group. Perforin expression is represented in three different ways.
Two-color analysis of CB lymphocytes and PBL for perforin (horizontal) and surface phenotype (vertical) showing the most characteristic differences in perforin positive T (B) and NK (C) cell subsets in various age groups. Isotype-matched murine control MoAb (A).
Two-color analysis of CB lymphocytes and PBL for perforin (horizontal) and surface phenotype (vertical) showing the most characteristic differences in perforin positive T (B) and NK (C) cell subsets in various age groups. Isotype-matched murine control MoAb (A).
Double-positive PBL.
In this representation, double-positive cells (perforin positive and surface marker positive) are represented as a fraction of total PBL counted (10,000 cells), which is set at 100% (Fig 3).
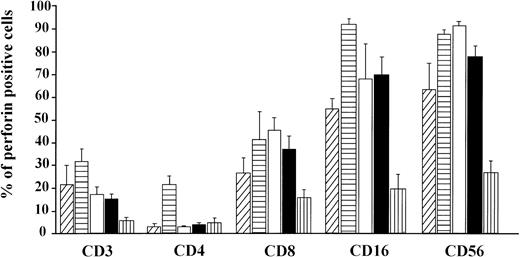
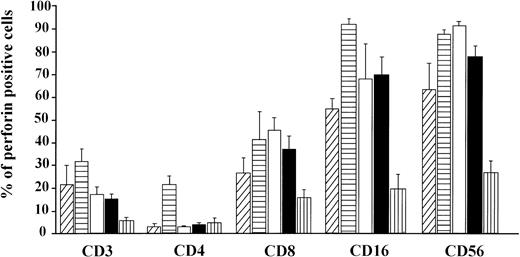
The percentage of double positive (perforin+ and surface marker+) lymphocytes (mean ± SE). CB lymphocytes (A, ▨;) and PBL from children (B, ▤), men (C, □), women (D, ▪), and elderly persons (E, ▥). Levels of significance (from P < .05 to P < .001) for: CD3 = (A) versus (B, C, D); (B) versus (D); (B, C, D) versus (E); CD4 = (B) versus (A, C, D, E); CD16 = (B) versus (A, C, D, E); (A) versus (E); (C) versus (D, E); (D) versus (E); CD8 = (A) versus (C, E); (B, C, D) versus (E); CD56 = (A) versus (C, E); (C) versus (D); (B, D) versus (E).
The percentage of double positive (perforin+ and surface marker+) lymphocytes (mean ± SE). CB lymphocytes (A, ▨;) and PBL from children (B, ▤), men (C, □), women (D, ▪), and elderly persons (E, ▥). Levels of significance (from P < .05 to P < .001) for: CD3 = (A) versus (B, C, D); (B) versus (D); (B, C, D) versus (E); CD4 = (B) versus (A, C, D, E); CD16 = (B) versus (A, C, D, E); (A) versus (E); (C) versus (D, E); (D) versus (E); CD8 = (A) versus (C, E); (B, C, D) versus (E); CD56 = (A) versus (C, E); (C) versus (D); (B, D) versus (E).
In the CB lymphocytes, two thirds of perforin positive cells belong to the NK subset (CD16; CD56; approximately 9% of cells) and one third to T lymphocytes (CD3+; approximately 5%). CD8+P+ are the predominant subset among perforin positive T lymphocytes (5.3%), and CD4+P+ cells account for only 0.8%. Percentages of CD16+P+ and CD56+P+ cells in this group are almost identical, indicating that almost all CD16+ cells are also CD56+P+.
Notable differences were found between PBL from children and all of the other groups in that the percentages of CD3+P+and CD16+P+ cells were significantly higher than in any other group. Furthermore, the most prominent finding in this group is a highly significant increase of CD4+P+ cells (8.9% of total PBL), which is 6 to 10 times higher than in any other group (P < .001). In all other groups, CD4+P+ cells were very low, approximately 1% of total PBL. In children, the percentage of CD16+P+ cells (25.6%) was significantly higher than in any other group.
In adults, the only difference between women and men was for cells of NK phenotype. The level of CD16+P+ and CD56+P+ cells was significantly higher in men than in women. Furthermore, the percentage of CD56+P+ cells in men (19.7%) was the highest in this investigation, significantly higher than in all other groups.
The percentages of total T cells and CD8+ cells that were perforin positive were by far the lowest in old persons, as were percentages of NK subpopulations of perforin positive cells (CD16+P+ and CD56+P+).
Frequency of perforin expressing cells in the various lymphocyte subsets.
The following data present perforin expression as the fraction of cells with a certain phenotype, eg, CD3. The percentage of perforin positive cells then represents a fraction of all CD3+ cells (Fig 4). Both cytotoxic T lymphocytes and NK cytolytic cells from CB lymphocytes are endowed with similar proportions of cells with the cytolytic molecule perforin as adults.
The frequency of perforin expressing cells (mean ± SE) in the various lymphocyte phenotypes in CB lymphocytes (A, ▨;) and PBL from children (B, ▤), men (C, □), women (D, ▪), and elderly persons (E, ▥). Levels of significance (from P < .05 toP < .001) for: CD3 = (A) versus (E); (B) versus (C, D, E); (C, D) versus (E); CD4 = (B) versus (A, C, D, E). CD8 = (A) versus (C, E); (B) versus (E); (C, D) versus (E); CD16 = (A) versus (B); (B) versus (C, D, E); (C, D) versus (E). CD56 = (A) versus (B, C, E); (B) versus (D, E); (C) versus (D, E); (D) versus (E).
The frequency of perforin expressing cells (mean ± SE) in the various lymphocyte phenotypes in CB lymphocytes (A, ▨;) and PBL from children (B, ▤), men (C, □), women (D, ▪), and elderly persons (E, ▥). Levels of significance (from P < .05 toP < .001) for: CD3 = (A) versus (E); (B) versus (C, D, E); (C, D) versus (E); CD4 = (B) versus (A, C, D, E). CD8 = (A) versus (C, E); (B) versus (E); (C, D) versus (E); CD16 = (A) versus (B); (B) versus (C, D, E); (C, D) versus (E). CD56 = (A) versus (B, C, E); (B) versus (D, E); (C) versus (D, E); (D) versus (E).
The highest level of perforin expression in T lymphocytes was found in children (31.3%), which is significantly higher than in all other groups. Careful analysis of this result shows that the perforin positive cells in the CD4+ cytolytic T lymphocytes subset (CD4+P+) contributes most of this increase. The percentage of perforin positive cells among CD8+cells is close to levels detected in both groups of adults, but their frequency in CD4+cells (21.3%) is five to eight times higher than in the other groups.
The percentage of perforin positive cells among CTL (CD3+P+ and CD8+P+) is the lowest in old persons. Similarly, by far the lowest levels of perforin positive NK cells (CD16+ = 19.1%; CD56+ = 26.3%) were found in this group (Fig 4).
There was no difference in perforin expression in CTL subpopulations between men and women. The only difference was for the CD56+ subset with significantly higher expression in the men's PBL (91.2% of cells perforin positive v 77.7% in women). This is the highest proportion of perforin positive cells in any cell population measured and indicates that in men almost all CD56+ cells contain perforin.
Level of perforin protein expression in T and NK cell subsets.
The procedure of double-labeling of PBL for simultaneous detection of intracellular protein (perforin) and cell surface antigens by flow cytometry, besides enabling the detailed analyses of perforin expressing cells, also permitted the measurement of MFI, which is supposed to be proportional to the number of perforin molecules present in the cells. By applying standard parameters for FSC, SSC, fluorescence intensity 1 (FL1), and FL2, we analyzed MFI for perforin in subsets of T lymphocytes (CD3+P+, CD4+P+, CD8+P+) and NK cells (CD56+P+, CD16+P+) (Table 1).
Perforin content in CB cells, for almost all subsets of T lymphocytes and NK cells, was generally similar to values obtained in adults. PBL of children had quantities of perforin similar to those of adults. The content of perforin was higher in all subsets of women's PBL compared with those from men, and the difference is significant for all subsets of T cells and for CD16+ NK cells.
By far the lowest levels of perforin were found in all subsets of PBL from old persons, which were significantly lower than in other groups.
Cytotoxicity assays.
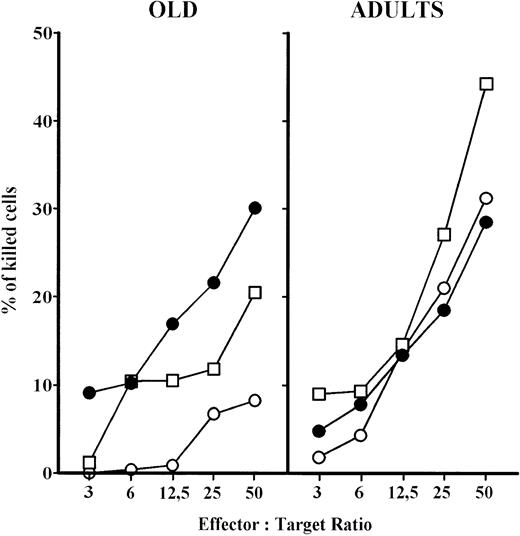
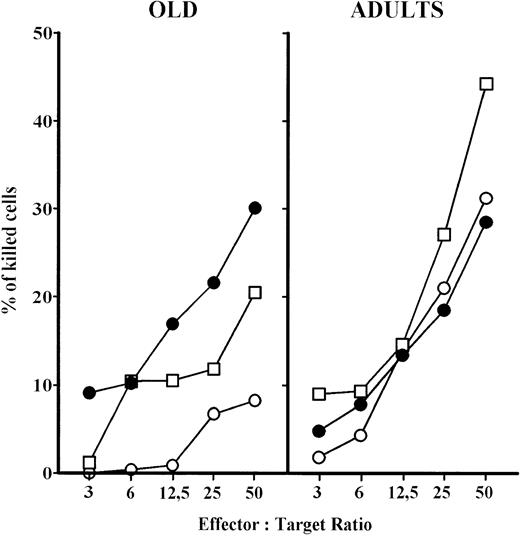
In a short-term (2 hours) cytotoxicity assay, PBL from aged persons have reduced cytotoxicity at all effector versus target ratios compared with adults (Fig 5). There was no difference in cytotoxicity between the 2- and 18-hour tests for adult persons. However, prolonged effector to target contact (18 hours) of PBL from aged persons greatly increased their cytotoxic activity, which reached the level of adults. The deficiency of aged PBL in short-term assay was partially corrected after 24 hours IL-2 culture, however the cytotoxicity was still lower than in adults (Fig 5). IL-2 culture also increased the cytotoxic potential of adults' PBL.
NK cytotoxicity of PBL from elderly persons increased in: (a) short-term assay, after previous IL-2 culture and (b) after prolonged contact (18 hours) with the target K-562 cells. Results are expressed as mean values, and each point is the average value of five to six samples. ○, short-term assay (2 hours); □, short-term assay (2 hours) with PBL cultured in IL-2 for 24 hours; •, long-term assay (18 hours).
NK cytotoxicity of PBL from elderly persons increased in: (a) short-term assay, after previous IL-2 culture and (b) after prolonged contact (18 hours) with the target K-562 cells. Results are expressed as mean values, and each point is the average value of five to six samples. ○, short-term assay (2 hours); □, short-term assay (2 hours) with PBL cultured in IL-2 for 24 hours; •, long-term assay (18 hours).
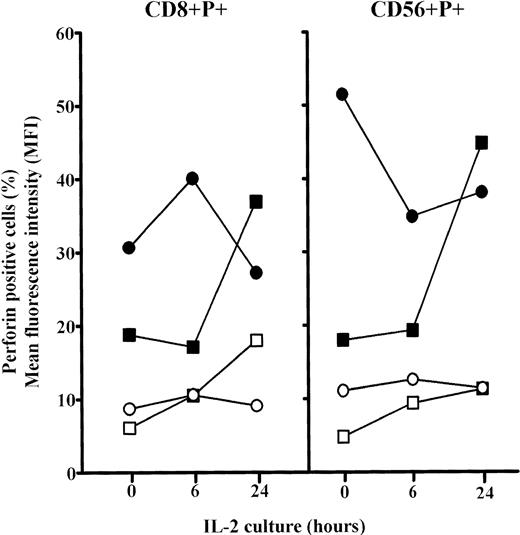
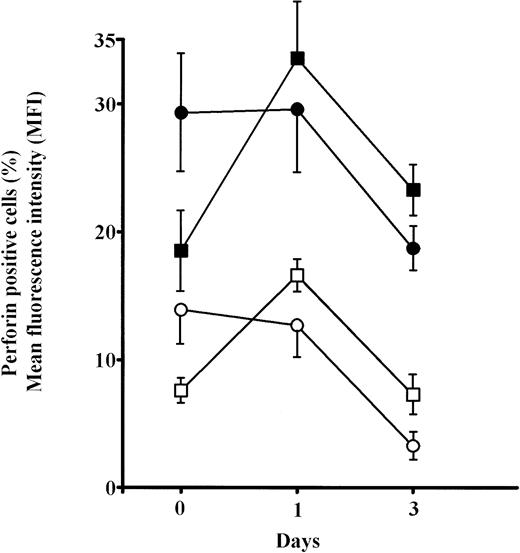
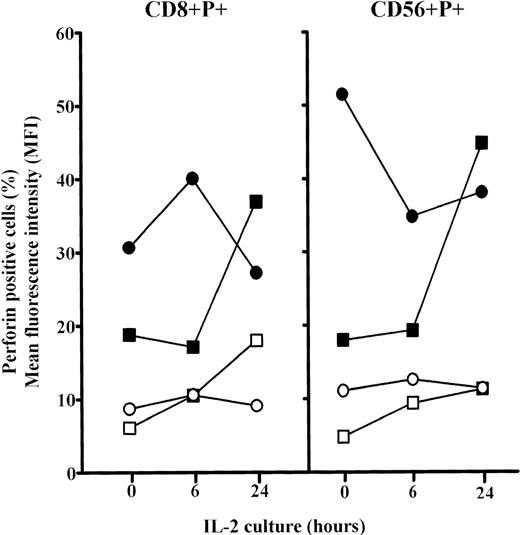
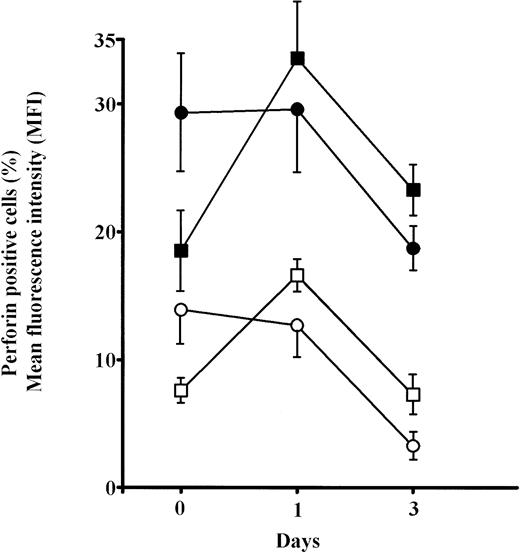
The analyses of the frequency of CD8+P+ and CD56+P+ cells in elderly persons showed significant increases for both after 6 hours culture in IL-2 (P< .01) and approached the levels in adults. Prolonged culture in IL-2 (24 hours) further increases these values (P < .001), but particularly MFI for perforin in both CD8+ and CD56+ cells in old persons (Fig6). Because the percentages of single positive CD8+ and CD56+ cells were not changed after 6 and 24 hours IL-2 culture (not shown), it is concluded that the proportion of cells that were double positive (CD8+P+ and CD56+P+) increased greatly.
Changes in the percentages and mean content of perforin (MFI) in subpopulations of perforin positive PBL (CD8+P+ and CD56+P+) cultured for 6 or 24 hours in high IL-2 medium. Results are expressed as mean values, and each point is the average value of five to six samples. Perforin positive cells: adults, ○; elderly persons, □. MFI: adults, •; elderly persons, ▪.
Changes in the percentages and mean content of perforin (MFI) in subpopulations of perforin positive PBL (CD8+P+ and CD56+P+) cultured for 6 or 24 hours in high IL-2 medium. Results are expressed as mean values, and each point is the average value of five to six samples. Perforin positive cells: adults, ○; elderly persons, □. MFI: adults, •; elderly persons, ▪.
Immunocytochemistry.
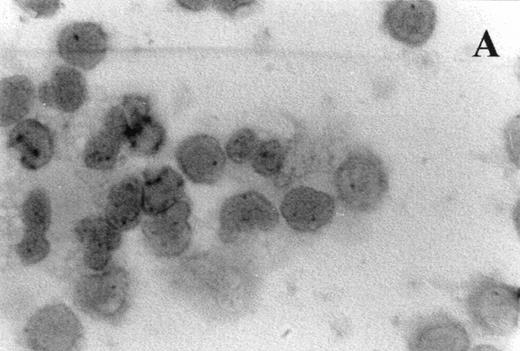
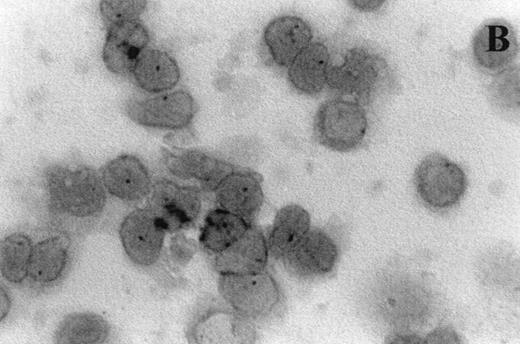
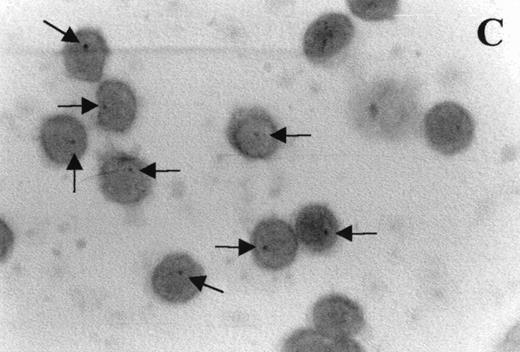
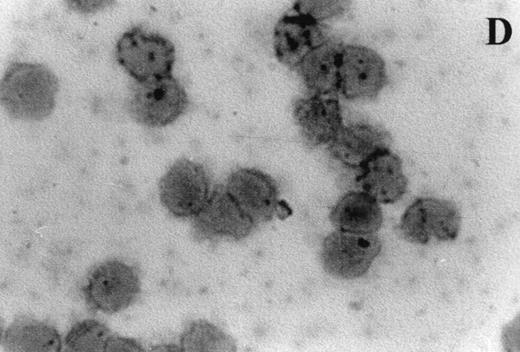
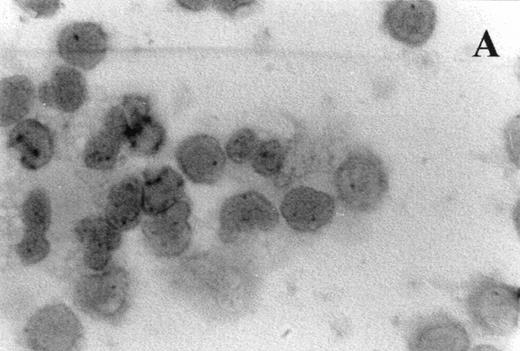
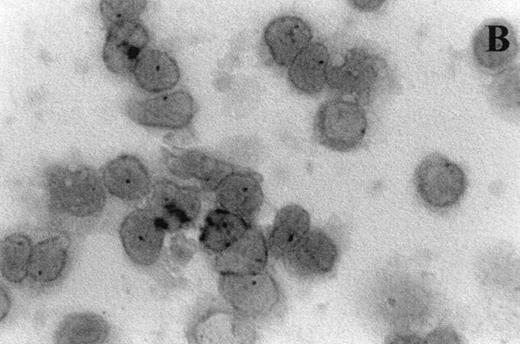
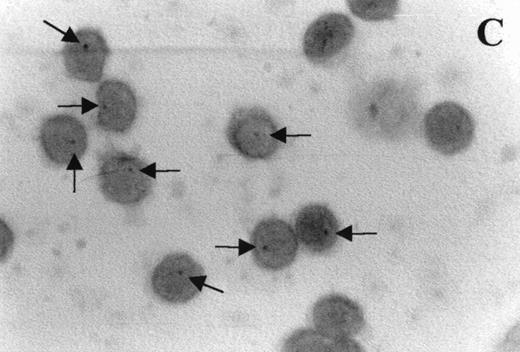
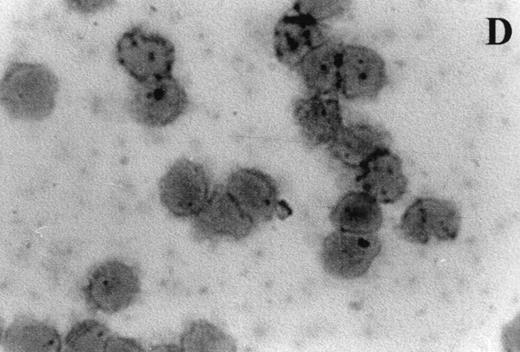
Immunocytochemistry clearly confirmed the results of FACS analyses (Fig 7). PBL from elderly persons (Fig 7C) exhibited very few and more lightly stained or smaller P+intracytoplasmic granules/cell compared with adults (Fig 7A). However, PBL of elderly persons after 24 hours IL-2 culture (Fig 7D) had numerous P+ granules, which were stained very strongly, and the number of granules was even higher than in the corresponding group of adults (Fig 7B).
Photomicrographs (1,000 × magnification) showing perforin expression in cytoplasmic granules. PBL from adults: (A) time 0; (B) 24 hours IL-2 culture. PBL from elderly persons: (C) time 0; (D) 24 hours IL-2 culture. Arrows in (C) indicate granules in the cytoplasm of elderly persons stained light, but clearly positive for perforin.
Photomicrographs (1,000 × magnification) showing perforin expression in cytoplasmic granules. PBL from adults: (A) time 0; (B) 24 hours IL-2 culture. PBL from elderly persons: (C) time 0; (D) 24 hours IL-2 culture. Arrows in (C) indicate granules in the cytoplasm of elderly persons stained light, but clearly positive for perforin.
Proliferation assay.
Alloantigens were found to be very strong stimulators of perforin expression in PBL of old persons. Short-term culture (24 hours) strongly increased both the percentage of perforin positive cells and MFI for perforin, even to higher levels than in adults (Fig 8). Both the percentage of perforin positive cells and MFI for perforin in aged persons were significantly higher (P < .01) after 24 hours culturing, compared with the initial values. In adults, these values were almost unchanged. In all groups, values for day 3 culture decreased significantly.
Changes in the percentage of perforin positive cells and mean content of perforin per cell in PBL from elderly persons cultured with mitomycin-C–treated allogeneic cells. Results are expressed as mean ± SE and each point is the average value of six samples. Perforin positive cells: adults, ○; elderly persons, □. MFI: adults, •; elderly persons, ▪.
Changes in the percentage of perforin positive cells and mean content of perforin per cell in PBL from elderly persons cultured with mitomycin-C–treated allogeneic cells. Results are expressed as mean ± SE and each point is the average value of six samples. Perforin positive cells: adults, ○; elderly persons, □. MFI: adults, •; elderly persons, ▪.
DISCUSSION
Previous reports have shown a steady decline in absolute numbers of both CD4+ and CD8+ T-cell subsets with advancing age,11,19 and this is reflected by a poorer response to mitogen.19 Superimposed on this decrease is a substantial increase in the proportion of T cells that express HLA-DR, a marker of T-cell activation. However, this is not associated with any change in the proportions of CD45RO+ cells,20and there are no data to indicate whether these cells are activated functionally. A more pronounced decrease with age in absolute B-cell numbers was also noted,11 paradoxically associated with increased serum levels of some Igs.21
However, absolute NK cell numbers did not decline with age and proportions of lymphocytes expressing CD16, CD56, or CD57 were significantly increased.11 Despite these relative increases, NK cell function on a per cell basis was either stable up to the age of 7022 or declined with age.23 When centenarians were analyzed as a separate group, they were found to have higher NK and redirected killing activity than middle-aged donors,11 but individuals surviving so long may not be representative of the elderly population as a whole.
Recently, we have been using extensively a cell membrane permeabilization method, adapted for flow cytometry, for investigations of perforin expression in various physiologic and pathologic conditions, including pregnancy, allotransplantation, and autoimmunity.18,24,25 Sumner et al,26 whose basic procedure we followed with some modifications, were able to directly correlate flow cytometric fluorescence intensities (MFI) with the absolute numbers of enzyme (beta-glucuronidase) and receptor molecules present in the T lymphocytes, thus establishing unequivocally the quantitative validity of the flow cytometry procedure at the molecular level. Both approaches, the detection of the percentages of perforin positive cells and of the mean perforin content per cell, were used in this investigation. In the latter case, MFI for perforin in different cell populations may not be strictly proportional to the number of perforin molecules per cell, as there are no direct data about the penetration of antiperforin antibody into the core of the cytoplasmic granules of permeabilized cells.
In unstimulated PBL, perforin is constitutively expressed in NK and γ/δ T cells (high level of expression) and in the subpopulation of the CD8+ cells expressing the CD11b antigen (moderate expression). However, perforin can be induced in the CD8+CD11b− subpopulation and in some CD4+ T cells.27 Perforin expressing CTL and NK cells are not homogenous cell populations, as we have suggested previously.28 CTL effectors can belong to class I and class II MHC-restricted subpopulations, ie, CD8+P+and CD4+P+, and NK effectors to CD56+P+ and CD16+P+subpopulations. MHC nonrestricted natural cytotoxic killing is characteristics of lymphokine-activated killer (LAK) T cells, CD3+γδTCR+CD4−CD8−P+cells and a subpopulation of CD3+αβTCR+CD4−CD8−, but P+ cells.29 30
Perforin expression in the CB lymphocytes and adult PB lymphocytes was studied by Berthou et al31 and our data are largely in agreement. Small differences could be a result of differences in the procedures used for simultaneous staining of intracellular and cell surface antigens. We have found that one third of perforin positive CB lymphocytes belong to T lymphocytes (approximately 5% of all cells), which is slightly higher than Berthou et al31 obtained (1%). The values are almost identical when perforin positive CD3 low+ and CD3+ cells from the investigation cited are taken together (≈6%). However, we did not perform triple staining as they did and showed that CD3 low+ cells are a subpopulation of NK cells. CB lymphocytes are endowed with mean levels of perforin per cell, which are close to those seen in adults. Therefore, our results also confirm that at least at the end of the pregnancy, the fetal immune system is endowed with a potent cytolytic armamentarium.
The most interesting result in children was the significantly higher level of perforin positive cells compared with all other groups. This is ascribed primarily to the unexpectedly high levels of CD4+P+ cells (≈10% of total lymphocytes) and additionally to the significantly higher levels of CD16+P+ cells. As mentioned, the CD4+P+ subset represents a subset of CD4+ T lymphocytes with cytotoxic potential and rapid granule-mediated killing of target cells bearing MHC class II molecules and the appropriate antigen. It has been shown that most human CD4+ CTL specific for human immunodeficiency virus (HIV) are perforin positive,32 as well as some of thyroid infiltrating lymphocytes in Hashimoto's thyroiditis.29 Recently, we have found an increase of this subpopulation in the acute phase of immunologically mediated kidney allograft rejection,24 in the active phase of disease in patients with multiple sclerosis,25 and in pathologic pregnancies.33 Very recent results from one of our laboratories34 offer an additional possible explanation of the role this cell subset could play in the downregulation of the afferent arm of the immune response through elimination of antigen-presenting cells. The immune system in early postnatal life is overwhelmed with new pathogens, and CD4+P+cells could be very useful in the downregulation of immune response through elimination of antigen-presenting cells, and in that way preventing immunologically mediated self-destruction. Alternatively, CD4+ cytotoxic T cells may be instrumental in eliminating virus-infected class II MHC expressing target cells, as children are exposed to many novel viruses within the first few years of life.
The CD16 molecule (IgG FcγRIIIA receptor) is a conventional transmembrane protein, with a distinct cytoplasmic domain.35 FcγRIIIA is expressed on NK cells36and links cellular and humoral immunity by serving as a bridge between specific antibody specificity and cytotoxic effector cell function. FcγRIIIA is exclusively responsible for antibody-dependent cell-mediated cytotoxicity (ADCC) and the CD16− NK subset lacks this function.37 ADCC plays a key role in immune defense against infectious diseases and in immune surveillance against malignant growth (for review, see Deo et al38). A role of lymphocyte-mediated ADCC in the children's group is suggested by the highest level of CD16+P+ cells in this age group, emphasizing the underlying importance of the combination of innate and adaptive immunity at the time of definitive maturation of the immune response. Fcγ receptors are of crucial importance in directing the uptake and destruction of viruses, bacteria, and a variety of infections and parasites and are involved in antibody-dependent killing of infected cells expressing viral antigens.38 FcγRIIIA-expressing NK cells isolated from HIV-seropositive individuals have been shown to be coated with anti-HIV antibodies and readily mediate lysis of HIV-infected or gp-120–coated target cells in vitro. Furthermore, this ADCC activity correlates inversely with disease progression.39
There was no difference seen between men and women when total perforin positive cells and double positive T lymphocytes and their subsets were analyzed. However, significant differences appeared in the percentages of double positive NK cells (both CD16+P+ and CD56+P+ cells), which were significantly more abundant in men (Fig 3). Conversely, in women, the levels of perforin expression per cell (MFI) were higher in all subpopulations of double positive T and NK cells, and the difference was significant, except for CD56+P+ cells. Substantial decreases of CD16+P+ cells were seen in PBL in the first trimester of human pregnancy, followed by great increases (to a significantly higher level than in nonpregnant women) at the end of the pregnancy.18,33 Because CD16+P+cells are almost absent from the pregnancy decidua (2% of decidual lymphocytes), despite the fact that 46% of decidual lymphocytes are CD56+P+ cells, it could be concluded that downregulation of perforin expression in the CD16+ cell subset could have a very important physiologic role in early pregnancy, when the fetoplacental allograft is most sensitive to the maternal rejection reaction. We have also found differences in NK subpopulations in multiple sclerosis (MS) patients. In the active phase of MS, a significant increase of CD16+P+ and decrease of CD56+P+ cells was found, which was accompanied by a sevenfold increase of CD16+ cells among P+ cells.25
There are very few data about the perforin expression in lymphocyte subsets in aged humans.40 In experiments in mice, the age-related decline in CTL activity was associated with a decreased proportion of killer cells among the target-binding population.13 The expression of perforin and granzymes genes was decreased in primary allogeneic CTL generated from spleen cells of aged compared with young mice. Furthermore, aging compromises the release of granzymes by CTL in mixed lymphocyte culture.14 15
A crucial point in the investigation of age-related decline of immune reactivity is the question whether aging affects the functional reactivity by decreasing the frequency of precursor cells within a given cell population and/or by decreasing the lytic potential of individual cells from the given cell population.
The senescent decline of CTL activity in mice was investigated by Horvath et al16 by detecting the expression of perforin proteins at the cellular level to determine whether perforin is expressed by fewer cells or whether expression is reduced in all cells, or both. A consistent decrease in the number of perforin positive granules, their size, and intensity of staining was found in aged mice. Furthermore, when stimulated in mixed lymphocyte culture, the cells from young mice showed much higher lytic activity (approximately sevenfold) and total P content (approximately 12-fold), while little difference was observed in the frequency of P+ cells (approximately twofold).16 The results of FACS analyses presented in this report clearly show that both the frequency of perforin positive cells and the content of perforin per cell are significantly lower in both T-cell and NK cell subsets of aged persons, compared with all other age groups. In three independent experiments, we have found in elderly persons significantly lower levels of both the percentage of P+ cells and MFI for P compared with adults.
The substantial reduction in the average P content per cell (judged by MFI) among effector cell populations in elderly persons might be responsible for the deficient lytic potential of these cells in short-term assays by limiting perforin-mediated lysis, which is one of the critical steps in cytolytic action.2 In our opinion, this deficiency in early spontaneous cytotoxicity (short-term assay) is the main difference in the NK cytotoxic potential of PBL from old persons compared with adults. This was unrecognized previously, as most investigations were performed using 18-hour assays,22 and our results clearly demonstrate the difference between 2-hour and 18-hour tests. Short-term contact is quite sufficient for adult PBL to express their full cytotoxic potential. Moreover, our preliminary results showed that in adults even 30 minutes is sufficient for effector cells to exert a cytotoxic effect, although of smaller magnitude (not shown). Prolonged contact (18 hours) of PBL from elderly persons with K562 target cells probably helps to upregulate the cytolytic machinery of effector cells, enabling them to reach the cytotoxic potential of adults.
Perforin and granzyme mRNA expression in human PBL are powerfully induced by IL-2 stimulation.41,42 There are also experimental data showing that P appears to be the limiting factor in NK-mediated cytolysis of NK sensitive targets in mice. Lymphocytes from perforin-deficient mice have no cytotoxic activity against YAC targets, while heterozygous perforin-deficient NK cells have about 50% of the lytic activity of wild-type cells.43 Our results clearly show that PBL from elderly persons have preserved the potential for upregulation of P protein expression in both CTL and NK cells and cytotoxic activity (Figs 5 and 6), after culture in 100 U/mL of IL-2. The effector populations from the same subjects used in the NK cytotoxicity experiments shown in Fig 5 were also used for analyses of perforin content in cell subsets shown in Fig 6. Accordingly, this increase in cytolytic cells and molecules correlates well with the increase in NK cytolytic activity of PBL from elderly persons. In the context of our study, it appears that in elderly people P is limiting possibly due to limiting IL-2.
It has been shown recently by Hamann et al44 that P containing CD8+ cells are effector T cells, while neither naive nor memory cells have significant perforin. They showed that CD8+ T cells with low P content (CD8+CD45RA−RO+) correspond to the memory type population, which does not exert cytolytic activity without prior in vitro stimulation, and this population could be a candidate for the predominant CD8+ population in elderly persons. Significant increases in the number of P+ cells and content of P per cell following 1-day MLR culture indicate that alloantigens are very strong stimulators of P expression in PBL of elderly persons.
It is well known that CMC is an important mechanism of tumor control in vivo, mediated by both CTL and NK cells, and both types of cells predominantly use the perforin-dependent pathway.45 By using perforin-deficient mice and an MHC class I-syngeneic lymphoma, Van den Broek et al46 showed that NK-mediated tumor growth control in vivo is perforin-dependent. Taking into account that perforin is also the major effector pathway used by CTL, it is possible that the high incidence of tumors in old age could partly be a result of a compromised early spontaneous cytotoxicity mediated by perforin. In this context, it would be of great interest to analyze perforin expression in centenarians, as many immunologic parameters in this group of exceptional individuals are much better than in other elderly people.47
ACKNOWLEDGMENT
The authors are grateful to Ivana Godnic for secretarial help and Davorka Percinic, Nadia Peraic, and Ksenija Tulic for technical assistance.
Supported by Grant No. 062001 from the Ministry of Science and Technology of the Republic of Croatia and by Grants No. CA39201-15 and CA57904-06 from the National Institutes of Health, Bethesda, MD.
Address reprint requests to Daniel Rukavina, MD, Department of Physiology and Immunology, Medical Faculty, University of Rijeka, B. Branchetta 20/1, HR-51000 Rijeka, Croatia; e-mail:Daniel.Rukavina@mamed.medri.hr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. The percentage of total perforin positive cells (mean ± standard error [SE]) in CB lymphocytes (A, ▨;) and PBL from children (B, ▤), men (C, □), women (D, ▪), and elderly persons (E, ▥). Levels of significance: (B, C, D) versus (A, E) P < .001; (A) versus (E) P < .05; (B) versus (C, D) P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2410/3/m_blod41904001x.jpeg?Expires=1767727873&Signature=O74Iq3TQdWwff-GuWBIZo7wn~ruMLe69ZfvsD8O96Ff~u196-hHUQdAw5cUsdKDs7RTZNduTye8MkplgD1kB0PDFX8Ketf0l87vgi2WEEBPORKm1Y5LBwLzCbd0BxqUd9T~gbsV642cBcwFjjMkFrPdRLfDe5zBD-wX4VuDsGmfrCnLaoMzYqSKjZeFAecidwk7jd74SC~G-~5zFNGeHzBGQtwaZi6SHJoy~8PQBUBQM2IWKMLAMHCyCP-Eo9SmrpZnufl7h0aQ5yWGkDN2YYF1x1XR1b303Yfms8JoXulfhhfMyETAyj3pRk9~cV2hLKdUyenKd1PYr3bMqrpbpsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. The percentage of total perforin positive cells (mean ± standard error [SE]) in CB lymphocytes (A, ▨;) and PBL from children (B, ▤), men (C, □), women (D, ▪), and elderly persons (E, ▥). Levels of significance: (B, C, D) versus (A, E) P < .001; (A) versus (E) P < .05; (B) versus (C, D) P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2410/3/m_blod41904001x.jpeg?Expires=1768579772&Signature=fI6MwpcAAO2eFiyA3BdHWE2gFKc72rIV~B7jz7Ci76KlnQY5c8lr8l1J5~Y~x0-IxTBq9i4dXP4MJX6cTUUrJhd~xtrEeJ-~lms1Motlm26aZ59yalmt10RcVsgIWp9vzutno~1nX02YmedBSCcnkwmG4ik8PIjqzLlyB-9kBvuwIPSNDHnqkrZSAKcW-44TCGXSnprQFtIVsnfMRaOImbp6ymlr7m1ywisqBJJagXqGC5emGpS9Ihmm-XMSV18UBLXA0Uhw2ZLz7LiI8hWBTk19G~c2BuRcTaAvYxTfzHALfG4L-1haYcdBv7ihSpOkznUqqi9HgeXRc-ZvrzqssA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)