Abstract
The present study was undertaken to assess the feasibility of using ferritin and transferrin receptor measurements on dried capillary blood spots to identify iron deficiency (ID) in public health surveys. Measurements on serum and blood spots prepared from venous blood were performed in 71 healthy subjects, 41 of whom were iron-replete and 30 who had ID, either without (n = 20) or with (n = 10) anemia. Parallel measurements were performed on hemolyzed whole blood and washed hemolyzed red blood cells to assess the erythrocyte contribution of ferritin and transferrin receptor to dried blood samples. The concentration of ferritin in dried blood samples was threefold higher than serum assays due to the release of ferritin from hemolyzed erythrocytes, which diminished the usefulness of ferritin measurements for detecting ID. On the other hand, there was negligible erythrocyte contribution to the measurement of transferrin receptor in dried blood spots. The most sensitive parameter in dried blood spots was the ratio of receptor/ferritin, which was suitable for identifying iron-deficiency anemia (IDA), but less reliable than serum assays for detecting milder ID without anemia. We conclude that tandem measurements of serum ferritin and transferrin receptor in dried blood spots can be used to facilitate the identification of IDA in epidemiologic studies.
© 1998 by The American Society of Hematology.
BASED ON A REVIEW of more than 500 studies published between 1960 and 1983, the global prevalence of anemia was reported to be approximately 30%, with a range from 8% to 36% depending on the economic status of the region.1Although the cause of the anemia was not determined in the majority of studies, the investigators estimated that iron deficiency (ID) was responsible in approximately 50% of the anemic individuals based on differences in anemia prevalence in various age and sex categories. In countries where the prevalence of anemia is highest, there are many causes of anemia other than ID, such as chronic bacterial infection, malaria, acquired immunodeficiency syndrome, malnutrition, folate or vitamin B12 deficiency, and inherited defects in hemoglobin synthesis, such as sickle cell anemia and thalassemia. The most widely used intervention strategy to reduce the high prevalence of anemia is to increase the intake of oral iron, which will be ineffective and possibly harmful if the anemia is not due to iron deficiency. Consequently, it is important to incorporate specific measures of iron status in population studies designed to assess or reduce the high prevalence of anemia.
There is a large array of laboratory measurements to identify and assess the severity of ID. Most prior surveys of iron status have required venous sampling to obtain sufficient blood for these determinations. In recent years, highly sensitive and specific immunoassays of iron status, such as the serum ferritin and serum transferrin receptor, have become available that require only a few microliters of serum and are therefore suitable for measurements on blood obtained by capillary sampling. Despite the advantage of fingerstick sampling, there is still a logistic problem, especially in rural areas, of processing samples in the field to obtain sera and transporting the latter to a central laboratory facility under controlled temperature.
The present study was undertaken to examine the feasibility of using small samples spotted onto filter paper for measurements of ferritin and transferrin receptor. This technology has been used extensively to detect inborn errors of metabolism in newborns and more recently in field studies to identify iodine deficiency.2 3 In the present study, venous blood from 71 subjects representing a wide spectrum of iron status was obtained to compare assay results on dried blood spots eluted from filter paper with measurements on serum. Additional assays were performed on whole blood (WB) and hemolyzed red blood cells (RBCs) to assess the erythrocyte contribution of ferritin and transferrin receptor to blood-spot measurements.
MATERIALS AND METHODS
Clinical samples.
Blood samples were obtained from 25 men and 46 women ranging in age from 19 to 71 years (mean, 27.9). All subjects without anemia were considered to be in good health. ID was defined as a serum ferritin concentration less than 12 μg/L and anemia as a hematocrit less than 37% in women and 40% in men.4 Based on these criteria, the participants were further divided for certain statistical analyses into 41 normal subjects, 20 subjects with ID defined as a low serum ferritin and normal hematocrit, and 10 subjects with iron-deficiency anemia (IDA) defined as a low serum ferritin and low hematocrit. All specimens were obtained according to procedures approved by the Human Subjects Committee of the Kansas University Medical Center.
Processing of blood specimens.
Eight-milliliter venous blood samples were collected into evacuated tubes containing potassium EDTA as the anticoagulant. After measurement of the microhemocrit, duplicate 25-μL samples of WB were spotted onto filter paper (Schleicher & Schuell #903, Keene, NH) and allowed to dry at room temperature for a minimum of 3 hours. The paper samples were stored for periods ranging up to 1 year in sealed plastic bags with 10 g of Drierite (calcium sulfate) (W.A. Hammond Drierite Co, Xenia, OH) desiccant at 4°C until the day of assay. The Drierite was contained in a separate porous package.
The remainder of the blood sample was fractionated for ferritin and transferrin receptor measurements on plasma, WB, and saline-washed RBCs. The latter two fractions were hemolyzed to assess the erythrocyte contribution of ferritin and transferrin receptor in dried blood spots that are fully hemolyzed when the sample dries. The WB samples were hemolyzed by adding an equal volume of distilled water and freezing at −30°C. To obtain the RBC samples, WB was centrifuged at 1,500g for 20 minutes, and after removing the plasma and buffy coat, the RBCs were washed twice with sterile normal saline. The cells were then lysed by adding 2 vol of distilled water and centrifuging at 10,000g for 30 minutes to remove the RBC membranes from the supernatant. The plasma, WB, and RBC samples were frozen and stored at −30°C until the day of assay.
Elution of blood spot.
Preliminary studies were performed to determine the optimal procedure for eluting the dried blood spot. Two drops of WB from two normal volunteers were spotted onto the filter paper in 16 replicate samples and allowed to dry overnight at room temperature. The blood spots were cut from the paper, placed in 1.5-mL microfuge tubes containing 1 mL of the following buffers, and mixed on an end-over-end hematology rotator for 2 hours at 4°C. Measurements of L-ferritin and transferrin receptor were used to compare the elution efficiency of four Tris buffers (Tris pH 8.0 plus 10 mmol/L Chaps, Tris plus 2% sodium dodecyl sulfate [SDS], Tris plus 1% NP40, and Tris plus 1% Brij 35) and four phosphate-buffered saline (PBS) buffers (PBS pH 7.2 plus 0.05% tween-20, PBS plus 10 mmol/L (3-[(3-Cholamidopropyl)-dimethylammonio]-1-propane sulfonate) (CHAPS), PBS plus 2% SDS, and PBS plus 1% NP40). Recovery was markedly reduced with buffers containing SDS, presumably due to protein denaturation. The Tris buffers also produced lower recoveries. Maximal recovery was achieved with the PBS-tween buffer, which was then used in all subsequent studies. Extending the elution time from 2 to 12 hours or increasing the elution temperature from 4°C to 25°C did not improve recovery significantly. For the selected elution protocol of 2 hours at 4°C in PBS-tween with end-over-end mixing, the elution efficiency, defined as the ratio of transferrin receptor or ferritin in dried blood spots to hemolyzed WB, ranged for 10 samples from 0.93 to 0.97, with a mean of 0.95 ± 0.01.
Effect of storage.
To assess the effect of storage under different conditions, venous blood from nine different volunteer subjects was collected in vacuum container tubes containing potassium EDTA. Twenty-five microliters of WB was used to prepare multiple dried spots from each sample. Six sets of dried blood spots were placed in each of six separate sealed plastic storage bags. Two of these bags were stored at 4°C, two at room temperature, and two at 37°C. One of the bags at each of these temperatures was stored with desiccant (calcium sulfate), the other without. At 1, 3, 7, 14, 21, and 28 days of storage, one blood spot was removed from each bag and assayed for L-ferritin and transferrin receptor. In a separate study, samples were stored at 4°C with desiccant for 1 year.
Variability studies.
To determine the intraassay variability of dried blood-spot measurements, 10 replicate samples of 25 μL from five subjects were spotted onto filter paper and allowed to dry overnight. The spots were eluted from the paper and the eluants were assayed in the same microtiter plate, each for ferritin and transferrin receptor concentrations. To determine interassay variability, dried blood-spot samples from seven subjects were measured in duplicate in six separate assays over a 2-week period.
Immunoassays.
Ferritin is composed of 24 subunits of two main types designated H and L, which are encoded by genes on separate chromosomes.5-7The L subunit predominates in liver and spleen ferritin and produces a basic isoferritin profile, whereas the ferritin in heart tissue and tumors contains predominantly H subunits and results in a more acidic isoferritin profile. Serum ferritin is rich in L subunits and is consequently measured by clinical assays developed against purified liver or spleen ferritin. In the present report, ferritin measured by such an assay is referred to as L-ferritin or simply ferritin, whereas the ferritin measured by an assay sensitive to the H-rich ferritin contained in heart is designated H-ferritin. Erythrocytes contain a preponderance of H-ferritin, which changes less with variations in body iron status than the smaller component of L-ferritin.8H-ferritin assays were performed in the present study in an attempt to assess the erythrocyte contribution of L-ferritin to measurements in dried blood spots.
Measurements of H-ferritin, L-ferritin, and transferrin receptor were performed by enzyme-linked immunoadsorbent assay (ELISA) using double monoclonal antibodies. The assay of L-ferritin was made by adding 200 μL of sample to duplicate microtiter wells precoated with monoclonal antibody in 0.2 mol/L carbonate buffer at pH 9.6 and incubating for 2 hours at room temperature as previously described.9 After washing the wells with PBS-tween, 200 μL of horse radish peroxidase (HRP)-conjugated antiferritin monoclonal antibody was added. After an additional 2-hour incubation at room temperature, the wells were again washed with PBS-tween, and 200 μL of o-phenylenediamine dihydrochloride substrate in citrate-phosphate buffer pH 5.0 was added. After 30 minutes of incubation in the dark, the color reaction was terminated by adding 50 μL of 2.5-mol/L sulfuric acid. The optical density was measured at 492 nm in a microplate reader and the ferritin values of the samples were determined from a standard curve of known concentrations of recrystallized human liver ferritin plotted against optical density. The concentration of ferritin standards was calibrated with the international World Health Organization (WHO) reference standard obtained from the National Bureau of Standards.10
The assay of H-ferritin was developed using purified human heart ferritin obtained by ultracentrifugation and gel filtration on Sepharose 6B as the ferritin source.11 Monoclonal antibodies were used for both the solid-phase and indicator reagent. The assay was performed by the same procedure used for the measurement of L-ferritin. Recrystallized human liver ferritin was nonreactive in the H-ferritin assay at all concentrations. On the other hand, 6% of the purified H-ferritin could be detected with the L-ferritin assay. This percentage is assumed to represent the minimal L-subunit composition of H-ferritin.12,13 The transferrin receptor was assayed as previously described using monoclonal antibodies against receptor purified from human placenta.14 The assay protocol was nearly identical to that used for the measurement of H- and L-ferritin.
Statistical analysis.
Because of the skewed distribution of L-ferritin in WB and plasma and the transferrin receptor/ferritin ratio, these data were converted to logarithms for certain statistical analyses and the results reconverted to antilogarithms. The degree of separation between the three categories of iron status, (normal, ID, and IDA) was assessed for various laboratory parameters by analysis of variance. The correlation between any two measurements was evaluated by Pearson’s product moment. Statistical calculations were performed using the Abstat statistical program (Anderson Bell, Parker, CO).
RESULTS
The normal subjects included 18 women and 23 men who had hematocrits of 40.6% ± 1.9% (mean ± 1 SD) and 45.4% ± 2.6%, and geometric mean plasma ferritin concentrations of 33.5 (16.4 to 68.3, ± 1 SD) and 53.1 (29.8 to 94.6) μg/L, respectively. The mean hematocrits in the 20 subjects with ID and the 10 subjects with IDA were 40.3% ± 1.6% and 30.5% ± 3.8%, respectively. All 30 subjects in the latter two groups were women.
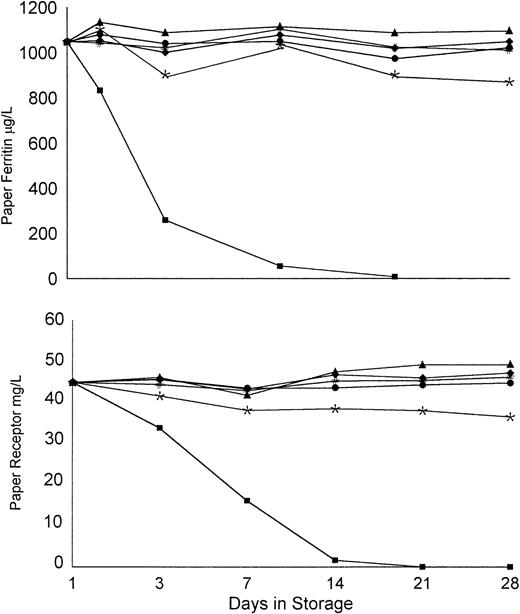
When blood spots were stored in sealed plastic bags at 4°C with or without desiccant, there was no significant decline in ferritin or transferrin receptor assays over 28 days (Fig1). When stored at room temperature with desiccant, the immunoreactivity in both assays remained constant, but there was a slight decline in activity when stored at room temperature without desiccant. Ferritin and transferrin receptor values decreased by approximately 10% when stored at 37°C for 28 days if the storage bag contained desiccant. However, without desiccant, there was a dramatic reduction in the concentration of both proteins. Thus, the combination of ambient humidity and high temperature resulted in a rapid deterioration in the immunoreactivity of both ferritin and transferrin receptor.
Stability of WB spots under different storage conditions. Individual points represent the total of six measurements on samples dried and stored in sealed bags at 4°C (▴, ⧫), room temperature (#, •), and 37°C (*, ▪). These were stored with dessicant (▴, #, *) and without dessicant (⧫, •, ▪).
Stability of WB spots under different storage conditions. Individual points represent the total of six measurements on samples dried and stored in sealed bags at 4°C (▴, ⧫), room temperature (#, •), and 37°C (*, ▪). These were stored with dessicant (▴, #, *) and without dessicant (⧫, •, ▪).
In a second study in which dried blood spots were stored at 4°C with desiccant for 1 year, there was no significant loss of immunoreactivity for either L-ferritin or transferrin receptor. In nine such measurements, values ranged from 95% to 107% of the original, with a mean of 98.4% ± 0.04%.
The intraassay variability for 10 measurements on each of five blood samples was 4.48%, with a range of 3.11% to 7.3%. The interassay variability for duplicate measurements on seven dried blood-spot samples measured over a period of 2 weeks averaged 5.55%, with a range of 2.7% to 9.6%.
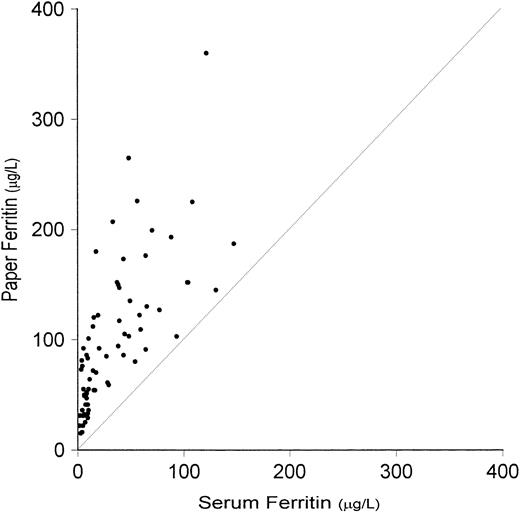
The immunoassay data for L- and H-ferritin and transferrin receptor in the normal, ID, and IDA subgroups of iron status and in the composite group of 71 subjects are listed in Table 1. The L-ferritin concentrations in WB, RBC, and paper samples were similar, and more than twofold higher than the plasma concentration, which reflects the contribution of L-ferritin from hemolyzed erythrocytes. The latter was more pronounced in subjects with ID. Thus, the WB and paper L-ferritin values were only two to three times greater than the serum concentration in normal subjects, but 10- and 20-fold higher in the ID and IDA groups, respectively (Fig2). The difference in the concentration of L-ferritin in WB and dried blood spots in the composite group was less than 5%, reflecting complete elution of L-ferritin from the dried blood spots.
Relationship between L-ferritin values determined on serum and on dried blood spots (paper). Interrupted line is the line of identity. Correlation coefficient is significant (r = .723,P < .001).
Relationship between L-ferritin values determined on serum and on dried blood spots (paper). Interrupted line is the line of identity. Correlation coefficient is significant (r = .723,P < .001).
Both L-ferritin and H-ferritin are released from hemolyzed erythrocytes, with the proportion of H-ferritin being considerably higher than L-ferritin.8 Compared with serum, L-ferritin measurements on hemolyzed samples (WB, RBC, and paper specimens in this study) are elevated, due both to the contribution of L-ferritin from erythrocytes and the fact that the L-ferritin assay measures a small percentage of H-ferritin. Assays of H-ferritin were performed to determine whether the erythrocyte contribution to the paper L-ferritin concentration could be estimated from the H-ferritin concentration. However, the relationship between L- and H-ferritin in the RBC samples varied with iron status. For example, the L-ferritin in RBC samples was 121.1 μg/L in normals as compared with 67.3 μg/L in ID, whereas the H-ferritin concentration was 599.3 and 530.2 μg/L, respectively (Table 1). As a result, when a constant ratio for L-/H-ferritin in erythrocytes of 0.18 based on values in the composite group (100.8/549.4) was used to estimate the erythrocyte contribution of L-ferritin to WB values, the calculated serum L-ferritin concentration was negative in more than half of the subjects with ID or IDA. When corrections were based on a lower L-/H-ferritin ratio of 6%, which represents the cross-reactivity of purified H-ferritin in the L-ferritin assay, the L-ferritin concentration in WB samples was not reduced appreciably. Consequently, we were unable to use the measurement of H-ferritin in WB or paper to derive a satisfactory estimate of serum L-ferritin concentration.
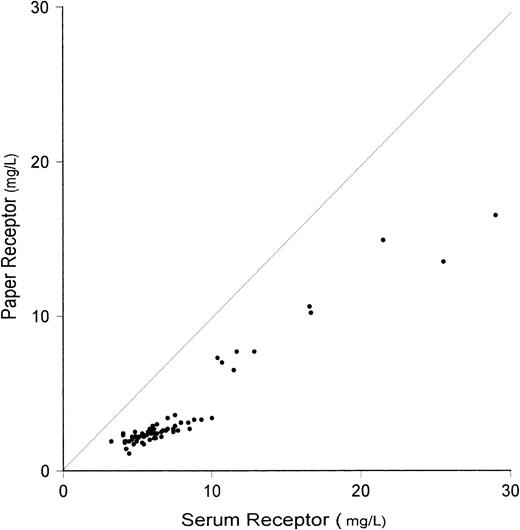
An impressive reciprocal change in serum transferrin receptor concentration with increasing ID has been reported previously15 16 and was observed again in the present study. The mean serum receptor concentration in IDA of 16.09 mg/L was threefold higher than the mean of 5.66 mg/L in normal subjects (Table1). In contrast with ferritin measurements, the concentration of transferrin receptor in RBC was negligible regardless of iron status. In the composite group of 71 subjects, the mean WB transferrin receptor concentration of 4.43 mg/L was similar to the serum receptor mean of 7.44 mg/L when the following correction was made for the volume displaced by RBCs. Based on the composite mean fractional hematocrit of 0.41, the serum transferrin receptor concentration predicted from the WB value was 4.43/0.59 − 0.05 = 7.46 mg/L, where 0.59 is the fractional plasmatocrit and 0.05 is the RBC receptor concentration. When this correction was applied, the paper values of transferrin receptor were 24%, 27%, and 11% lower than WB values in normal subjects, ID, and IDA, respectively. A highly significant correlation was observed between WB and paper receptor values (Fig3).
Relationship between measurements of transferrin receptor performed on serum and on dried blood spots (paper). Interrupted line represents the line of identity. Correlation is highly significant (r = .968, P < .001).
Relationship between measurements of transferrin receptor performed on serum and on dried blood spots (paper). Interrupted line represents the line of identity. Correlation is highly significant (r = .968, P < .001).
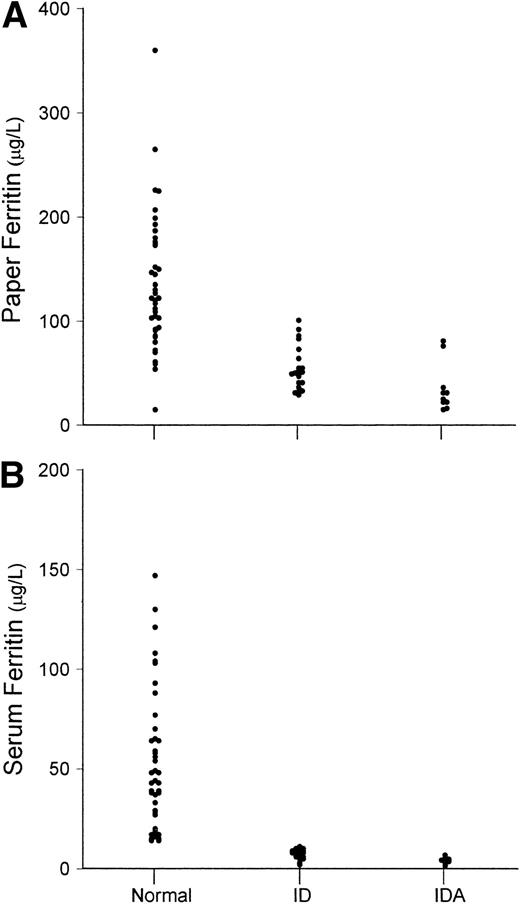
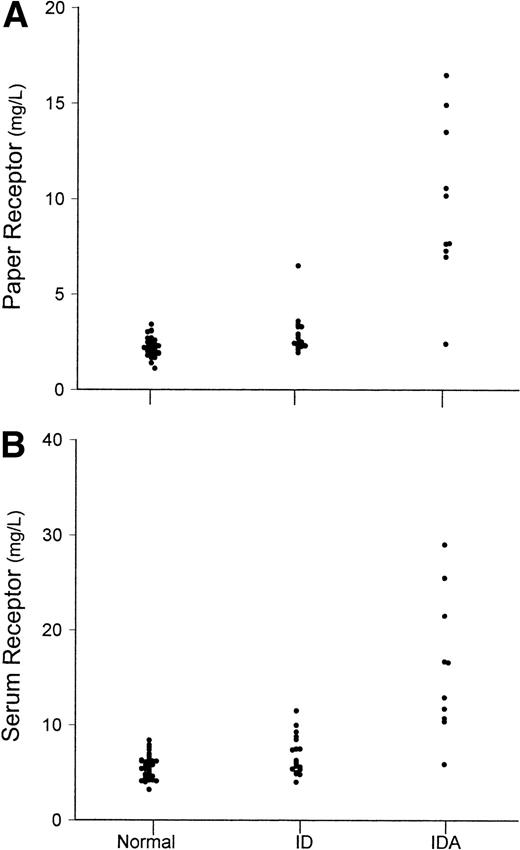
An important aspect of any new measurement or combination of measurements for assessing iron status in prevalence studies is the extent to which ID and IDA can be distinguished from iron-replete individuals. Because of the significant and variable contribution of L-ferritin in erythrocytes to the paper L-ferritin values, the distinction between ID or IDA and normal subjects was less satisfactory than with serum ferritin measurements (Fig4). On the other hand, the separation between normal and IDA based on transferrin receptor measurements was similar for serum and paper assays (Fig 5). With both paper and serum determinations, only one of 10 subjects with IDA had transferrin receptor concentrations in the normal range.
Values of (A) dried paper samples of blood L-ferritin and (B) serum L-ferritin. Values are shown separately for normal subjects (N), subjects with ID, and subjects with IDA.
Values of (A) dried paper samples of blood L-ferritin and (B) serum L-ferritin. Values are shown separately for normal subjects (N), subjects with ID, and subjects with IDA.
Values of (A) dried paper samples of blood transferrin receptor and (B) serum transferrin receptor. Values are shown separately for normal subjects (N), subjects with ID, and subjects with IDA.
Values of (A) dried paper samples of blood transferrin receptor and (B) serum transferrin receptor. Values are shown separately for normal subjects (N), subjects with ID, and subjects with IDA.
One of the more difficult problems in identifying IDA in population studies conducted in developing countries is the presence of chronic illnesses or infections, which falsely elevate the serum ferritin and lessen its diagnostic utility. Recent clinical studies have indicated that the serum transferrin receptor is typically elevated in IDA regardless of associated illness and that the ratio of receptor/ferritin is more efficient for identifying IDA than either the transferrin receptor or ferritin alone.16 17 Although none of our subjects had associated illnesses, it was of interest to compare the diagnostic efficiency of serum and paper measurements of receptor/ferritin ratio.
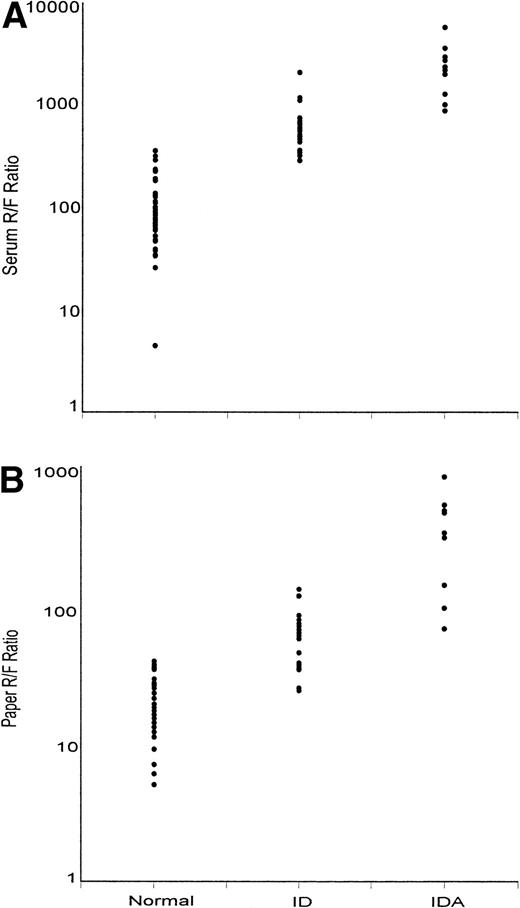
The geometric mean receptor/ferritin ratio varied widely depending on iron status from a mean of 128 in normal subjects to 936 in ID and 3,839 in IDA (Table 1). Because of the erythrocyte contribution of L-ferritin to the paper values, the mean ratio with the paper samples was only one tenth that observed with serum measurement in the composite group of subjects, although the degree of separation and extent of overlap between the three study groups was similar for serum and paper measurements (Fig 6). When evaluated by analysis of variance, the separation between normal, ID, and IDA using the logarithm of the receptor/ferritin ratio was similar for serum and paper (F = 132.8 and 105.3, respectively;P < .001). There was no overlap between normal and IDA with either paper or serum determinations.
Values of receptor/ferritin ratio (R/F ratio) from (A) serum and (B) dried paper samples of blood. Values are shown separately for normal subjects (N), subjects with ID, and subjects with IDA.
Values of receptor/ferritin ratio (R/F ratio) from (A) serum and (B) dried paper samples of blood. Values are shown separately for normal subjects (N), subjects with ID, and subjects with IDA.
DISCUSSION
There has been a marked increase in recent years in the use of dried serum or blood spots collected on filter paper for a variety of laboratory measurements. The earliest and most extensive use of this technology was in screening cord blood for neonatal metabolic disorders such as phenylketonuria18,19 and congenital hypothyroidism.20,21 With the availability of increasingly sensitive immunologic and molecular probes, blood-spot technology has been extended to a broad range of medical applications. The technique is used in screening for infectious diseases such as Toxoplasma gondii,22 human immunodeficiency virus,23and Pseudomonas aeruginosa,24 and it has been used successfully to monitor drug therapy such as cyclosporine25and phenytoin.26 In nutritional screening programs, dried blood-spot determinations of thyrotropin have been used for the detection of iodine deficiency in developing countries.2
There are several advantages in using dried blood spots. Perhaps the most important is in eliminating the need for venous blood sampling, which is often a technical challenge in infants and young children. Venous sampling is also more costly than capillary sampling in epidemiologic surveys because of the disposable sampling equipment required for the former. Another important advantage of using dried blood spots is the ease in transporting and storing specimens before analysis. Even if the temperature of paper samples must be controlled, the cost of transporting specimens is considerably lower for paper samples than for larger volumes of WB or serum.
The most efficient method for blood-spot tests is to place a drop or two of capillary blood directly onto the filter paper. If a quantitative measurement is required, a paper punch can be used to obtain a constant amount of the spotted blood sample for elution. We used this approach initially, cutting 8-mm paper discs from the center of a larger dried blood spot. However, when discs were cut at varying distances from the center of the spot, we noted significant differences in both the protein and hemoglobin concentrations, presumably due to variations in the rate of diffusion in the paper. In subsequent studies, we therefore elected to use a precisely measured 25-μL blood spot and elute the entire sample. This approach requires the use of heparinized microhematocrit tubes or specialized microvette collection tubes containing potassium EDTA. The former are especially convenient in field studies if anemia is determined by microhematocrit. The use of a precisely measured volume of blood also requires disposable pipettes for pipetting devices and a certain degree of technical skill, but neither of these constraints outweighs the advantage of the increased accuracy and reproducibility achieved by using a precisely measured sample volume.
A major consideration in designing the present study was the published evidence that sufficient amounts of L-ferritin are contained in erythrocytes to influence assays in specimens containing hemolyzed RBCs such as dried WB samples.8 12 Our attempts to derive a suitable correction for the erythrocyte contribution of L-ferritin using measurements of H-ferritin proved unsuccessful because of variations in the ratio of H-/L-ferritin in erythrocytes with differences in iron status. Ferritin measurements alone in dried blood spots are unsuitable for distinguishing between normal subjects and individuals with either ID or IDA (Fig 4).
In contrast to ferritin, the concentration of transferrin receptor in erythrocytes was negligible. Consequently, measurements of transferrin receptor in dried blood samples were equivalent to serum measurements when a correction was made for the plasma displaced by RBCs. However, clinical studies to date do not support the use of isolated measurements of transferrin receptor to identify ID or IDA. The major value of receptor measurements is to identify IDA when the serum ferritin is falsely elevated because of ongoing inflammation or chronic infection. Because the serum transferrin receptor remains normal with inflammation, but increases with the onset of tissue ID, recent clinical studies have shown that the ratio of transferrin receptor/serum ferritin can be used to detect IDA even in the presence of other chronic infection or inflammatory disease.16 27 An important finding in the present study was that the receptor/ferritin ratio in dried blood spots was as useful in identifying IDA as measurements on serum. With both paper and serum determinations, complete separation between normal and IDA was achieved using the receptor/ferritin ratio. Thus, the combined determination of ferritin and transferrin receptor in dried blood samples could be potentially useful if our present findings are confirmed in future studies performed under field conditions.
A reasonable alternative is to use serum or plasma samples spotted on filter paper, rather than WB. Our recent studies indicate that the data obtained with this approach is equivalent to measurements of ferritin and transferrin receptor performed directly on serum.28 In a recent study, a measured volume of serum, spotted and dried on filter paper, was found to give values comparable to traditional serum measurements.29 The disadvantage with this approach is it requires centrifugation of blood samples, which often poses a significant logistic problem in a field setting. The advantage of using plasma is the ability to identify both ID and IDA, which cannot be accomplished with dried blood spots. The choice between measurements on whole serum and either dried blood or plasma spots will depend to a large extent on the location of the survey and local laboratory facilities.
Address reprint requests to James D. Cook, MD, Department of Medicine, University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS 66160-7233.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.