Abstract
Recently, a C3-binding cyclic synthetic peptide (Compstatin) has been identified that binds to complement component C3 and inhibits complement activation. Here we have examined the influence of Compstatin on complement activation and its indirect effects on cellular responses in whole blood in two models for extracorporeal circulation. Compstatin effectively inhibited the generation of C3a and sC5b-9 and the binding of C3/ C3 fragments to the polymer surface. As a result of the inhibition of complement activation, the activation of polymorphonuclear leukocytes (PMNs; as assessed by the expression of CD11b) and the binding of these cells (CD16+) to the polymer surface were almost completely lost. In contrast, blood cell counts were not affected. Using surface plasmon resonance technology, we have confirmed that Compstatin exerts its inhibitory effect on complement activation by binding to native C3. These data show that complement activation, leading to activation and binding of PMNs to the biomaterial surface, can be abolished by the addition of Compstatin. The properties of Compstatin make Compstatin a promising drug for use in extracorporeal circuits to avoid bioincompatibility reactions, eg, during cardiopulmonary bypass, but also a favorable precursor peptide for the development of an anticomplement drug for oral use.
© 1998 by The American Society of Hematology.
THE COMPLEMENT SYSTEM is known to be activated during extracorporeal circulation, eg, during dialysis, plasmapheresis, and cardiopulmonary bypass surgery. In particular, complement-related complications resulting from bioincompatibility reactions accompanying cardiopulmonary bypass (CPB) have gained recent attention. Two major areas of concern are neurological sequelae and postperfusion inflammation reactions that may lead to lung damage.1-5 During CPB, complement activation is likely to contribute to both of these conditions by activating platelets and leukocytes, through which the ongoing “whole body” inflammation is supported.2,6 During CPB the blood in the oxygenator is in direct contact with two phases: the polymer surface and the oxygen gas surface. Both interfaces are known to activate complement by the alternative pathway, thereby generating C3a and soluble C5b-9.7,8 Classical pathway activation has also been reported.9
New inhibitors of complement activation have been shown to decrease the severity of bioincompatibility reactions during extracorporeal treatment.10 Several types of inhibitors have been developed in recent years: recombinant natural inhibitors of complement,11 antibodies to complement components,10 and protease inhibitors.12,13However, a specific small-molecular-weight complement inhibitor has not been available until recently. A recent study has described a specific peptide inhibitor with a completely different mechanism of action.14 This peptide (Compstatin) was developed from a clone of a phage-displayed random peptide library that was screened for binding to C3b, a fragment of complement component C3. Compstatin consists of 13 amino acid residues (ICVVQDWGHHRCT-NH2), whose ring is maintained by a disulphide bond between the two cysteines. Further studies on the substitution analogs and the solution structure of the peptide have indicated that the type-1 β-turn segment of the peptide is critical for the preservation of its conformational stability and probably forms the C3 binding site.15
In this study we have used two recently developed models for extracorporeal circulation to test the ability of Compstatin to alleviate the complement-related side effect of such procedures. The results obtained in this study suggest that Compstatin can be used as an effective complement inhibitor during extracorporeal circulation.
MATERIALS AND METHODS
Peptide Synthesis and Purification
Compstatin and control peptide (IAVVQDWGHHRAT-NH2) were synthesized as described before.14 Both peptides were synthesized on an Applied Biosystems peptide synthesizer (model 431A; Foster City, CA) using a F-moc amide resin. The crude peptides were purified on a C18 column (Waters Associates, Milford, MA). Disulfide oxidation was performed by stirring a 0.15 mmol/L solution of the peptide in 0.1 mol/L ammonium bicarbonate, pH 8.0, and bubbling with oxygen at 22°C for 48 hours. The identity and the purity of the peptides were checked by analytical high-pressure liquid chromatography (HPLC) and laser desorption mass spectrometry.16 17
Complement Purification
C3 and factor B were prepared from human plasma according to Hammer et al18 and Lambris and Müller-Eberhard,19respectively. Factor D was purified from peritoneal fluid from patients with renal failure as described by Catana and Schifferli.20C3 was digested with trypsin to C3a and C3b (1 % (wt/wt) for 5 minutes at room temperature), and the fragments were separated by gel filtration as described by Bokisch et al.21
Preparation of Blood
Sixty milliliters of blood was collected from healthy blood donors and immediately mixed with 30-180 IU (approximately 210 to 1,300 μg) sodium heparin from porcine intestinal mucosa (Bioiberica, Sociedad Anònima, Spain). Heparin-coated equipment was used for this procedure. In analyses in which Compstatin and control peptide were used, they were used in concentrations up to 250 μmol/L (ie, a 46-fold molar excess assuming a C3 concentration of 1 g/ L).
Plasmon Resonance Measurements
The experiments were performed on a Biacore 2000 instrument (BiaCore AB, Uppsala, Sweden). An alternative pathway convertase was constructed as described elsewhere (manuscript in preparation); 300 μg per cm2 of C3b was coupled by amine bonds to CM5 chips according to the manufacturer’s recommendation. Factor B (58 μg/mL) was injected into the flow cell. As a working buffer, phosphate-buffered saline (PBS) containing 1 mmol/L Ni2+was used.22 When the binding of factor B to C3b had reached equivalance, factor D (10 μg/mL) was injected. This resulted in the formation of the alternative pathway C3 convertase C3b, Bb. To deposit additional C3b molecules, native C3 (132 μg/mL) was injected. The newly attached C3b was used for another cycle of factor B, factor D, and C3 addition. After a second or a third cycle, the convertase was incubated with 70 μmol/L of Compstatin followed by C3 in the presence of either Compstatin or control peptide (70 μmol/L). No attachment of C3 occured in the absence of ions (Ni2+).
Experimental Models
Tubing loops model.
A modification of the model previously described was used.23,24 Nine-milliliter tubing loops (diameter = 6.3 mm, length = 300 mm, surface area = 59.3 cm2) were filled with 5 mL of blood (area: volume = 11.9 cm2/mL) that had been mixed with heparin immediately after the blood had been withdrawn from the blood donor. The samples were then thereafter closed into circuits with connectors of stainless steel furnished with immobilized heparin,23 giving a gas volume of approximately 4 mL. The tubing loops were rotated vertically (at 32 rpm, linear flow rate 0.3 L/min) during incubation in a water bath for up to 60 minutes at 37°C. After incubation, 250 μL of 0.2 mol/L EDTA was added to the blood to give a final EDTA concentration of 10 mmol/L. The 0-minute sample was not added to the tubing loop but was instead immediately added to a tube containing EDTA, because 0-minute samples that had been allowed to come into contact with the tubing had previously been shown to give results similar to background levels.23 The blood samples were centrifuged at 4°C (3,290g, 20 minutes), and the plasma was collected. Each piece of tubing was washed 3 times with PBS containing 0.05% Tween 20 (vol/vol) and 0.02% Antifoam (vol/vol; Pharmacia Upjohn AB, Sweden). Antifoam was added to avoid bubbles in the tubing. The tubing loops were stored at −70°C until analysis. The experiments were performed 3 to 4 times for each time point, using blood from different donors.
Microscope slide model.
This model is described in detail in a manuscript under preparation. Slides were prepared as follows: two polymethylmethacrylate (PMMA) rings with a height of 5 mm, outer diameter of 24 mm, and an inner diameter of 19 mm (surface area = 8.1 cm2) were fixed onto a polystyrene microscope slide. This device was coated with heparin, as described previously.23 The two wells formed by the rings were filled with 1 mL of whole blood containing 3 IU heparin. The wells were closed with a polyvinyl chloride (PVC) microscope slide (test slide), and the device was then mounted on the outer rim of a plastic disc (30 cm in diameter), which was rotated at 32 rpm in a 37°C water bath. After incubation for up to 1 hour the blood was removed and the loosely attached PVC test slide was washed twice in veronal-buffered saline, pH 7.4, containing 0.75 mmol/L Ca2+ and 2.5 mmol/L Mg2+. The test slide was then dried at room temperature and stored at −70°C until further use.
Enzyme Immunoassays (EIA) for Detection of C3a and sC5b-9
PBS containing 1% (wt/vol) bovine serum albumin (BSA) and 0.1% (vol/vol) TWEEN 20 was used as a working buffer, and PBS containing 0.1% (vol/vol) TWEEN 20 was used as a washing buffer in all three EIAs described below.
EIA for detection of C3a.
This assay is a sandwich EIA that uses the monoclonal antibody (MoAb) 4SD17.3 as the capture antibody. EDTA plasma was diluted 1/500 and analyzed as described previously.25 Bound C3a was detected with biotinylated rabbit anti-C3a followed by horseradish peroxidase (HRP)–conjugated streptavidin (Amersham, UK). Zymosan-activated serum,26 calibrated against a solution of purified C3a,21 served as standard and the values are given in ng/mL.
EIA for detection of sC5b-9.
A modification of the EIA, described by Mollnes et al,27was used to measure sC5b-9. Plasma samples, diluted 1/5, were added to microtiter plates coated with anti-neoC9 MoAb MCaE11. sC5b-9 was detected by polyclonal anti-C5 antibodies diluted 1/500, followed by HRP-conjugated anti-rabbit Ig diluted 1/500 (both from Dako A/S, Glostrup, Denmark). Zymosan-activated serum defined as containing 40,000 arbitrary units (AU) per mL served as the standard. Heparin, at the concentrations used in this study, did not interfere with the performance of any of the EIAs.23
EIA for Determination of Surface-Bound C3/C3 Fragments
Lengths of PVC tubing were cut into 3-cm pieces and a tube stopper was applied to one end of each tubing piece. One hundred microliters of HRP-labeled rabbit anti-human C3c (Dako A/S), diluted 1/250 in working buffer, was incubated in each tubing piece for 60 minutes at room temperature. The tubing pieces were then washed 3 times in washing buffer, and the color reaction was initiated by the addition of 100 μL of 1,2-phenylendiamine dihydrochloride (Fluka, Buchs, Switzerland) then stopped with 100 μL of 1mol/L H2SO4 after 5 minutes. Absorbance was measured at 492 nm. Binding of C3/C3 fragments in plasma, containing 10 mmol/L EDTA, was used as a control and was subtracted from the values obtained without EDTA. Bound C3/C3 fragments were presented as mean492A ±SEM, obtained from three pieces of tubing.
Immunochemical Staining of Microscope Slides
The microscope slides (described above), which had been stored at −70°C, were immersed in ice-cold 50% (vol/vol) acetone for 30 seconds, followed by another incubation for 5 minutes in ice-cold 100% (vol/vol) acetone. All subsequent steps were performed at room temperature. The slides were dried and washed twice with PBS for 5 minutes, then incubated in 0.3% (vol/vol) H2O2 in PBS for 15 minutes, washed twice with PBS for 5 minutes, and blocked with 4% BSA in PBS for 5 minutes. Subsequently, the slides were:
Incubated with primary polyclonal antibodies (DAKO A/S) for 30 minutes: anti-CD11b, anti-CD16, and anti-CD41, each diluted 1/50 or anti-CD14, diluted 1/25 in PBS.
Incubated with anti-mouse Ig (DAKO A/S), diluted 1/40, for 30 minutes.
Incubated with mouse peroxidase-antiperoxidase (PAP; Serotec, Kidlington, UK), diluted 1/250, for 30 minutes.
Incubated in darkness for 15 minutes with a mixture of 10 mg 3-amino-9-etylcarbazol (AEC) predissolved in 6 mL of dimethylsulfoxide (DMSO); 50 mL of 0.02 mol/L NaAc (pH 5.5); and 8 μL of 30% (vol/vol) H2O2. The color reaction was started by the addition of the AEC solution. Each incubation step was followed by a washing step, which included 3 to 5 rinses in PBS. All incubations were done at room temperature. The slides were finally stained in hematoxylin solution (Harris type; Sigma, St Louis, MO). Four exposures for each well were analyzed, and the number of positive cells per three to four experiments was presented as x ± SEM/ 7mm2.
Flow Cytometry
The expression of CD11b (CR3) on PMNs was quantitated using flow cytometry measurements. Briefly, 100 μL of blood was incubated for 15 minutes at 37°C with monoclonal anti-CD11b conjugated with phycoerythrin (Becton Dickinson, Franklin Lakes, NJ), followed by the addition of lysis reagent (Becton Dickinson). In a few experiments the cells were double-labeled in addition to the anti-CD11b label by adding 100 μL of anti-CD16 antibody conjugated with fluorescein isothiocyanate (FITC; Becton Dickinson) before lysis.
The samples were analyzed using an Epics Profile XL-MCL cytometer (Coulter Electronics, Hialeah, FL). Data processing from 5,000 cells was performed with the XL software (Coulter Electronics). In this system each cell is represented by a point in a rectangular coordinate system, and the instrument gives the percentage of positive cells, mean fluorescence intensity (MFI), complexity (right angle scatter), and mean cell size (forward angle scatter) of the cell population within the field. A discrimination frame was placed around the neutrophil cluster using right angle scatter and forward angle scatter. Analytical markers were set in the phycoerythrin channel to measure the CD11b MFI. The same sample settings were used throughout the experiment. All experiments were run in duplicate.
Statistical Analyses
Results were expressed as mean ±SEM. Statistical significance was calculated with Student’s t-test, using Statview 4.0 (SAS Institute, Inc, Capy, NC) for Macintosh (Apple Computer, Inc, Cupertino, CA).
RESULTS
Inhibition of the Alternative Pathway Convertase-Mediated Cleavage of C3 by Compstatin
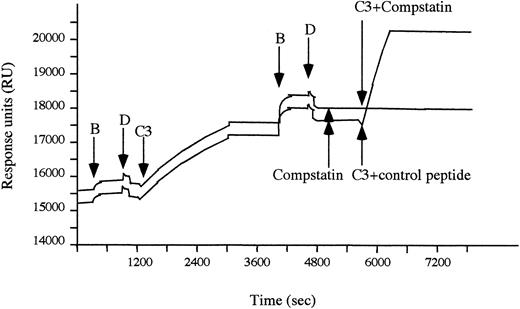
An alternative pathway C3 convertase was formed on a CM5 chip in the BiaCore instrument. Cleavage of C3 by the convertase (C3b,Bb), resulting in deposition of C3b on the chip, was monitored by plasmon resonance. Alternative pathway convertase complexes, which were preincubated with 70 μmol/L of Compstatin, still cleaved native C3 (Fig 1). In contrast, the cleavage of native C3 was completely inhibited when C3 was added to the surface-bound convertase complexes in the presence of Compstatin, whereas the control peptide had no effect.
The stepwise generation of alternative pathway convertases monitored by plasmon resonance. C3b was conjugated to a CM 5 chip by amide bonds and used to create an initial alternative pathway convertase by sequential addition of factors B and D (as indicated by arrows) in the presence of 1 mmol/L Ni2+. The active convertase was thereafter subjected to native C3, which subsequently bound to the surface in the form of C3b. When the cycle with factors B and D was repeated, additional alternative pathway convertase complexes were generated. These convertase complexes were preincubated with Compstatin (70 μmol/L) followed by native C3 added in the presence of either Compstatin (70 μmol/L) or control peptide (70 μmol/L).
The stepwise generation of alternative pathway convertases monitored by plasmon resonance. C3b was conjugated to a CM 5 chip by amide bonds and used to create an initial alternative pathway convertase by sequential addition of factors B and D (as indicated by arrows) in the presence of 1 mmol/L Ni2+. The active convertase was thereafter subjected to native C3, which subsequently bound to the surface in the form of C3b. When the cycle with factors B and D was repeated, additional alternative pathway convertase complexes were generated. These convertase complexes were preincubated with Compstatin (70 μmol/L) followed by native C3 added in the presence of either Compstatin (70 μmol/L) or control peptide (70 μmol/L).
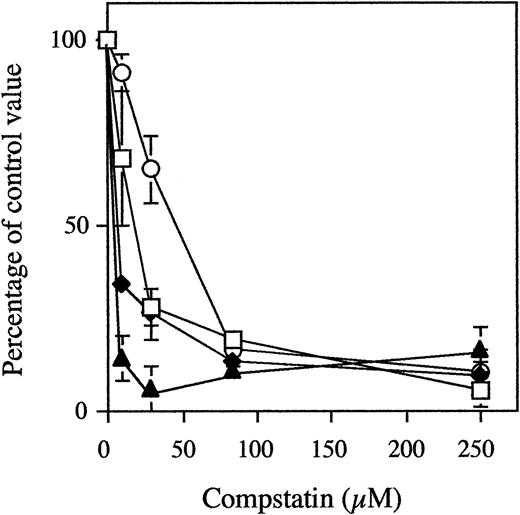
Dose-Dependent Inhibition of Complement Activation by Compstatin
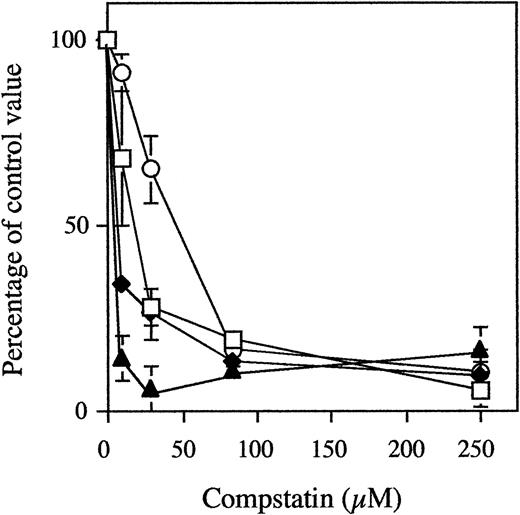
In three different experiments using three different blood donors, PVC tubing loops containing 5 mL of blood were rotated for 60 minutes at 37°C with 0 to 250 μmol/L (a 46-fold molar excess when compared with C3) of Compstatin or control peptide (Fig 2). In the loops containing 250 μmol/L control peptide, the levels of C3a increased from 141 ± 18 to 2,887 ± 603 ng/mL and those of sC5b-9 from 39 ± 6 to 491 ± 141 AU/mL (similar values were obtained for buffer controls). In contrast, in tubing loops containing 250 μmol/L Compstatin, the levels of C3a and sC5b-9 were reduced to 271 ± 106 ng/mL (5% of controls) and 83 ± 20 AU/mL (10%), respectively.
Generation of fluid-phase C3a (open squares) and sC5b-9 (closed diamonds), binding of C3/C3 fragments (open circles) to the biomaterial surface, and expression of CD11b on circulating PMNs (closed triangles) in whole blood during incubation in the tubing loop model. Whole blood containing 0.4 IU of heparin/mL was rotated for 60 minutes at 37°C in PVC tubing loops filled with 5 mL fluid (4 mL of gas). The peptides were present at concentrations of up to 250 μmol/L (a 46-fold excess when compared with plasma C3). Data are the mean of three experiments and are presented as the mean percentage ± SEM of the values obtained with 250 μmol/L of control peptide.
Generation of fluid-phase C3a (open squares) and sC5b-9 (closed diamonds), binding of C3/C3 fragments (open circles) to the biomaterial surface, and expression of CD11b on circulating PMNs (closed triangles) in whole blood during incubation in the tubing loop model. Whole blood containing 0.4 IU of heparin/mL was rotated for 60 minutes at 37°C in PVC tubing loops filled with 5 mL fluid (4 mL of gas). The peptides were present at concentrations of up to 250 μmol/L (a 46-fold excess when compared with plasma C3). Data are the mean of three experiments and are presented as the mean percentage ± SEM of the values obtained with 250 μmol/L of control peptide.
Surface-bound C3/C3 fragments in the above experiments were analyzed by EIA. The binding increased from 492A = 0.03± 0.01 to492A = 0.68 ± 0.12 in the absence of peptide. In the presence of 250 μmol/L Compstatin, the binding only reached 0.10 ± 0.02 (11%).
Analysis by flow cytometry showed that the expression of leukocyte CD11b MFI increased from 17 ± 5 to 59 ± 10 in blood without Compstatin. Double staining of the cells showed that 95% of the cells were CD16+, ie, PMNs. Already in the presence of 28 μmol/L of Compstatin the expression was reduced to 20 ± 3 (7%) and in the presence of 250 μmol/L Compstatin MFI to 25 ± 9 (19%).
The platelet count dropped by 24% during incubation in the absence of peptide, a drop that was not affected by increasing concentrations of Compstatin (Table 1). The leukocyte and the erythrocyte counts were not affected during the 60-minutes incubation.
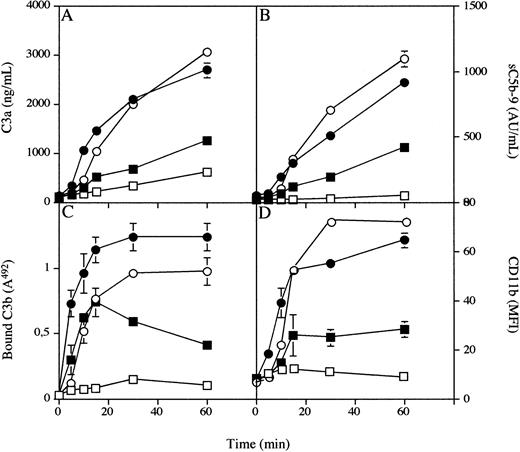
Time-Dependent Inhibition of Complement Activation by Compstatin
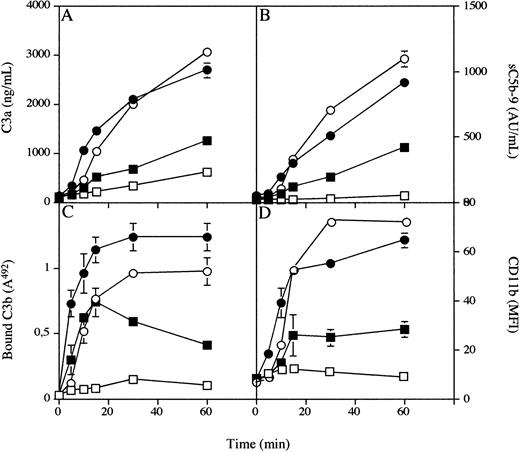
PVC tubing loops containing 5 mL of blood from a single donor were rotated for 5, 10, 15, 30, or 60 minutes at 37°C in the absence or presence of 43 μmol/L Compstatin (Fig 3). Before the experiment, the C3a and sC5b-9 levels in untreated blood were 111 ± 4 ng/mL and 36 ± 0.2 AU/mL, respectively. These levels increased continuously to 2,698 ± 149 ng/mL and 913 ± 1 AU/mL at 60 minutes in samples without peptide but reached only 1,245 ± 14 and 423 ± 4 in the presence of Compstatin. The corresponding values for the binding of C3/C3 fragments at 60 minutes, as reflected by the binding of anti-C3c, were 492A = 1.2 ± 0.1 for the control and 492A = 0.41 ± 0.1 in the presence of Compstatin.
Generation of fluid phase C3a (A) and sC5b-9 (B) binding of C3 fragments to the biomaterial surface (C), and expression of CD11b on circulating PMNs in whole blood (D) during incubation in the tubing loop model. Whole blood containing 0.4 IU of heparin/mL in the presence (squares) or absence (circles) of 43 μmol/L Compstatin (an eightfold molar excess over C3) was rotated up to 60 minutes at 37°C in PVC tubing loops filled with 5 mL fluid (4 mL of gas). Open symbols represent experiments performed with 10 mmol/L EGTA, and filled symbols represent those without EGTA present.
Generation of fluid phase C3a (A) and sC5b-9 (B) binding of C3 fragments to the biomaterial surface (C), and expression of CD11b on circulating PMNs in whole blood (D) during incubation in the tubing loop model. Whole blood containing 0.4 IU of heparin/mL in the presence (squares) or absence (circles) of 43 μmol/L Compstatin (an eightfold molar excess over C3) was rotated up to 60 minutes at 37°C in PVC tubing loops filled with 5 mL fluid (4 mL of gas). Open symbols represent experiments performed with 10 mmol/L EGTA, and filled symbols represent those without EGTA present.
Analysis by flow cytometry showed that the expression of leukocyte CD11b MFI increased continuously during incubation, from the initial value of 8 ± 1 to a maximum value of 65 ± 3 after 60 minutes. In the presence of peptide inhibitor the MFI increased only to a maximum of 28 ± 3 after 60 minutes.
The same experiments were repeated in the presence of 10 mmol/L EGTA. In the absence of peptide the added values for the four parameters were similar to those without EGTA. In the presence of 43 μmol/L Compstatin, the levels of C3a and sC5b-9 were further reduced to 619 ± 29 ng/mL and 50 ± 7 AU/mL, respectively, after 60 minutes. Binding of C3/C3 fragments were reduced to 492A = 0.1 ± 0 and CD11b to 9 ± 3.
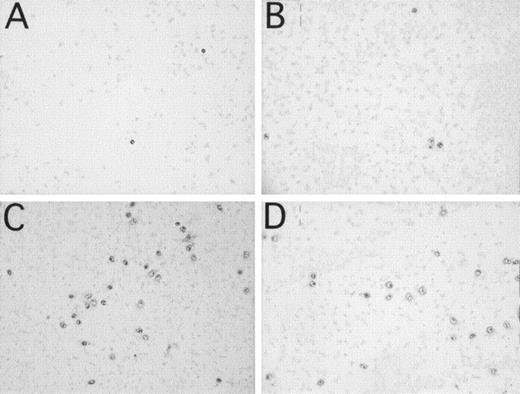
The Effect of Compstatin on the Binding of CD16+, CD11b+ Cells to a PVC Surface
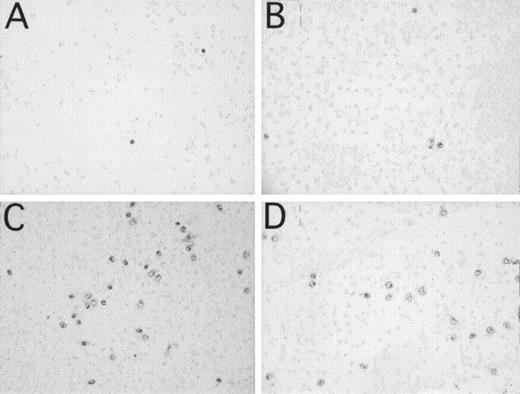
One milliliter of blood, containing 3 IU/mL of heparin, was incubated in the microscope slide model for 60 minutes. More than 95% of the bound leukocytes were polynuclear leukocytes, as assessed by hematoxylin staining. These cells were CD11b+ (131 ± 56 cells / 7 mm2; Fig 4C) and CD16+ (130 ± 16 cells / 7 mm2; Fig 4D). Less than 5% of the cells were CD14+ (not shown), indicating binding of a small amount of monocytes to the surface. Bound platelets (no shown) formed a film over the whole slide. The addition of 250 μmol/L Compstatin inhibited binding of CD11b+ (13 ± 3; P < .01; Fig 4A) and CD16+ cells (19 ± 6; P < .001; Fig 4B) to the surface, whereas binding of platelets was unaffected (not shown). Forty-three micromoles per liter Compstatin had no significant effect (not shown).
Binding of leukocytes and platelets to PVC microscope slides identified by immunochemical staining after incubation of 1 mL of whole blood containing 3 IU of heparin/mL in the microscope slide model. (A and C) represent CD11b+ leukocytes. (B and D) represent CD16+ PMNs. Panels (A and B) are experiments with 250 μmol/L Compstatin and (C and D) with the same concentration of control peptide.
Binding of leukocytes and platelets to PVC microscope slides identified by immunochemical staining after incubation of 1 mL of whole blood containing 3 IU of heparin/mL in the microscope slide model. (A and C) represent CD11b+ leukocytes. (B and D) represent CD16+ PMNs. Panels (A and B) are experiments with 250 μmol/L Compstatin and (C and D) with the same concentration of control peptide.
DISCUSSION
We have recently described a synthetic cyclic peptide, Compstatin, that specifically inhibits C3 activation.14 This peptide has been shown to affect both the classical and the alternative pathway by inhibiting the C3 convertase-mediated cleavage of native C3 to C3a and C3b. The inhibition did not involve direct blocking of the protease cleavage site, because trypsin cleavage was not affected. A more likely explanation is that the Compstatin bound to a site on C3 that is important for interaction with the C3 convertase complexes. These studies, however, did not completely exclude the possibility that the peptide exerted its effect on C3 convertases. The conclusions of these previous studies were confirmed in the present study by plasmon resonance. An alternative pathway C3 convertase was formed by purified components on a Biacore chip. The cleavage of purified C3 to C3a and C3b was completely inhibited when the peptide was allowed to bind to native C3, but preincubation of the convertase had no effect (Fig 1).
To investigate the biological effects of the peptide, we have used two simple models of extracorporeal circuits that relate to procedures such as dialysis, plasmapheresis, and cardiopulmonary bypass.23,24 Both models involve systems that are partially filled with blood and rotated at 37°C. In these cases, complement activation takes place both on the blood/polymer surface and at the blood/air interfaces.23 The blood is freshly drawn and the concentration of heparin kept to as low as possible (0.4 IU/mL), thus minimizing the interference of the heparin with other components of the blood.
When added to the tubing model, Compstatin inhibited complement activation, as indicated by the dose-dependent reduction in the generation of C3a and sC5b-9 and binding of C3b to the surface. The inhibition tended to be effected in two steps. An 8-fold molar excess of Compstatin (as compared with plasma C3) produced a significant inhibition of complement activation, but total inhibition required an approximately 40-fold excess. In contrast, when the classical pathway was inhibited by Ca2+ chelation in combination with peptide added at eightfold excess, a complete inhibition of C3a and sC5b-9 generation and of C3b binding was achieved, indicating that the classical pathway requires a higher dose for a complete inhibition. The properties of C3 that were induced by Compstatin were similar to those of a dysfunctional form of C3 that has been found in three siblings of a family in which one sister suffered from an atypical form of systemic lupus erythematosus.28 29 This dysfunctional C3 is almost completely resistant to cleavage by the alternative pathway convertase but less resistant to the classical pathway convertase, and its cleavage by trypsin is unaffected. Localization of the mutation in the dysfunctional C3 might be one way to understand the mechanism by which Compstatin inhibits C3 activation.
In a previous study using the same two experimental models and recombinant sCR1, we have shown that complement activation, initiated by the biomaterial and the gas surface, led to a sequence of events: binding of C3/C3 fragments to the biomaterial surface, generation of fluid-phase C3a and sC5b-9, upregulation of CD11b/CD18 of PMNs and monocytes, and binding of these cells to the polymer via C3 fragments.24 Addition of sCR1, at a dose of 100 μg/mL (0.5 μmol/L), resulted in complete inhibition of all the above markers of complement activation. In comparison, 65 μg/mL of Compstain (43 μmol/L) was required to achieve similar effects. Not unexpectedly, the biological effect produced by Compstatin was similar to that of sCR1. Both inhibitors apparently abrogate the upregulation of CD11b on PMNs as well as the binding of these cells to the polymer surface. It suggests that complement activation represents the major cause of inflammation in these models and presumably during extracorporeal circulation.
It is interesting that the inhibition obtained at an eightfold excess of peptide to plasma C3 only affected the alternative pathway and did not significantly reduce complement activation or the complement-mediated biological effects induced by the polymer surface. In contrast, Ca2+ chelation, which blocks the classical pathway, does not reduce complement activation, although the activation of the alternative pathway has a lag phase of 5 to 10 minutes before it accelerates. These data therefore indicate that a polymer (biomaterial) is able to activate complement by both pathways and that the classical pathway alone is able to maintain a substantial bioincompatibility reaction. Furthermore, these data support the concept that the classical pathway is initiated before the alternative pathway and might, under conditions of no inhibition, be the reaction that deposits C3b on the surface and eventually triggers the alternative pathway. In general, alternative-pathway activation has been reported to occur on biomaterials,30 31 but this study indicates that classical pathway activation occurs at least on initial contact between blood and polymer.
In recent years, various types of heparin coatings of CPB circuits have been shown to decrease bioincompatibility. Nevertheless, complete inhibition of complement activation in heparin-coated cardiopulmonary circuits has not been achieved, and the degree of inhibition obtained has varied from report to report.8 32-34 One reason the heparin coatings should not be expected to inhibit complement activation completely is that the activation that takes place on the gas surface cannot be inhibited by surface-conjugated heparin. The results of this study suggest that the addition of a complement inhibitor could further improve the biocompatibility. Compstatin, which is easy to manufacture, is a possible candidate for such an inexpensive inhibitor.
The platelet count dropped by 24% during incubation for 60 minutes in the loop model in the absence of peptide because of binding of platelets to the polymer surface. This drop was not affected by Compstatin at concentrations up to 250 μmol/L. Similarly, the leukocyte and erythrocyte counts were not affected, which suggests that Compstatin was not toxic to blood cells at the concentrations used in this study. The fact that the dose of Compstatin needed for inhibition of complement activation in serum is similar to the one in whole blood14 indicates that Compstatin is not accumulated in blood cells. Thus, combined with the high affinity for native C3, the steady-state concentration in vivo is likely to be close to the concentration of circulating C3, ie, 5.4 μmol/L. In that case, the concentration of free Compstatin would approach the therapeutic range of another cyclic synthetic peptide, cyclosporin, which has an upper therapeutic level of approximately 0.5 μmol/L.
Soluble CR1 and other recombinant complement inhibitors have been shown to inhibit complement activation in vivo in a variety of animal models.35,36 Such drugs will be instrumental in alleviating complement-dependent injury and inflammation in a variety of acute and chronic inflammatory and autoimmune disorders. Some of these belong to the hematological field, ie, different types of hemolysis including paroxysmal nocturnal hemoglobinuria.37 38 A further step forward will be the introduction of an anticomplement drug for oral use. Compstatin produces biological effects that are very similar to those of sCR1. Unlike the recombinant proteins that can only be administered parentally, Compstatin is a markedly less complex molecule that has the potential to be given orally.
ACKNOWLEDGMENT
We acknowledge the expertise of Dr Jerker Westin who performed the BiaCore analyses. We also thank Lynn Spruce for peptide synthesis and Dr Deborah McClellan for editorial assistance.
Supported by the Swedish Board for Industrial and Technical Development; the Göran Gustavsson Foundation; the Swedish Medical Research Council, Grant Nos. 5647 and 11578; and National Institutes of Health Grant AI 30040 to J.D.L.; and Cancer and Diabetes Centers Core Support Grants CA 16520 and DK 19525.
Address reprint requests to Prof John D. Lambris, Laboratory of Protein Chemistry, The Department of Pathology and Laboratory Medicine, University of Pennsylvania, 410 Johnson Pavilion, Philadelphia, PA 19104; e-mail: lambris@mail.med.upenn.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.