Abstract
The murine Ly6-E gene is transcriptionally induced by interferon-α/β (IFN-α/β) and IFN-γ in a variety of distinct cell types. The mechanism of IFN inducibility in B-cell lines was investigated by deletion analysis of the promoter and by identifying DNA binding proteins in mobility shift assays. A region located in the distal part of the promoter at −2.3 kb contributed to inducibility by both types of IFNs. This region contains a novel element in addition to the previously well-characterized IFN-stimulated response element (ISRE). The probes containing ISRE detected IFN-inducible complexes in mobility shift assays and the signal transducer and activator of transcripition–1 was found to be in these complexes from cells treated with either type of IFN. An additional element present in the proximal part of the promoter at position −109 is also required for IFN-α/β–mediated induction. These data suggested a cooperative interaction between these physically disparate regulatory regions. A crucial role for HMGI(Y) protein in this cooperative multiprotein complex is supported by the evidence that inhibition of HMGI(Y) expression via antisense RNA results in the loss of IFN-α/β–mediated induction of the Ly6-E gene. These results show the complexity involved in achieving cell-type specificity in IFN-mediated gene regulation.
INTERFERONS (IFNs) ARE a heterogenous family of cytokines which regulate a number of cellular functions including antiviral, antiproliferative, and immunoregulatory activities. Most of these activities are accomplished by the products of genes that are transcriptionally activated by IFNs.1 The study of the mechanism of activation of IFN-inducible genes has led to the dramatic discovery of the JAK-STAT pathway involved in signal transduction pathways of numerous cytokines and growth factors. The latent transcription factors termed “signal transducers and activators of transcription” (STATs) preexist in the cytoplasm2 and on stimulation with IFN they are activated by tyrosine phosphorylation catalyzed by Janus kinases (JAK).3 On activation they multimerize and translocate to the nucleus where they bind to the target DNA sequences and enhance the transcription of regulated genes.2
Two of the best characterized regulatory elements are IFN-stimulated response element (ISRE)4 and IFN-γ activation site (GAS).5 IFN-α/β induces the formation of ISGF3 complex which binds ISRE and is composed of three subunits: STAT1, STAT2, and a 48-kD DNA binding protein (ISGF3γ). IFN-γ treatment leads to the formation of γ-activated factor (GAF) that binds to the GAS element and is responsible for the upregulation of the IFN-γ–inducible primary response genes. This response occurs within minutes and without synthesis of intermediate signaling molecules. GAF is formed by the dimerization of STAT1α subunits via SH2 domain-phosphotyrosine interactions.6 ISRE has been shown to be essential for responsiveness to IFN-α/β2 but for certain genes such as major histocompatibility complex (MHC) class I7 and Igκ light chain,8 the response to IFN-γ is also mediated by an ISRE. An IFN-γ–inducible complex containing STAT1 homodimers and p48 (ISGF3γ) has been shown to bind ISRE.9
Immunoregulatory functions of IFNs include induction of various cell surface molecules crucial for cell-to-cell interactions, eg, MHC class I and class II molecules. In addition to the essential interaction between antigen receptors on T lymphocytes and MHC molecules on antigen presenting cells, a number of accessory molecules are also required for T-cell activation. Among these accessory molecules expressed on T cells is the Ly-6A/E antigen, a member of a murine multigene family. The Ly-6–encoded proteins are anchored on the cell surface via a carboxyterminal phosphatidylinositol moiety10 and various members are expressed at crucial times during hematopoiesis and immune responses. The Ly-6A/E is one of the best characterized markers for the hematopoietic pluripotent stem cells in fetal and adult mice.11
Given their suspected important roles in leukocyte development, it was reasonable to expect human homologues of some of these molecules. In fact, recently the E48 gene homologous to mouse ThB antigen12 and the 9804 gene homologous to mouse differentiation antigen TSA-1/Sca-213 were characterized and found to be localized on human chromosome 8, the syntenic locus of mouse chromosome 15, where the murine Ly-6 genes have been mapped.14 Like some of their murine counterparts, the 9804 gene is also responsive to IFNs.13 Interestingly, this gene is also inducible by retinoic acid during differentiation of acute promyelocytic leukemia cells.15
The Ly-6E antigen is found to be associated with tyrosine kinases in T cells16 and reduced expression of Ly-6E via antisense RNA in a T-cell clone causes impairment of functional responses as well as the inhibition of enzymatic activity of fyn tyrosine kinase.17 Interaction of Ly-6E with tyrosine kinases provides an explanation for signal transduction properties of these molecules in lymphocytes. Cross-linking of the Ly-6E molecules on murine B lymphocytes causes a marked increase in the concentration of intracellular free calcium in the absence of phosphatidylinositol turnover. This rise in calcium, in the presence of a costimulator like interleukin-4, IFN-γ,18 or protein kinase C activator phorbol myristate acetate,19 provides a signal for Ly-6E–mediated B-cell proliferation.
Because IFNs are the principal physiological inducers of the Ly-6E gene, we have been studying the underlying molecular mechanism. We previously characterized a GAS element necessary for IFN-α/β– and IFN-γ–mediated induction in fibroblasts.20,21 Almost all of the studies which have characterized the IFN signal transduction pathway have been performed in fibroblast and epithelial cell lines. Although the general biological effects of IFNs are quite similar, several observations suggest some important differences in the mechanism of gene activation by IFNs in different cell types. In some cell lines the IFN-mediated induction of certain genes is a primary response without the need for ongoing protein synthesis,22whereas an intermediate protein(s) needs to be synthesized for the induction of the same genes in other cell types.23Furthermore, the sizes of proteins isolated from myeloid and lymphoid cells that bind to the same ISRE were different.24 Unlike fibroblasts, the analysis of Ly-6E gene expression in the B-cell line A20-2J by Northern blots showed very delayed kinetics of induction by IFNs. Moreover, a genomic construct lacking the GAS site of the Ly-6E promoter was inducible by IFNs in this cell line.25 These results suggested that distinct regulatory elements of the Ly-6E promoter mediate IFN responsiveness in this cell line.
To define these elements we used a combination of genomic deletions and a reporter gene linked to various fragments of the Ly-6E promoter. This analysis has identified a 56-bp regulatory region located at −2.3 kb that contains a functional ISRE and an additional novel element necessary for the IFN-γ responsiveness in A20-2J cells. This region binds IFN-α/β– and IFN-γ–inducible complexes which contain STAT1. However, for IFN-α/β inducibility an additional element located in the proximal part of the promoter at position −109 is also required. One interpretation of these results is that a cooperative interaction between the protein complexes binding to these physically disparate regions is required for IFN-mediated induction of Ly-6E gene in B cells. The supportive evidence for the assembly of such a multiprotein complex promoted by the HMGI(Y) protein is provided by the direct binding of HMGI(Y) to one of the regulatory sequences and by the lack of IFN-α/β inducibility after inhibition of HMGI(Y) expression accomplished by antisense RNA. In summary, distinct regulatory mechanisms exist for the IFN-α/β– and IFN-γ–mediated induction of Ly-6E gene in B cells.
MATERIALS AND METHODS
Cell lines.
A20-2J and BALB/3T3 cells were obtained from the American Type Culture Collection (Rockville, MD). Murine B lymphoma (A20-2J) and fibroblast (BALB3T3) cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum.
Cytokines.
Recombinant murine IFN-γ was purchased from GIBCO-BRL (Gaithersburg, MD). Recombinant murine IFN-α/β was provided by LEE Biomolecular (San Diego, CA).
Stable transfections.
Stable transfection was achieved by cotransfection with 20 μg of the test plasmid and 2 μg of the pSV2neo plasmid. Approximately 2 × 107 cells in phosphate-buffered saline (PBS) containing 2 mmol/L β-mercaptoethanol were electroporated at 320 V and 960 microfarads with a Bio-Rad Gene pulser (Hercules, CA). The cells were then plated in DMEM containing 10% fetal bovine serum. After 36 to 48 hours the cells were collected, counted, and replated in fresh medium containing 3 mg/mL G418 in 96-well culture plates at a density of 104 per well. After 2 weeks individual transfectants could be expanded for analysis.
Transient transfections.
Cells (3 × 106) were obtained and resuspended in 1 mL serum-free DMEM with 10 mmol/L HEPES, pH 7.4. DNA (10 μg), DEAE-Dextran (250 μg/mL), and chloroquine (0.1 mmol/L) were added to the cells. After incubation at 37°C for 30 minutes the cells were washed twice with serum-free DMEM containing HEPES. The cells were resuspended in 10 mL complete DMEM and split into two separate flasks, one for a control and the other for treatment with IFN. Cells treated with IFN-γ received 500 U/mL of IFN 20 hours after the transfection was initiated and assays were performed after 48 hours. Assays for chloramphenicol acetyl transferase (CAT) activity were as previously described.21 For luciferase assays the cells were collected by centrifugation and rinsed twice in PBS. The cell pellets were resuspended in 400 μL of 1× Lysis Reagent (Luciferase Assay System; Promega Corp, Madison, WI) and the suspension was incubated at room temperature for 15 minutes. The lysate was centrifuged for 1 minute in a microfuge to pellet cell debris. After measurement of protein concentration by Bradford assay, appropriate aliquots of the cell lysate were mixed with 100 μL of the luciferase substrate at room temperature and activity measured in a luminometer.
DNA mobility shift assays (DMSA).
DMSA were performed with nuclear extracts prepared as described (Khodadoust and Bothwell, submitted). Probes were prepared using synthetic oligonucleotides or restriction fragments and end labeled with 32P using Klenow DNA polymerase. Desired fragments were gel purified on an 8% nondenaturing polyacrylamide gel and eluted by soaking in Tris EDTA (TE) buffer. Nuclear extract (protein concentration, ∼2 μg/μL) was incubated with the probe in the presence of 1 μg of nonspecific competitor poly (dI.dC):poly(dI.dC) duplex in a 10-μL binding reaction. The binding buffer was 20 mmol/L HEPES (pH 7.9), 4% ficoll, 1 mmol/L MgCl2, 40 mmol/L KCl, 0.1 mmol/L EGTA, and 0.5 mmol/L dithiothreitol (DTT). Bound complexes were separated from free probe by electrophoresis for 2 hours at 180 V on a 4% nondenaturing polyacrylamide gel in 0.25× Tris-borate EDTA (TBE) buffer and identified by autoradiography. The sequences of the duplex oligonucleotides used for competition were as follows (consensus sequences are underlined): for the high-affinity sis-inducible factor binding element (SIE), 5′ GTCGACATTTCCCGTAAATC 3′ 26; for the ISRE element (O15), 5′ GATCCTTCAGTTTCGGTTTCCCT 3′ 27; for the G element, 5′ AAGCTTCTGCTC AGAATTTATGCATATTCCTGTAAGTGAGATCT3′ (Khodadoust and Bothwell, submitted). Bacterially derived rhuHMG-I was a gift from Dr Nancy Ruddle (Yale University). Binding assays involving rhuHMG-I consisted of about 15 ng of purified protein, 0.5 μg of Poly (dG-dC), and 1.25 μg of acetylated bovine serum albumin. In some experiments nuclear extracts were incubated with anti-STAT1 or anti-STAT3 sera for 120 minutes at 4°C before addition to the binding reactions.
Antibodies.
Anti-STAT1 and anti-STAT3 peptide polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Cytofluorometric analysis.
Transfected cells were analyzed for surface expression of Ly-6A by immunofluorescence using a FACSscan (Becton Dickinson, Mountain View, CA). Monoclonal antibody (MoAb) 34-11-3 recognizes Ly-6A antigen and MoAb SK70.94 recognizes Ly-6E antigen. Binding was detected with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse secondary antibody.
Intact gene deletion constructs.
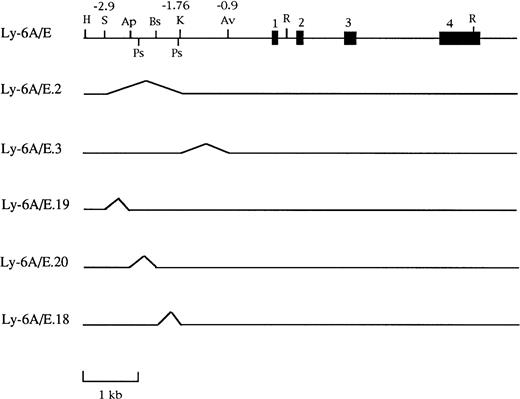
The intact gene described in Khan et al20 was the parent construct for six deletions made in the 5′ flanking region with enzymes that cleaved the construct only once. Thus a double digest followed by religation was used to generate the following constructs diagrammed in Fig 1: Ly-6A/E.3,KpnI + AvaI produces a 850-bp deletion; Ly-6A/E.2, SpeI + KpnI produces a 1,150-bp deletion; Ly-6A/E.1, SpeI + AvaI produces a 2,000-bp deletion; Ly-6A/E.19, SpeI + ApaI produces a 379-bp deletion; Ly-6A/E.18, ApaI + BstEII produces a 387-bp deletion; and Ly-6A/E.20, BstEII + KpnI produces a 383-bp deletion.
Schematic representation of genomic deletion constructs used to define the IFN responsive region in the 3.2-kb 5′ flanking sequence of the Ly-6E gene. The map shows the intact gene coding for the Ly-6A antigen that was present in all these constructs. The region between the two PstI sites is the PstI fragment used for further subcloning. Restriction sites in the map are abbreviated as follows: H, HindIII; S, SpeI; Ap,ApaI; Ps, PstI; Bs, BstEII; K, KpnI; Av,AvaI; R, EcoRI.
Schematic representation of genomic deletion constructs used to define the IFN responsive region in the 3.2-kb 5′ flanking sequence of the Ly-6E gene. The map shows the intact gene coding for the Ly-6A antigen that was present in all these constructs. The region between the two PstI sites is the PstI fragment used for further subcloning. Restriction sites in the map are abbreviated as follows: H, HindIII; S, SpeI; Ap,ApaI; Ps, PstI; Bs, BstEII; K, KpnI; Av,AvaI; R, EcoRI.
Reporter constructs.
Construction of Ly-6E-CAT plasmids, which are derivatives of pUC-CAT and contain deletions of the Ly-6E promoter, was described previously.20 The TKCAT construct was obtained from B.M. Peterlin28 and the pTKLuxbu+ was obtained from J. Hambor (unpublished) and incorporated in the study by Gao and Kavathas.29 Both contain the basal thymidine kinase promoter linked to the reporter gene. Tri-TKCAT was derived by subcloning a DNA fragment containing the trimer sequence20 into the HindIII-XbaI sites of the polylinker of TKCAT upstream of the TK promoter. Five constructs were made by polymerase chain reaction (PCR) using the HaeIII (150) pTKLuxbu+ as template. The PCR products were cloned directly into HindIII-BglII of pTKLuxbu+. All five constructs had the same 5′ primer and differed by the following 3′ primers: 5′ wildtype+Xho CTCAAGCTTAGGTTTCTGTTTCCCCTCGAGAGAACTCT, 3′ + κB TCTAGATCTTAGGGGGATTCCAAGTG, 3′ − κB TCTAGATCTAAGTGTATCAGGTAGTTG, 3′ GMb* TCTAGATCTAGGGGGATTCCAAGTGTATGTCGACGGTTATCAGAC, 3′ BG1 TCTAGATCTGACTGCCAGTCACATGAT, 3′ BG2 TCTAGATCTGGTT ATCAGACTGCCAGTCGACATGATTT.BglII, XhoI, and SalI sites are underlined. BG1 results in a deletion of sequence relative to BG2. BG2 contains a SalI site as a consequence of a single insertion of C residue.
CAT assay.
Cell extracts were made from untreated and IFN-treated cells and assayed for CAT activity as described by Gorman et al30with some modifications.20 Cells were treated with 1,000 U/mL of IFN-α/β or 500 U/mL of IFN-γ. Acetyl coenzyme A was from Sigma Chemical Co (St Louis, MO) and [14C] chloramphenicol (60 mCi/mmol) from Dupont NEN Products (Boston, MA).
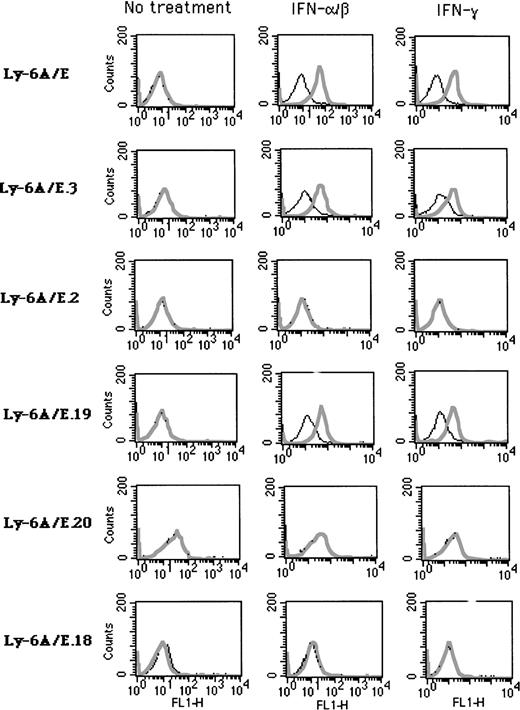
IFN inducibility of transfected chimeric Ly-6A/E deletion constructs (shown in Fig 1). Stable clones derived from A20-2J cells were analyzed by cell surface staining with MoAb 34-11-3 directed specifically against Ly-6A antigen. Cells were mock treated or treated with IFN-α/β (1,000 U/mL for 24 hours) or IFN-γ (500 U/mL for 24 hours). Plain lines indicate cells stained with secondary antibody only as control (FITC-conjugated rabbit anti-mouse Ig); bold lines indicate cells stained with 34-11-3 followed by secondary antibody.
IFN inducibility of transfected chimeric Ly-6A/E deletion constructs (shown in Fig 1). Stable clones derived from A20-2J cells were analyzed by cell surface staining with MoAb 34-11-3 directed specifically against Ly-6A antigen. Cells were mock treated or treated with IFN-α/β (1,000 U/mL for 24 hours) or IFN-γ (500 U/mL for 24 hours). Plain lines indicate cells stained with secondary antibody only as control (FITC-conjugated rabbit anti-mouse Ig); bold lines indicate cells stained with 34-11-3 followed by secondary antibody.
RESULTS
Identification and localization of regulatory elements necessary for the IFN response of the Ly-6E gene in the A20-2J B cells.
The A20-2J B cells express no detectable endogenous Ly6-E antigen on the cell surface in the absence of IFN, but treatment with IFN-α/β or IFN-γ for a period of about 16 hours leads to very high levels of expression as can be shown by staining with a MoAb. The Ly6-A is the allelic counterpart of Ly-6E and differs from it by a single amino acid which can be distinguished by specific MoAbs. To take advantage of this, we constructed chimeric constructs containing various deletions of the Ly-6E promoter linked with exons 2-4 of the Ly-6A genomic clone (Fig 1). This allowed us to distinguish the expression of the transfected Ly-6E/A chimeric gene from the endogenous Ly-6E gene product. Stable A20-2J transfectants of these genomic clones were generated and response to IFN was assayed by cell surface expression of Ly6-A antigen by specific MoAb 34-11-3. Previous analysis of the Ly-6E promoter in fibroblasts had identified the region between −1760 and −900 (KpnI-AvaI) to be required for induction by both types of IFNs.19 20 By contrast, deletion of this region (Ly-6A/E.3 construct) had no adverse effect on the inducibility of the transfected gene by IFN-α/β or IFN-γ in A20-2J cells (Fig 2, Table 1). On the other hand, the Ly-6A/E.2 construct which contains a deletion between −2900 to −1760 (SpeI-KpnI) showed no response to either IFN.
Analysis of Stable A20-2J Transfectants
| Construct . | Neo R . | Basal . | IFN-α/β . | IFN-γ . |
|---|---|---|---|---|
| Ly-6A/E | 8 | 0 | 8 | 8 |
| Ly-6A/E.3 | 5 | 0 | 4 | 4 |
| Ly-6A/E.2 | 11 | 0 | 0 | 0 |
| Ly-6A/E.19 | 13 | 0 | 7 | 7 |
| Ly-6A/E.20 | 11 | 0 | 0 | 0 |
| Ly-6A/E.18 | 8 | 0 | 0 | 0 |
| Construct . | Neo R . | Basal . | IFN-α/β . | IFN-γ . |
|---|---|---|---|---|
| Ly-6A/E | 8 | 0 | 8 | 8 |
| Ly-6A/E.3 | 5 | 0 | 4 | 4 |
| Ly-6A/E.2 | 11 | 0 | 0 | 0 |
| Ly-6A/E.19 | 13 | 0 | 7 | 7 |
| Ly-6A/E.20 | 11 | 0 | 0 | 0 |
| Ly-6A/E.18 | 8 | 0 | 0 | 0 |
Estimation of Ly-6A expression was performed by FACS analysis and those clones that showed at least threefold induction were considered inducible.
Abbreviations: NeoR, total number of clones analyzed; basal, the number of clones that expressed basal levels of Ly-6A antigen; IFN-γ, the number of clones showing IFN-γ–inducible Ly-6A; IFN-α/β, the number of clones showing IFN-α/β–inducible Ly-6A expression.
Because the region between −2900 and −1760 contained the necessary elements for IFN responsiveness in A20-2J cells, it was dissected further by deletion analysis. The deletion construct (Ly-6A/E.19) which lacks the region between −2900 to −2519 (SpeI-ApaI) was IFN inducible, whereas both deletion constructs Ly-6A/E.20 and Ly6A/E.18 were unable to respond to either IFN treatment. These results localized the crucial sequences for IFN-α/β– and IFN-γ–mediated induction between −2519 and −1760. The next question was whether this region alone was sufficient for IFN inducibility. Because most of this region is contained within a PstI fragment (−2400 to −1800), this fragment was subcloned into plasmids TK-CAT and pCAT-5 which contain viral thymidine kinase promoter and the minimal Ly-6E promoter (−900) linked to a CAT reporter gene,20 respectively. This fragment conferred IFN-γ inducibility to the heterologous TK promoter, but no induction was observed with IFN-α/β.25However, when the same PstI fragment was linked to the homologous Ly-6E minimal promoter sequence, a response to IFN-α/β was readily observed.25 These results suggested that all the sequences required for IFN-γ response are localized in thePstI fragment, whereas the additional element(s) required for IFN-α/β response is present in the basal promoter region downstream of −900. These regions were further analyzed separately.
Analysis of the distal regulatory region to define the minimal sequence essential for IFN-γ inducibility.
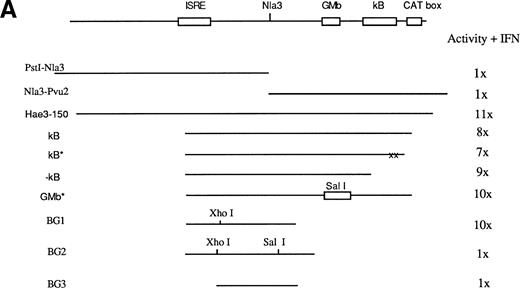
To characterize the sequences for IFN-γ response contained within the PstI fragment, several smaller fragments from this region were subcloned upstream of the TK promoter linked to a luciferase reporter gene (TK-Luxbu+). These constructs were then analyzed in transient transfections in A20-2J cells. Among the various fragments examined, only an HaeIII 150-bp fragment was capable of a strong response to IFN-γ (Fig 3). Computer analysis of this region identified an ISRE, NF-κB, NF-GMb, and CAT box sites (Fig 3). Several constructs were generated to determine functional significance of each of these elements in IFN responsiveness. Initially, we hypothesized the involvement of NF-κB site in combination with ISRE. To test this we used PCR to generate three different NF-κB site constructs: κB contains ISRE and NF-κB site, κB* contains mutated NF-κB site, and -κB construct lacks NF-κB site. As shown in Fig 3, the HaeIII (150 bp) fragment showed 11-fold induction in response to IFN-γ. To our surprise, all three κB constructs showed very similar inducible activities, which suggests the lack of any contribution from NF-κB site to IFN-γ response. Similarly disruption of the NF-GMb site by substitution with a SalI site did not cause any impairment of the IFN response.
Map of the distal regulatory region responsible for IFN inducibility of the Ly-6E promoter in B cells. (A) Various constructs used to characterize this region and their response to IFN-γ assayed by luciferase activity is shown as well as the HaeIII 150-bp fragment showing maximum IFN inducibility. Computer analysis of this sequence identified potential sites for three DNA binding proteins: a CAT box, NF-κB, and a GMb site defined in the promoter of the GM-CSF gene. Constructs κB, κB*, −κB, and GMb* were designed to address their function. Astericks represent mutated sites and −κb construct lacks NF-κB site. BG1 and BG2 sequences were generated by PCR to further define the functional sites. BG2 contains additional 8 bp relative to BG1 and an insertion of a nucleotide (C) 3′ toNlaIII site to generate SalI site. BG1 was fully inducible, whereas BG2 was completely devoid of inducibility. The increase in luciferase activity is shown on the right and is representative of five experiments. (B) At the bottom, the minimal sequence required for IFN-γ inducibility is shown.
Map of the distal regulatory region responsible for IFN inducibility of the Ly-6E promoter in B cells. (A) Various constructs used to characterize this region and their response to IFN-γ assayed by luciferase activity is shown as well as the HaeIII 150-bp fragment showing maximum IFN inducibility. Computer analysis of this sequence identified potential sites for three DNA binding proteins: a CAT box, NF-κB, and a GMb site defined in the promoter of the GM-CSF gene. Constructs κB, κB*, −κB, and GMb* were designed to address their function. Astericks represent mutated sites and −κb construct lacks NF-κB site. BG1 and BG2 sequences were generated by PCR to further define the functional sites. BG2 contains additional 8 bp relative to BG1 and an insertion of a nucleotide (C) 3′ toNlaIII site to generate SalI site. BG1 was fully inducible, whereas BG2 was completely devoid of inducibility. The increase in luciferase activity is shown on the right and is representative of five experiments. (B) At the bottom, the minimal sequence required for IFN-γ inducibility is shown.
Because both the constructs PstI-NlaIII andNlaIII-PvuII (Fig 3) lacked IFN inducibility, the region around NlaIII site seemed to be crucial. The constructs BG1, BG2, and BG3 (Fig 3) were used to further define the necessary element around NlaIII site. The BG1 construct had full IFN-γ inducibility, whereas the BG2 construct was completely devoid of IFN-γ responsiveness. This result is quite significant considering the only difference between the two constructs is the insertion of a single C (underlined below) immediately 3′ to the NlaIII site to create a SalI site. This result confirmed the necessity of this sequence located around the NlaIII site. The BG3 construct was generated via a HindIII-Xho1 deletion of BG1 to eliminate the ISRE sequence. Loss of IFN-γ response confirmed the functional importance of this ISRE. In summary, this extensive deletion analysis identified a minimal 56-bp regulatory region of the Ly-6E promoter for IFN-γ inducibility in A20 cells. The sequence of this region is shown below:
The involvement of the ISRE in IFN-γ responsiveness is not surprising, but the need for an additional novel element in A20-2J B cells is quite interesting. The cell-type specificity of this region for IFN-γ responsiveness was assessed by transient transfections in other cell lines and results are summarized in Table 2. Consistent strong responses were observed in two B-cell lines, A20-2J and M12. Weaker but significant activity was observed in two T-cell lines, BW5147 and EL-4. No inducible activity was observed in J774 macrophage cell line or BALB/3T3 fibroblast cell line.
Cell-Type Specific IFN-γ Responses
| Cells . | Type . | Fold Induction . | Experiments . |
|---|---|---|---|
| A20-2J | B | 5.2-15 | 11 |
| M12 | B | 4-10 | 6 |
| EL4 | T | 1.3, 2.3, 5.8 | 3 |
| BW5147 | T | 1.8, 4.6, 6.8 | 3 |
| J774 | MΦ | 1.0, 1.2, 2.0 | 3 |
| BALB3T3 | Fibroblast | 1.2, 1.4, 0.8 | 3 |
| Cells . | Type . | Fold Induction . | Experiments . |
|---|---|---|---|
| A20-2J | B | 5.2-15 | 11 |
| M12 | B | 4-10 | 6 |
| EL4 | T | 1.3, 2.3, 5.8 | 3 |
| BW5147 | T | 1.8, 4.6, 6.8 | 3 |
| J774 | MΦ | 1.0, 1.2, 2.0 | 3 |
| BALB3T3 | Fibroblast | 1.2, 1.4, 0.8 | 3 |
Transient transfections were performed with either theHaeIII (150) or BG1 luciferase reporter construct. No significant differences were noted when these constructs were used.
Detection of IFN-α/β– and IFN-γ–inducible nuclear factors with probes derived from the distal regulatory region.
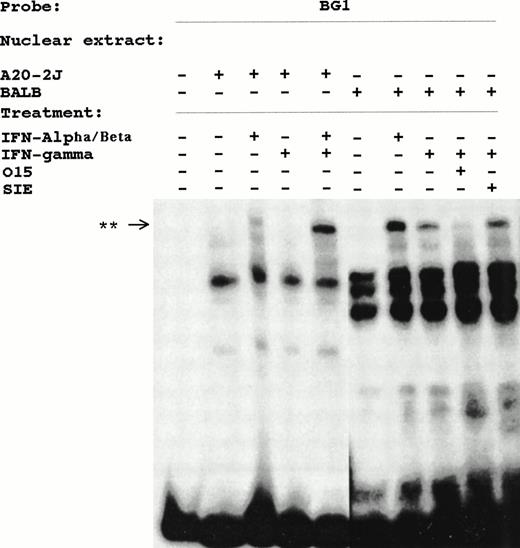
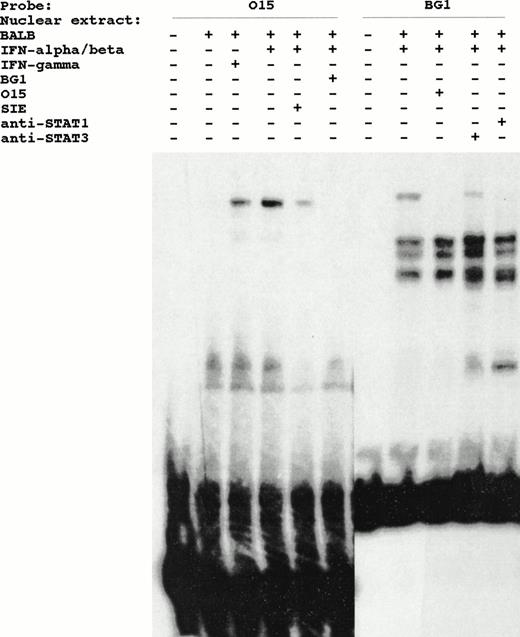
DNA mobility shift analysis was performed using the BG1 probe and nuclear extracts from untreated, IFN-α/β–treated and IFN-γ–treated BALB/3T3 cells. This analysis identified induced complexes from both IFN-α/β– and IFN-γ–treated cells (Fig 4). A similar complex was also observed in IFN-α/β–treated A20-2J nuclear extracts. Pretreatment of A20-2J cell with IFN-γ (24 hours) before stimulating with IFN-α/β for 15 minutes resulted in tremendous enhancement of this inducible complex. The binding specificity of this complex was shown by the complete inhibition of this complex in the presence of a competitor sequence derived from the ISRE of the ISG-15 gene designated O15 in Fig4. ISG-15 ISRE is the highest affinity site known for ISGF3γ (p48). A sequence corresponding to the c-fos SIE did not inhibit this complex. The electrophoretic mobility of the induced complex detected by BG1 probe is identical to the ISGF-3 complex that binds to the O15 probe (Fig 5). Cross-competition with BG1 sequence inhibited the formation of ISGF-3 complex by O15 probe. Furthermore, formation of the complex by Ly-6E BG1 probe was specifically inhibited by anti-STAT1 antibody (Fig 5). No inhibition was observed with anti-STAT3 antibody (Fig 5) or anti-STAT2 antibody (data not shown). No complexes having the expected mobility of IRF-1 or IRF-2 were observed and no supershifts were observed with anti–IRF-1 or anti–IRF-2 antibodies (data not shown).
INF-α/β– and INF-γ–inducible complexes detected in DMSA using BG1 probe and nuclear extracts from BALB/3T3 or A20-2J cells. Cells were treated with IFN-α/β or IFN-γ for 15 minutes where indicated. The specificity of these complexes is shown by competition with O15 or SIE oligonucleotides. **, Inducible complex.
INF-α/β– and INF-γ–inducible complexes detected in DMSA using BG1 probe and nuclear extracts from BALB/3T3 or A20-2J cells. Cells were treated with IFN-α/β or IFN-γ for 15 minutes where indicated. The specificity of these complexes is shown by competition with O15 or SIE oligonucleotides. **, Inducible complex.
IFN-inducible DNA binding complex detected by BG1 probe of Ly-6E is identical to the inducible complex detected by O15 probe derived from ISG-15 gene. Identity and specificity of the binding complex is shown by the ability of these sequences to cross-compete with each other when present in a 100-fold molar excess (lanes 6 and 9). Effect of anti-STAT1 and anti-STAT3 antibodies on the formation of these complexes by BG1 probe is also shown (lanes 10 and 11).
IFN-inducible DNA binding complex detected by BG1 probe of Ly-6E is identical to the inducible complex detected by O15 probe derived from ISG-15 gene. Identity and specificity of the binding complex is shown by the ability of these sequences to cross-compete with each other when present in a 100-fold molar excess (lanes 6 and 9). Effect of anti-STAT1 and anti-STAT3 antibodies on the formation of these complexes by BG1 probe is also shown (lanes 10 and 11).
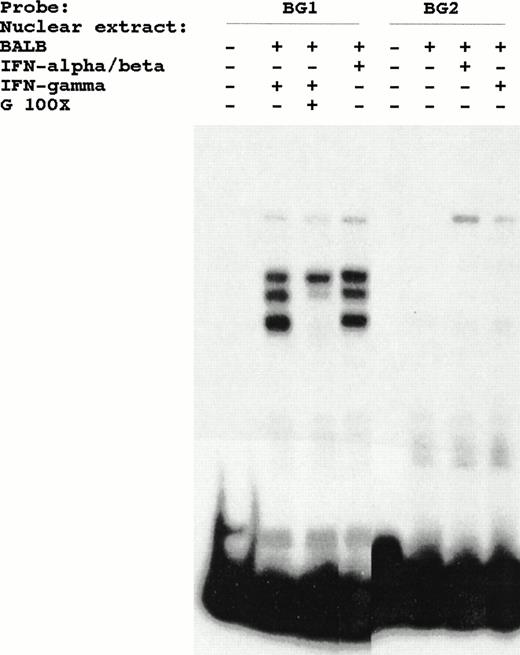
The constitutive DNA protein interactions observed with BG1 probe are lost as a consequence of the BG2 mutation, but inducible ISRE binding proteins are still detected with BG2 probe (Fig 6). Because the BG2 mutation lacks functional responsiveness, the constitutive proteins that bind to this region might play a role in IFN inducibility.
Lack of effect of BG2 mutation on the binding of IFN-inducible complex in DMSA. The constitutive proteins, detected by BG1 probe, do not bind the mutated BG2 probe.
Lack of effect of BG2 mutation on the binding of IFN-inducible complex in DMSA. The constitutive proteins, detected by BG1 probe, do not bind the mutated BG2 probe.
Binding of HMGI(Y) protein to the distal regulatory region.
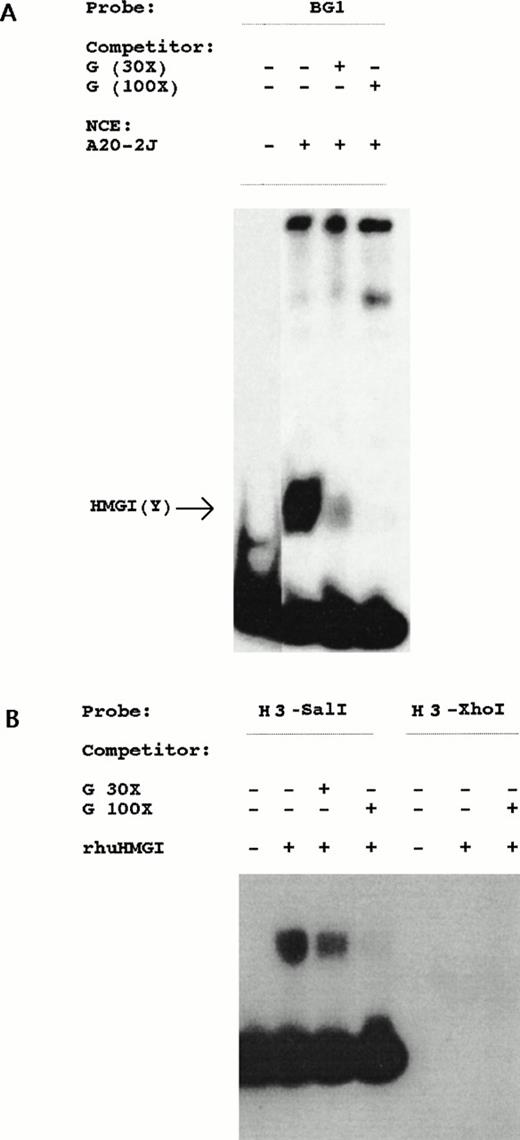
Our analysis of the Ly-6E gene in T cells led to the identification of a distinct regulatory region (designated G region) which contains a binding site for high-mobility group protein HMGI(Y) as well as a GAS and octamer sites (Khodadoust and Bothwell, submitted). To investigate its role in B cells, conditions favorable for detection of HMGI(Y)-like protein binding were used in DNA mobility shift assays. A fast migrating complex charasteristic of HMGI(Y) was detected with BG1 probe from nuclear extracts of A20-2J cells (Fig 7). This complex can be specifically competed by oligonucleotides corresponding to the HMGI(Y) binding site from the G region active in T cells. Presence of HMGI(Y) binding site in the BG1 probe was further confirmed by the specific binding of a recombinant human HMGI(Y) protein in DMSA. Recombinant protein was used to further localize the binding site by two different probes derived from this region. HindIII-SalI probe was bound whereas HindIII-XhoI probe which contains ISRE failed to bind rhuHMGI(Y) protein (Fig 7). Hence, HMGI(Y) binding sequence exists in a 23-bp fragment immediately 3′ to the ISRE betweenXhoI and SalI sites. The above-mentioned G region DNA sequence competed with this interaction as well in a dose-dependent manner.
Localization of HMG-I binding region. (A) Detection of an HMGI(Y)-like protein by BG1 probe from nuclear extracts of A20-2J cells. Competition with increasing amounts (30- and 100-fold molar excess) of unlabeled G region oligonucleotide shows specificity of the retarded band. (B) Recombinant human HMGI(Y) protein binds only to theHindIII-SalI probe (sequence shown in Fig 3) derived from the regulatory region. HindIII-XhoI probe containing ISRE was unable to bind rhHMGI(Y) protein. Competition with unlabeled G region oligonucleotide inhibited the complex in a dose-dependent fashion.
Localization of HMG-I binding region. (A) Detection of an HMGI(Y)-like protein by BG1 probe from nuclear extracts of A20-2J cells. Competition with increasing amounts (30- and 100-fold molar excess) of unlabeled G region oligonucleotide shows specificity of the retarded band. (B) Recombinant human HMGI(Y) protein binds only to theHindIII-SalI probe (sequence shown in Fig 3) derived from the regulatory region. HindIII-XhoI probe containing ISRE was unable to bind rhHMGI(Y) protein. Competition with unlabeled G region oligonucleotide inhibited the complex in a dose-dependent fashion.
Identification of an additional IFN-α/β–responsive element within the Ly-6E minimal promoter.
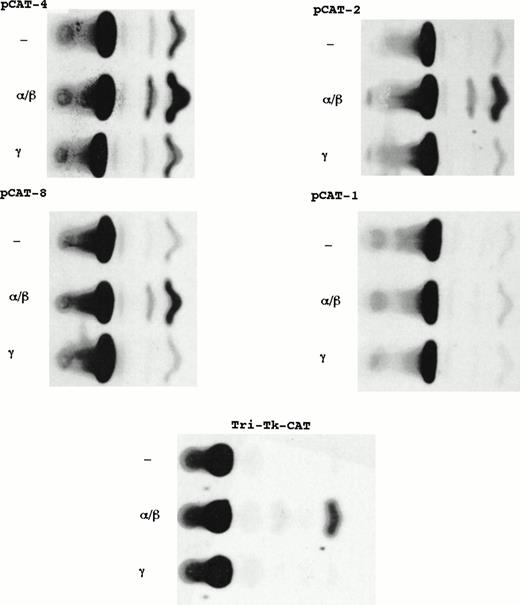
Comparison of the results of constructs containing distal regulatory region (PstI fragment) linked to homologous minimal Ly-6E promoter with that of heterologous TK promoter suggested an additional element for IFN-α/β responsiveness in the minimal promoter downstream of −900. Chimeric constructs containing successive deletions of the Ly-6E promoter linked to CAT gene were used to derive stable transfectants of A20-2J cells. Individual clones were used for basal and IFN-inducible expression. Figure8 and Table 3 show comparison of CAT activities from extracts of untreated, IFN-α/β– and IFN-γ– treated cells. All the chimeric constructs with deletions from −900 to −113 were inducible by IFN-α/β but not by IFN-γ.
Localization of an IFN-α/β–responsive element within the Ly-6E minimal promoter. Comparison of CAT activities from representative stable clones derived from transfection of A20-2J cells with constructs containing fragments of Ly-6E promoter linked to CAT reporter gene. Plasmids pCAT-4 and pCAT-2 contain sequences up to −420 and −167, respectively. Plasmids pCAT-8 and pCAT-1 contain sequences up to −113 and −81, respectively. Tri-TK-CAT has a triple repeat of the sequence between −113 and −88.
Localization of an IFN-α/β–responsive element within the Ly-6E minimal promoter. Comparison of CAT activities from representative stable clones derived from transfection of A20-2J cells with constructs containing fragments of Ly-6E promoter linked to CAT reporter gene. Plasmids pCAT-4 and pCAT-2 contain sequences up to −420 and −167, respectively. Plasmids pCAT-8 and pCAT-1 contain sequences up to −113 and −81, respectively. Tri-TK-CAT has a triple repeat of the sequence between −113 and −88.
Analysis of Stable A20-2J Transfectants
| Construct . | Neo R . | CAT Expression . | ||
|---|---|---|---|---|
| Basal . | IFN-α/β . | IFN-γ . | ||
| pCAT-5 (−900) | 11 | 9 | 9 | 0 |
| pCAT-4 (−420) | 5 | 4 | 4 | 0 |
| pCAT-2 (−167) | 4 | 4 | 4 | 0 |
| pCAT-3 (−138) | 4 | 4 | 4 | 0 |
| pCAT-8 (−113) | 5 | 4 | 4 | 0 |
| pCAT-1 (−81) | 3 | 3 | 0 | 0 |
| TK-CAT | 9 | 7 | 0 | 0 |
| Tri-TK-CAT | 6 | 6 | 6 | 0 |
| Ly-6E expression | ||||
| HMGI-C antisense | 11 | 0 | 0 | 5 |
| Construct . | Neo R . | CAT Expression . | ||
|---|---|---|---|---|
| Basal . | IFN-α/β . | IFN-γ . | ||
| pCAT-5 (−900) | 11 | 9 | 9 | 0 |
| pCAT-4 (−420) | 5 | 4 | 4 | 0 |
| pCAT-2 (−167) | 4 | 4 | 4 | 0 |
| pCAT-3 (−138) | 4 | 4 | 4 | 0 |
| pCAT-8 (−113) | 5 | 4 | 4 | 0 |
| pCAT-1 (−81) | 3 | 3 | 0 | 0 |
| TK-CAT | 9 | 7 | 0 | 0 |
| Tri-TK-CAT | 6 | 6 | 6 | 0 |
| Ly-6E expression | ||||
| HMGI-C antisense | 11 | 0 | 0 | 5 |
Quantitative stimulation of CAT activity was performed by densitometric scanning of autoradiographs and only those clones which showed at least threefold induction were considered inducible. Estimation of Ly-6E expression was performed by FACS analysis.
Abbreviations: NeoR, total number of clones analyzed; basal, the number of clones that expressed basal CAT activity; IFN-γ, the number of clones showing IFN-γ–inducible CAT activity; IFN-α/β, the number of clones showing IFN-α/β–inducible CAT activity.
There is an element (TGGAAAAGGTTAAG) with homology to the ISRE located between −109 and −95 of the Ly-6E promoter. To determine whether this element was responsible for the observed IFN-α/β–mediated induction, a recombinant construct containing three tandem repeats of the sequence between −113 and −88 fused to the same TK promoter (TK-CAT) was generated. Plasmids TK-CAT and Tri-TK-CAT were used in stable transfections. Although there was some clonal variation in the transfectants derived from TK-CAT, none of the clones showed any significant induction by either IFN-α/β or IFN-γ treatment. All the clones transfected with Tri-TK-CAT showed marked inducibility after treatment with IFN-α/β only; none of them responded to IFN-γ (Fig 9 and Table 3). In summary, this analysis identified a functional element for IFN-α/β response in A20-2J cells located between −113 and −88 of the Ly-6E promoter. Similar results were obtained in M12 B-cell line as well (data not shown). However, none of the deletion constructs containing sequences from −900 to −113 of the Ly-6E promoter or the Tri-TK-CAT showed any responsiveness to IFN-α/β in BALB/3T3 fibroblast or BW5147 T-cell lines in transient or stable transfections (data not shown).
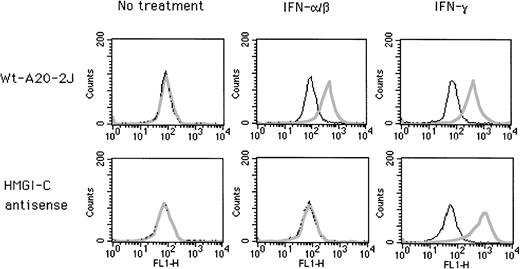
Effect of antisense HMGI-C on IFN inducibility of endogenous Ly-6E gene in A20-2J cells. Wild-type cells and clones stably transfected with antisense plasmid DNA were analyzed by cell surface staining with Sca-1 antibody. Plain lines indicate control cells stained with secondary antibody (FITC-conjugated rabbit anti-rat Ig) only; bold lines indicate cells stained with Sca-1 before secondary antibody.
Effect of antisense HMGI-C on IFN inducibility of endogenous Ly-6E gene in A20-2J cells. Wild-type cells and clones stably transfected with antisense plasmid DNA were analyzed by cell surface staining with Sca-1 antibody. Plain lines indicate control cells stained with secondary antibody (FITC-conjugated rabbit anti-rat Ig) only; bold lines indicate cells stained with Sca-1 before secondary antibody.
Transfection of A20-2J cells with antisense HMGI-C construct prevents IFN-α/β inducibility of the endogenous Ly-6E gene.
HMGI(Y) protein is essential for the transcriptional activity and IFN inducibility of the Ly-6E gene in T cells (Khodadoust and Bothwell, submitted). We suspected a functional role for the HMGI(Y) protein in B cells as well because of its binding site in the distal regulatory region necessary for IFN inducibility. The synthesis of HMGI(Y) proteins can be successfully blocked by using an antisense cDNA construct.31 This construct was used to derive stable transfectants of A20-2J B cells and cell surface expression of endogenous Ly-6E antigen was analyzed by fluorescence-activated cell sorting (FACS) (Fig 9). In 5 out of 11 clones IFN-γ inducibility of the Ly-6E gene was normal but IFN-α/β inducibility was completely abolished (Table 3).
Because IFN-γ was able to induce endogenous Ly-6E genes in five clones, it is very unlikely that these results are due to some nonspecific effects of antisense RNA on some other aspect of Ly-6 gene expression. The specific loss of IFN-α/β inducibility is quite interesting because only this response requires two distinct elements located more than 2 kb apart in the Ly-6E promoter. Assembly of a multiprotein complex between proteins binding to these elements mediated by the binding of HMGI(Y) protein is suggested.
DISCUSSION
Ly-6 genes are regulated in a complex fashion, as shown by the diversity of both the tissues and the various developmental stages in which these antigens are expressed. This complexity is further highlighted by the recent discovery of human homologues of some of these antigens. The human E48 antigen homologue of mouse ThB antigen is involved in keratinocyte cell-cell adhesion and is expressed at high levels in squamous cell carcinomas.12 The other homologous gene 9804 is not only inducible by IFNs, but interestingly, is expressed in acute promyelocytic leukemia cells after they are induced to differentiate by retinoic acid.15 These characteristics of the Ly-6 gene expression make them an interesting model for studying gene regulation.
We previously characterized a GAS-containing region of the Ly-6E promoter located at −1.2 kb responsible for induction by both IFN-α/β and IFN-γ in murine fibroblasts.20,21 To our surprise, this region of the Ly-6E promoter was dispensable for IFN-mediated induction in A20-2J B lymphocytes as shown by genomic deletions.25 In this study we have identified two novel overlapping regulatory mechanisms in the A20-2J B cells used by the Ly-6E gene for IFN-α/β and IFN-γ inducibility. A distal regulatory region located at −2.3 kb is important for response to both types of IFNs and contains two functional elements: an ISRE that plays a role in inducibility by either IFN, and a novel element that is necessary only for IFN-γ–mediated induction. Further analysis of the promoter identified a third element with homology to ISRE in the proximal minimal promoter for IFN-α/β responsiveness in B cells. An interaction between the proteins binding to these physically disparate elements was suggested by the presence of a binding site for high-mobility group HMGI(Y) protein in the distal regulatory region. The functional significance of this protein for the IFN-α/β inducibility of Ly-6E gene in A20-2J cells was investigated by antisense RNA–mediated inhibition of HMGI(Y) expression. Interestingly, the induction of endogenous of Ly-6E gene by IFN-α/β was completely abrogated, whereas the response to IFN-γ was preserved as analyzed by FACS staining of surface expression of the protein on stable transfectants.
IFN-γ inducibility.
Genomic deletion analysis in the A20-2J cell line has shown that a region spanning from −2519 to −1760 of the Ly-6E promoter is necessary for both IFN-α/β and IFN-γ inducibility. By reporter gene analysis a minimal region of 56 bp essential for IFN-γ inducibility was localized at position −2300. This region contains two functional elements, an ISRE at its 5′ end and a novel element at its 3′ end. The sequence of this ISRE GGTTTCTGTTTTC is very similar to the consensus sequence YAGTTTCNNTTTYY.32,33 This region detected IFN-α/β– and IFN-γ–inducible complexes from nuclear extracts. These complexes can be competed by a typical ISRE site derived from ISG-15 gene. The presence of STAT-1 in this complex is shown by the ability of anti-STAT1 antibody to disrupt this complex. However, the sequence derived from a conventional STAT1 binding site was not able to compete, illustrating the lack of direct DNA binding of STAT1 in this region. Presence of STAT1 has been reported previously in a number of complexes (eg, GAF, IRBFα, IRBFγ, and FCRFγ) involved in IFN-γ responsiveness.34
As expected, deletion of the ISRE resulted in the loss of IFN-γ inducibility of the reporter gene and abolished the binding of IFN-γ–inducible complex as well. Our functional data is consistent with previous reports showing that IFN-γ inducibility of MHC class I, 9-27, and guanylate-binding protein (GBP) genes require an ISRE element. However, for these genes IFN-γ inducibility was attributed to the binding of IRF-1 and no IFN-γ–inducible complexes have been reported for these genes.7,35-37 The consensus core recognition sequence, considered necessary for the binding of IRF-1 and IRF-2, is not present in the Ly-6E ISRE element. In addition, no complexes having the expected mobilities of IRF-1 or IRF-2 were detected with probes from this region in DMSA and antibodies against IRF-1 and IRF-2 failed to cause any supershifts (data not shown). The most likely explanation for these data is that the Ly-6E ISRE is detecting an IFN-γ–inducible complex consisting of STAT1 homodimers and DNA-binding subunit p48 as described.9 This conclusion is supported by the ability of Ly-6 BG1 oligonucleotide to inhibit the binding of ISGF-3 complex to the ISG-15 ISRE–containing probe.
The role of an additional novel element present at the 3′ end of this distal regulatory region for IFN-γ response in B cells remains unclear, but its functional importance cannot be ignored because insertion of a single nucleotide in this element completely abolished IFN-γ inducibility. This element failed to detect any IFN-inducible complex, but it is the site for the binding of specific constitutive proteins in DMSA. Further characterization of this element might provide insights in cell-type specificity observed in the IFN-γ response of Ly-6E gene.
IFN-α/β inducibility.
The above-mentioned ISRE located in the distal regulatory region of the Ly-6E promoter was found to be required for IFN-α/β inducibility as well. An IFN-α/β–induced protein complex bound this region and could be competed with a sequence derived from the ISRE of ISG-15 gene. Furthermore, anti-STAT1 antibody was able to disrupt this complex. Quite unexpectedly, this distal region was unable to confer IFN-α/β inducibility to a heterologous TK promoter. However, linking this fragment with minimal proximal Ly-6E promoter restored responsiveness to IFN-α/β. This result suggested a necessary cooperative interaction between the distal ISRE and an additional element within the minimal promoter of the Ly-6E gene. This second element was identified by stable transfections of A20-2J cells with recombinant plasmids containing progressive 5′ deletions of the Ly-6E promoter linked to a reporter CAT gene.
All the constructs with sequences from −900 to −113 bp were inducible with IFN-α/β. Sequence comparison showed a stretch of homology to the ISRE at −109 to −95 bp. To address the functional significance of this element, a triple repeat of the sequence between −113 and −88 was linked to the same TK promoter. This trimerized element conferred strong IFN-α/β inducibility to TK promoter in B cells only. The deletion constructs containing the sequences downstream of −900, which include this ISRE-like region or the Tri-TK-CAT plasmid, were not inducible by IFN-α/β in the two fibroblast cell lines BALB/3T3 and Ltk−20 and the BW5147 T-cell line.
This extensive analysis of the Ly-6E promoter identified two elements, one distal and one proximal, essential for IFN-α/β inducibility in B cells. The distal element is present in the context of a broad regulatory region. This regulatory region is comprised of at least three distinct functional elements: an ISRE at its 5′ end required for response to both types of IFNs, a novel element at its 3′ end necessary only for IFN-γ inducibility, and a site for HMGI(Y) binding in the middle. Because the two elements required for IFN-α/β inducibility are physically located more than 2 kb from each other in the Ly-6E promoter, a cooperative interaction between the proteins binding to these elements might be mediated by protein-protein interactions. The presence of an HMGI(Y) binding site in the distal regulatory region was curious because it had been shown to mediate such interactions by bending DNA to bring physically disparate regions in close proximity.38 Moreover, our analysis of the Ly-6E gene in T cells also defined the need for the binding of HMGI(Y) protein for the assembly of an enhanceosome-like complex required for IFN-inducible expression (Khodadoust and Bothwell, submitted). To test its function in B cells, inhibition of expression of HMGI(Y) protein was achieved by antisense HMGI-C RNA in A20-2J cells as described.31 Surprisingly, this led to the specific loss of IFN-α/β–mediated induction of the endogenous Ly-6E gene while leaving IFN-γ responsiveness intact. The preservation of IFN-γ responsiveness in the same stable clones argues strongly against any nonspecific effects of antisense RNA on some other aspect of Ly-6 gene expression. This result provides support for the hypothesis that assembly of a multiprotein complex mediated by HMGI(Y)-like protein is required for IFN-α/β inducibility of Ly-6E gene in B lymphocytes. These data highlight the complex fashion in which multiple constitutive and inducible proteins binding to different regulatory elements interact with each other to achieve cell-type specific IFN-regulated transcription of a single gene.
ACKNOWLEDGMENT
We thank Ray Reeves for the recombinant HMGI protein and Alfredo Fusco for the antisense HMGI-C plasmid.
Supported by Public Health Service Grant No. GM40924 from the National Institutes of Health.
Address reprint requests to Alfred L.M. Bothwell, PhD, Section of Immunobiology, Yale University School of Medicine, 310 Cedar St, New Haven, CT 06520-8011; e-mail: alfred.bothwell@yale.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal