Abstract
CD28 is a major coreceptor that regulates cell proliferation, anergy, and viability of T cells. The negative selection by T-cell receptor (TCR)-induced cell death of immature thymocytes as well as of activated human antigen-specific T-cell clone, requires a costimulatory signal that can be provided by CD28. Conversely, CD28-mediated signals increase expression of Bcl-XL, a survival gene, and promote survival of naive T cells cultured in the absence of antigen or costimulation. Because CD28 appears to both protect from, or induce T-cell death, one important question is to define the activation and cellular parameters that dictate the differential role of CD28 in T-cell apoptosis. Here, we compared different CD28 ligands for their ability to regulate TCR-induced cell death of a murine T-cell hybridoma. In these cells, TCR triggering induced expression of Fas and FasL, and cell death was prevented by anti-Fas blocking monoclonal antibody (MoAb). When provided as a costimulus, both CD28 MoAb and the B7.1 and B7.2 counter receptors downregulated, yet did not completely abolish T-cell receptor–induced apoptosis. This CD28 cosignal resulted in both upregulation of Bcl-XL and prevention of FasL expression. In marked contrast, when given as a single signal, CD28 MoAb or B7.1 and B7.2 induced FasL expression and resulted in T-cell death by apoptosis, which was dependent on the level of CD28 ligation. Furthermore, triggering of CD28 upregulated FasL and induced a marked T-cell death of previously activated normal peripheral T cells. Our results identify Fas and FasL as crucial targets of CD28 in T-cell death regulation and show that within the same cell population, depending on its engagement as a single signal or as a costimulus together with the TCR, CD28 can either induce a dose-dependent death signal or protect from cell death, respectively. These data provide important insights into the role of CD28 in T-cell homeostasis and its possible implication in neoplastic disorders.
© 1998 by The American Society of Hematology.
LYMPHOID DEVELOPMENT and cell mass equilibrium kinetics are determined by cell proliferation and differentiation processes, but also by cell death. Apoptosis is an active form of cell death characterized on the basis of morphological alterations, including chromatin condensation and DNA fragmentation.1 During thymic development, the elimination of self-reactive immature T cells through a mechanism called clonal deletion is ensured via apoptosis.2-4 Apoptosis is also implicated in the maintenance of self-tolerance in the peripheral immune system.5 Apoptotic signals implicate at least two families of effectors: cytoplasmic proteases related to the interleukin-1β (IL-1β)–converting enzyme (ICE)6 and the Bcl2 family, a set of interacting proteins with death-promoting as well as death-inhibiting properties.7 ICE-family proteases are likely to act on a number of downstream elements leading to multiple damage pathways, such as activation of endogenous endonucleases responsible for internucleosomal DNA fragmentation.8-10 Conversely, expression levels together with post-translational modifications of Bcl2-related proteins dictate cell survival.11,12 Bcl2 and Bcl-XL are potent inhibitors of apoptosis13 and compete in vivo with death agonists, Bax or Bad, through heterodimerization12,14and/or independent function.15 The recent identification of new related members of this family has added to the complexity of the system.16 17
Both the thymic and peripheral deletion of T cells rely on the T cell receptor/CD3 complex (TCR). Antibodies to the TCR complex induce apoptosis in immature T cells in thymic cultures.18 TCR triggering by CD3 monoclonal antibodies (MoAbs) or by superantigens of previously activated mature T cells induce apoptosis by activation-induced cell death (AICD).19-23 AICD results from the induction of the Fas/Fas ligand (FasL) pathway.24-26 Fas (CD95) is a member of the tumor necrosis factor receptor (TNFR) superfamily.27 FasL expression is induced upon CD3 crosslinking, which also upregulates Fas. Oligomerization of Fas by FasL initiates a cascade of protease activation that results in cell death.28,29 Induction of Fas-FasL–dependent AICD can be mimicked by CD3 triggering of T-cell hybridomas.30 Recently, the role of Fas in clonal deletion has been debated,31 32 suggesting that multiple members of the TNFR superfamily might be involved.
The full activation of mature T cells is controlled by antigen-presenting cells and requires costimulatory signals in addition to the TCR engagement by the peptide/major histocompatibility complex. CD28 is one such major coreceptor that functions to increase cytokines and cytokine receptor induction through both transcriptional and post-transcriptional mechanisms.33-35 Clonal deletion of immature T cells in the thymus also requires costimulatory signals, which can be provided by CD28.36,37 Hence, CD28 was proposed to play an important role in negative selection of immature T cells. Conversely, several recent reports showed that CD28-mediated signals increase expression of Bcl-XL and promote survival of TCR-activated T cells.38-40 CD28 was also found to prevent apoptosis triggered upon TNFR-2 crosslinking.41 Thus, CD28 appears to act both as a protector or activator of apoptosis, but the mechanism of this differential function in apoptosis remains to be defined.
To analyze how CD28 can differently regulate T-cell death, we used a previously described murine T-cell hybridoma stably transfected by the human CD28 cDNA.42 Antigen-induced cell death can be mimicked by CD3 triggering in this experimental system,30,43 which also expresses a functional CD28 molecule.42 43 We show that both CD28 MoAb or its major ligands B7.1 and B7.2 can trigger a Fas-dependent death-signaling pathway, whereas after CD3 stimulation, CD28 signals downregulate T-cell death by increased Bcl-XL and reduced FasL expression.
MATERIALS AND METHODS
Materials.
The CD28 (248; CD28.2) murine CD3ε (145-2C11), human CD3 (289) and CD5 (C11E.4) MoAbs have been described previously44 and were used at 1/200 ascite dilution or 50 ng/mL for the 248 and 145-2C11 MoAbs, respectively, and at 25 μg/mL for the others. The Fas MoAb (clone Jo2) was obtained from Pharmingen (Cliniscience, France), and the FasL and Bcl-XL, BcL2, and poly (adenine diphosphate [ADP]-ribose) polymerase (PARP) polyclonal antisera were purchased from Transduction Laboratories (Interchim, France), Santa Cruz (Tebu, France), and Boehringer Mannheim (Mannheim, Germany), respectively. The goat antimouse Ig is a polyclonal antiserum and was used as a 1/50 dilution. The CTLA4 Ig fusion protein is a kind gift of P. Linsley (Bristol Myers Squibb, Seattle, WA) and was used at 10 μg/mL.
Cells and cell cultures.
L cells and L cells stably transfected with B7.1 (LB7.1) or B7.2 (LB7.2) cDNAs were previously described.45 DWT6.11 is a previously described murine hybridoma T-cell clone derived by transfecting the human CD28 gene into the DC27.1 hybridoma.42 These cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum (FCS) and sodium pyruvate (1 mmol/L). Peripheral blood mononuclear cells were obtained from normal blood donors, heparinized, washed in phosphate-buffered saline (PBS) and resuspended in RPMI supplemented with 10% FCS.
Cell death assays were conducted with 5 × 105cells/well of a 24-well plate in 1 mL total volume. For DNA or protein isolation, cells were cultured in 12-well plates at 1 × 106 cells/well in 2 mL total volume.
Detection of apoptosis.
Apoptosis was evaluated combining flow cytometry and DNA fragmentation analysis by electrophoresis.
In parallel with trypan blue staining experiments, cell loss was determined by quantitative analysis by flow cytometry to measure the percentage of subdiploid DNA after propidium iodine staining, as previously described.43 46 Briefly, the DNA content of cell nuclei was determined with propidium iodide staining and a FACScan cytometer using the Lysis II software (Becton Dickinson, Mountain View, CA).
DNA was extracted as previously described.47 Briefly, cells were washed in PBS, pelleted, and then resuspended in 40 μL of 0.2 mol/L Na2HPO4/0.1 mol/L citric acid (192:8) at room temperature for 60 minutes. After centrifugation at 2,000gfor 30 minutes, supernatants were transferred to new Eppendorf tubes and 3 μL of 0.25% NP-40 in distilled water was added followed by 3 μL of RNAse A (10 mg/mL). After incubation at 37°C for 60 minutes, 3 μL of proteinase K (10 mg/mL) was added and samples were incubated for 30 minutes at 50°C, after which 5 μL of loading buffer (0.25% bromophenol blue, 40% glycerol) was added, and aliquots were loaded onto a 1% agarose gel containing 5 μg/mL of ethidium bromide. After electrophoresis, DNA was visualized under ultraviolet (UV) light.
For Hoechst 33258 (Sigma, Saint Quentin Fallavier, France) staining, cells were washed in PBS, fixed and permeabilized simultaneously in 2% paraformaldehyde, plated on glass coverslips, air dried, washed in PBS, incubated for 5 minutes in 1 μg/mL Hoechst, and washed twice in PBS. Coverslips were mounted in Mowiol and analyzed by using a Leica TCS 4D confocal microscope (Leica, Heidelberg, Germany).
Polymerase chain reaction (PCR) analysis and primers.
Total RNA was extracted by the single-step method using Trizol reagent (GIBCO BRL, Life Technologies, Cergy Pontoise, France) as previously described.48 Briefly, RNA (1 μg) was reverse transcribed into cDNA by using an oligodT primer (Promega, Charbonnierés, France) and Moloney Murine Leukemia Virus (M-MLV) reverse-transcriptase (GIBCO BRL, Life Technologies) in a total volume of 20 μL. First strand cDNA was diluted by addition of 80 μL H2O and 2 μL of this dilution was used for PCR amplification. The PCR reaction was performed for 25 cycles for β2m and 33 cycles for Fas and FasL under standard conditions (preheating 5 minutes at 94°C, denaturation 50 seconds at 94°C, annealing 45 seconds at 65°C, extension 45 seconds at 72°C) in a final volume of 25 μL containing 10 mmol/L Tris-Hcl pH 8.3 at room temperature, 50 mmol/L KCL, 1.5 mmol/L MgCl2, 200 μmol/L of each dNTPs, 2.5 U Taq polymerase (GIBCO BRL, Life Technologies), and 25 ng of each amplification primers (Genset, Paris, France). After the amplification, 12.5 μL of the PCR products were run on a 1.5% agarose gel and stained with ethidium bromide. Integrated intensity of the specific band was determined by using the BioImage (Millipore Corp, Molshein, France). Oligonucleotide sequences were β2M: 5′ TGA CCG GCT TGT ATG CTA TC, 3′ CAG TGT GAG CCA GGA TAT AG; FAS: 5′ ATC CGA GCT CTG AGG AGG CGG GTT CAT GAA AC, 3′ GGA GGT TCT AGA TTC AGG GTC ATC CTG; FASL: 5′ CAG CTC TTC CAC CTG CAG AAG G, 3′ AGA TTC CTC AAA ATT GAT CAG AGA GAG;IL-2: 5′ GAC ACT TGT GCT CCT TGT CA; 3′ TCA ATT CTG TGG CCT GCT TG.
Western blot analysis.
For Western blot analysis, cells were washed with PBS and lysed in 1% Triton X-100, 50 mmol/L HEPES pH 7, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 10% glycerol, 1 mmol/L sodium orthovanadate, 100 mmol/L sodium fluoride, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 10 mmol/L dithiothreitol, 1 mmol/L phenylmethyl sulfonyl fluoride buffer, followed by centrifugation at 4°C (13,000g for 15 minutes). The amount of total proteins was determined by the Bradford method using a protein assay dye reagent (Bio-Rad, Iury Sur Seine, France). Equal protein amounts (20-50 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinyldifluoride (PVDF) membranes (Millipore), immunoblotted with specific antibodies, and visualized by chemiluminescence according to the manufacturer’s instructions (Amersham, Arlington Heights, IL).
RESULTS
CD28 can both induce T-cell death or reduce CD3-triggered cell death.
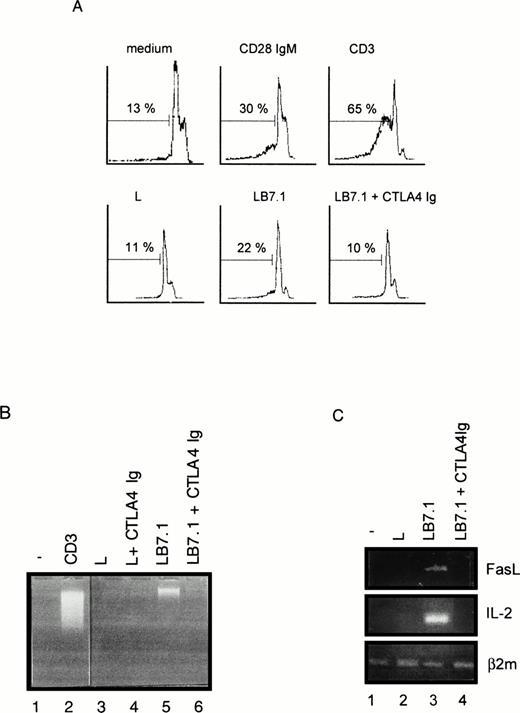
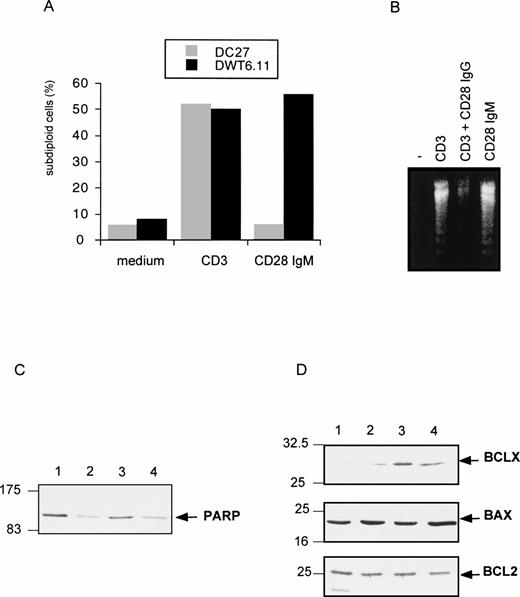
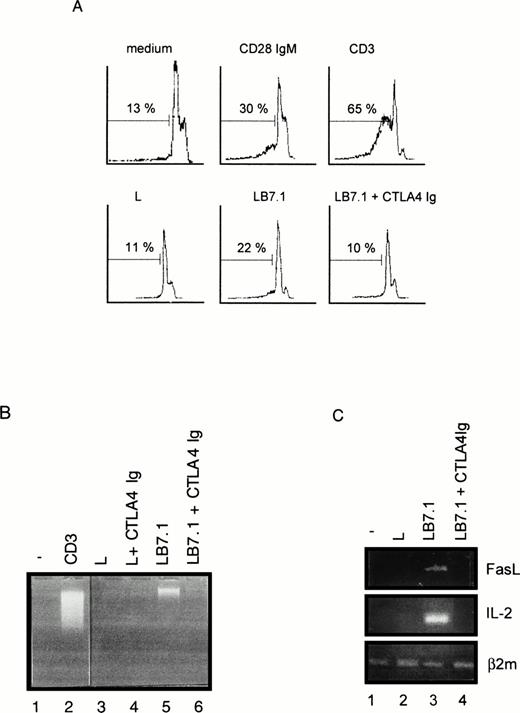
To investigate the regulation of T-cell apoptosis by CD28 we used DWT6.11, a murine T-cell hybridoma stably transfected with the human CD28 cDNA.42 Cell death was assessed by propidium iodide staining of permeabilized cells followed by cytofluorometry. The subdiploid cell population reflected cells undergoing DNA fragmentation and apoptosis.46 As shown in Fig 1A and B, CD3 MoAb induced rapid cell death compared with untreated cells or with cells treated with an irrelevant isotype-matched control MoAb (not shown), hence confirming previous reports.43 49-51Costimulation with the CD28 IgG MoAb but not with the irrelevant isotype-matched control MoAb significantly reduced, yet did not completely abolish, CD3-triggered cell death. Surprisingly, when DWT6.11 cells were incubated with the CD28.2 IgG MoAb alone, a low but reproducible cell death was observed (Fig 1A, B, and 2), and the CD28 IgM MoAb induced cell death to the same extent as the CD3 MoAb (Fig 1A, B, and 3A). Also, rather than reducing cell death, the CD28 IgM MoAb further increased CD3-induced cell death (Fig 1B). When triggered by the CD28 IgM, the parental untransfected DC27 hybridoma cell line retained a similar viability as untreated cells (Fig 3A), indicating that cell death induced by this MoAb was strictly dependent on CD28 expression. Cell death induced on CD28 triggering by the IgM MoAb was similar to that seen in CD3-treated cultures and resulted in the typical morphological alterations seen in apoptotic cells (eg, membrane blebbing and disintegration of cells and nuclei into small vesicles). Cell death and changes in morphology coincided with the DNA fragmentation detected in cytoplasmic preparations (Fig 3B) and appeared as soon as 6 hours after treatment of DWT6.11 cells with CD3 or CD28 IgM MoAb (Fig 3B). When combined with CD3 MoAbs, CD28 IgG MoAbs significantly reduced DNA fragmentation. In marked contrast, the CD28 IgM induced DNA fragmentation on its own (Fig 3B), and further increased CD3-induced DNA fragmentation (not shown).
Flow cytometric analysis of a PI-stained CD28 transfected murine T-cell hybridoma. DWT6.11 cells were seeded for 8 hours in a 24-well plate in the presence of the indicated MoAbs as described in the Materials and Methods. (A) PI staining versus the number of nuclei in one representative experiment. Numbers above histograms indicate the percentage of apoptotic nuclei. (B) Results are presented as averaged percentage (±standard deviation [SD]) of subdiploid nuclei obtained from six independent experiments.
Flow cytometric analysis of a PI-stained CD28 transfected murine T-cell hybridoma. DWT6.11 cells were seeded for 8 hours in a 24-well plate in the presence of the indicated MoAbs as described in the Materials and Methods. (A) PI staining versus the number of nuclei in one representative experiment. Numbers above histograms indicate the percentage of apoptotic nuclei. (B) Results are presented as averaged percentage (±standard deviation [SD]) of subdiploid nuclei obtained from six independent experiments.
Cell death (A), DNA fragmentation (B), and FasL mRNA expression (C) are increased on CD28 crosslinking. (A) DWT6.11 cells were seeded for 12 hours in a 24-well plate in the presence of the indicated MoAbs and PI stained followed by cytofluorometry as described in the Materials and Methods. The results are presented as averaged percentage of subdiploid nuclei obtained from three independent experiments (±SD). (B) DNA was extracted from the cells collected at 8 hours of incubation and analyzed on a 1% agarose gel containing ethidium bromide.(C) The cells were collected after a 6-hour incubation period, followed by mRNA extraction, reverse transcription, and PCR using FasL, IL-2, or β2 microglobulin primer pairs as described in the Materials and Methods. Amplification products were analyzed on a 1% agarose gel followed by ethidium bromide staining. Integrated intensity of the specific band was determined using the BioImage (Millipore Corp). Results were normalized to the relative levels of β2m and are presented as histograms. One representative experiment out of two is shown. Where indicated, the X symbol indicates that goat antimouse polyclonal antiserum was added.
Cell death (A), DNA fragmentation (B), and FasL mRNA expression (C) are increased on CD28 crosslinking. (A) DWT6.11 cells were seeded for 12 hours in a 24-well plate in the presence of the indicated MoAbs and PI stained followed by cytofluorometry as described in the Materials and Methods. The results are presented as averaged percentage of subdiploid nuclei obtained from three independent experiments (±SD). (B) DNA was extracted from the cells collected at 8 hours of incubation and analyzed on a 1% agarose gel containing ethidium bromide.(C) The cells were collected after a 6-hour incubation period, followed by mRNA extraction, reverse transcription, and PCR using FasL, IL-2, or β2 microglobulin primer pairs as described in the Materials and Methods. Amplification products were analyzed on a 1% agarose gel followed by ethidium bromide staining. Integrated intensity of the specific band was determined using the BioImage (Millipore Corp). Results were normalized to the relative levels of β2m and are presented as histograms. One representative experiment out of two is shown. Where indicated, the X symbol indicates that goat antimouse polyclonal antiserum was added.
CD28 IgM MoAb induces a CD28-dependent cell death (A), with DNA fragmentation (B), PARP downregulation (C), and BclXL upregulation (D). The untransfected (DC27) and CD28 stably transfected (DWT6.11) murine hybridoma T cells were treated as described in Fig 1. After an 8-hour incubation period, cells were harvested, PI stained, and analyzed by cytofluorometry (A), or DNA from DWT6.11 cells was extracted followed by electrophoretic analysis on a 1% agarose gel stained with ethidium bromide (B), or proteins were extracted followed by SDS-PAGE fractionation and immunoblotting of the same membrane with the indicated polyclonal serum, successively (C and D): lane 1, untreated cells; lane 2, anti-CD3 plus anti-CD5; lane 3, anti-CD3 plus anti-CD28 IgG; and lane 4 anti-CD28 IgM alone. Results are representative of at least three independent experiments.
CD28 IgM MoAb induces a CD28-dependent cell death (A), with DNA fragmentation (B), PARP downregulation (C), and BclXL upregulation (D). The untransfected (DC27) and CD28 stably transfected (DWT6.11) murine hybridoma T cells were treated as described in Fig 1. After an 8-hour incubation period, cells were harvested, PI stained, and analyzed by cytofluorometry (A), or DNA from DWT6.11 cells was extracted followed by electrophoretic analysis on a 1% agarose gel stained with ethidium bromide (B), or proteins were extracted followed by SDS-PAGE fractionation and immunoblotting of the same membrane with the indicated polyclonal serum, successively (C and D): lane 1, untreated cells; lane 2, anti-CD3 plus anti-CD5; lane 3, anti-CD3 plus anti-CD28 IgG; and lane 4 anti-CD28 IgM alone. Results are representative of at least three independent experiments.
CD28 regulates Fas-mediated programmed cell death.
We next investigated the expression and function of effectors known to regulate T-cell apoptosis. The PARP is a substrate for the cysteine protease CPP32 or caspase-3,52,53 one of the various downstream cysteine protease effectors triggered after activation-induced cell death.8 Immunoblotting experiments showed that PARP protein level was severely downregulated after stimulation with CD3 MoAb but not on costimulation with CD28 IgG MoAb (Fig 3C). In contrast, the CD28 IgM MoAb induced a clear downregulation of PARP (Fig 3C). CD28 was previously proposed to rescue from CD3-induced apoptosis by upregulation of the survival factor Bcl-XL.38 Indeed, CD28 IgG together with CD3 MoAbs induced a marked upregulation of this factor without any significant impact on either BCL2 or Bax expression (Fig 3D). However, the CD28 IgM MoAb, on its own, significantly upregulated Bcl-XL (Fig 3D), indicating that Bcl-XLupregulation was not sufficient for cell survival. Indeed, we have shown previously43 that the CD28 IgG MoAb could reduce CD3-triggered apoptosis in absence of a significant increase of Bcl-XL. Rather, as shown in Fig 4A by reverse transcriptase-polymerase chain reaction (RT-PCR) determination, the CD28 IgG MoAb repressed induction of FasL expression by CD3, while it augmented IL-2 expression. In parallel, the CD28 IgM MoAb induced an early upregulation of FasL and IL-2 expression (Fig 4A). To investigate the functional implication of FasL upregulation in CD28-triggered cell death, cells were preincubated in the presence of a Fas-blocking MoAb before induction by the CD28 IgM MoAb. Figure 4B shows that the Fas MoAb blocked most of the CD28-induced cell death signaling, indicating that CD28 can trigger a Fas-dependent cell death.
Expression of FasL and IL-2 mRNA after CD28 and/or CD3 stimulation (A) and the role of Fas in CD28-induced cell death (B). (A) DWT6.11 cells were left untreated (lane 1) or were incubated in the presence of CD3 plus CD5 (lane 2), CD3 plus CD28 IgG (lane 3), or CD28 IgM alone (lane 4) for 2 and 6 hours as indicated. After these incubation periods, cells were harvested and mRNA was extracted, reverse transcribed, and analyzed by PCR using FasL, IL-2, or β2 microglobulin primer pairs as described in the Materials and Methods. Amplification products were analyzed on a 1% agarose gel, followed by ethidium bromide staining. (B) DWT6.11 cells were incubated in the presence of the indicated amount of the Fas blocking MoAb (Jo2), and either left untreated or treated with the CD3 and CD28 IgM MoAb. After an 8-hour incubation period, cells were PI stained followed by cytofluorometry. Results from a representative out of three independent experiments are shown.
Expression of FasL and IL-2 mRNA after CD28 and/or CD3 stimulation (A) and the role of Fas in CD28-induced cell death (B). (A) DWT6.11 cells were left untreated (lane 1) or were incubated in the presence of CD3 plus CD5 (lane 2), CD3 plus CD28 IgG (lane 3), or CD28 IgM alone (lane 4) for 2 and 6 hours as indicated. After these incubation periods, cells were harvested and mRNA was extracted, reverse transcribed, and analyzed by PCR using FasL, IL-2, or β2 microglobulin primer pairs as described in the Materials and Methods. Amplification products were analyzed on a 1% agarose gel, followed by ethidium bromide staining. (B) DWT6.11 cells were incubated in the presence of the indicated amount of the Fas blocking MoAb (Jo2), and either left untreated or treated with the CD3 and CD28 IgM MoAb. After an 8-hour incubation period, cells were PI stained followed by cytofluorometry. Results from a representative out of three independent experiments are shown.
Collectively, these data show that the Fas-FasL pathway represent an important target of CD28 signaling, being more or less involved depending on cell stimulation conditions.
CD28-mediated T-cell death is regulated by increased ligation.
Because the CD28 IgM MoAb induced a more pronounced cell death signal than the CD28 IgG (Fig 1 and 2A), we hypothesized that this cell death process was dependent on the CD28 aggregation level. To test this hypothesis, cells were treated with the CD28 IgG MoAb followed by an Ig goat antiserum. The CD28 IgG induced a significant cell death compared with the CD5 control MoAb, which was enhanced further by the goat antiserum (Fig 2A). Neither crosslinking the CD5 control antibody nor using the Ig goat antiserum alone affected cell viability. Concurrently with the determination of cell death by propidium iodide staining of permeabilized cells, cytoplasmic DNA was extracted from cells treated with these various antibodies, and analyzed by electrophoresis (Fig2B), confirming that the CD28 IgG antibody induces a slight DNA fragmentation that was increased on crosslinking by the goat antiserum. Also, the CD28 IgG MoAb induced a barely detectable level of FasL expression, which was markedly upregulated after crosslinking by the Ig goat antiserum (Fig 2C). We concluded that the degree of CD28 aggregation could contribute to determine the extent of cell death induced on activation.
B7.1 and B7.2 can induce FasL expression and cell death, but can reduce T-cell death in costimulation with CD3.
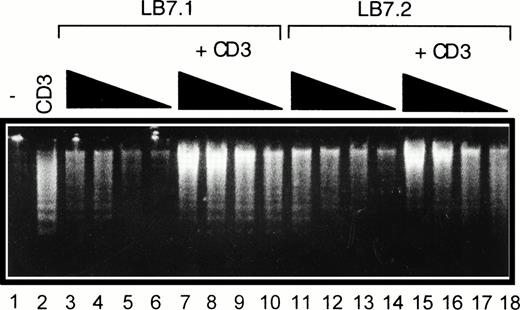
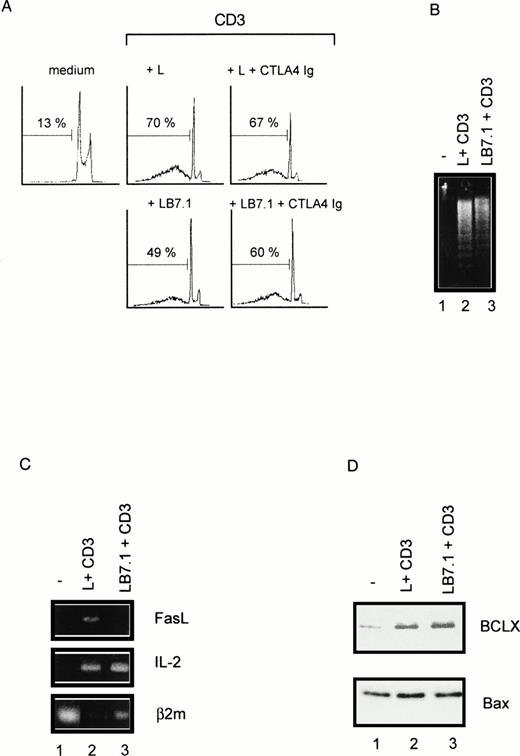
We have previously reported that CD28 signaling was differently induced on triggering with CD28 IgG MoAb or with its ligand B7-1.45Thus, we determined the ability of L cells stably expressing a transfected B7-1 cDNA to trigger cell death. As shown in Fig 5A, significant cell death was found on coculture of DWT6.11 with LB7.1 cells compared with untreated cells or cells cocultured in the presence of L cells. This signal was inhibited in the presence of a recombinant CTLA4 Ig fusion protein, which displays high affinity for B7.1 and B7.2 and prevents the CD28/B7.1 interaction, which indicated that cell death was specifically triggered by B7.1-dependent signals (Fig 5A). Similarly, electrophoretic analysis showed the presence of cytoplasmic fragmented DNA in cells cocultured with LB7.1 (Fig 5B) but not L cells, which was prevented by the presence of CTLA4Ig in the cell culture. The extent of cytoplasmic DNA fragmentation was augmented by increasing the number of LB7.1- or LB7.2-stimulating cells (Fig 6). RT-PCR experiments also showed that LB7.1 but not L cells upregulated Fas and FasL expression, which was prevented by pretreating the cells with CTLA4Ig (Fig 5C). Together, these results showed that the CD28 ligand B7.1 and B7.2 can trigger T-cell death.
Cell death (A), DNA fragmentation (B), and FasL mRNA (C) are induced by B7.1 expressing cells. (A) DWT6.11 cells were seeded for 12 hours in a 24-well plate in the presence of MoAbs or in the presence of either L cells (L) or L cells expressing a transfected B7.1 cDNA (LB7.1), as indicated, with a 3:1 (DWT6.11:L) cell ratio. After this incubation period, cells were collected and PI stained followed by cytofluorometry as described in the Materials and Methods. PI staining versus the number of nuclei is shown. Numbers above histograms indicate the percentage of apoptotic nuclei. (B) DNA was extracted from the collected cells at 8 hours of incubation and analyzed onto a 1% agarose gel containing ethidium bromide. (C) mRNA was extracted from cells collected at 6 hours of incubation, followed by reverse transcription and PCR using FasL, IL-2, or β2 microglobulin primer pairs as described in the Materials and Methods. Amplification products were analyzed on a 1% agarose gel, followed by ethidium bromide staining. The results from one representative experiment out of three are shown. Where indicated, the CTLA4 Ig recombinant protein was added 15 minutes before treatment.
Cell death (A), DNA fragmentation (B), and FasL mRNA (C) are induced by B7.1 expressing cells. (A) DWT6.11 cells were seeded for 12 hours in a 24-well plate in the presence of MoAbs or in the presence of either L cells (L) or L cells expressing a transfected B7.1 cDNA (LB7.1), as indicated, with a 3:1 (DWT6.11:L) cell ratio. After this incubation period, cells were collected and PI stained followed by cytofluorometry as described in the Materials and Methods. PI staining versus the number of nuclei is shown. Numbers above histograms indicate the percentage of apoptotic nuclei. (B) DNA was extracted from the collected cells at 8 hours of incubation and analyzed onto a 1% agarose gel containing ethidium bromide. (C) mRNA was extracted from cells collected at 6 hours of incubation, followed by reverse transcription and PCR using FasL, IL-2, or β2 microglobulin primer pairs as described in the Materials and Methods. Amplification products were analyzed on a 1% agarose gel, followed by ethidium bromide staining. The results from one representative experiment out of three are shown. Where indicated, the CTLA4 Ig recombinant protein was added 15 minutes before treatment.
Regulation of DNA fragmentation in a CD28-transfected murine T-cell hybridoma by B7.1- or B7.2-expressing cells. DWT6.11 cells were seeded for 8 hours in a 24-well plate in the presence or absence of CD3 MoAb and/or of L cells expressing a transfected B7.1 cDNA (LB7.1) or B7.2 cDNA (LB7.2), as indicated. After this incubation period, DNA was extracted and analyzed on a 1% agarose gel containing ethidium bromide. Lane 1, untreated cells; lane 2, anti-CD3; lanes 3 to 6, LB7.1 cells alone at the DWT6.11:LB7.1 cell ratio 2:1, 4:1, 10:1, and 20:1, respectively; lanes 7 to 10, plus anti-CD3; lane 11 to 14, LB7.2 cells alone at the DWT6.11:LB7.2 cell ratio 2:1, 4:1, 10:1, and 20:1, respectively; and lanes 15 to 18, plus anti-CD3.
Regulation of DNA fragmentation in a CD28-transfected murine T-cell hybridoma by B7.1- or B7.2-expressing cells. DWT6.11 cells were seeded for 8 hours in a 24-well plate in the presence or absence of CD3 MoAb and/or of L cells expressing a transfected B7.1 cDNA (LB7.1) or B7.2 cDNA (LB7.2), as indicated. After this incubation period, DNA was extracted and analyzed on a 1% agarose gel containing ethidium bromide. Lane 1, untreated cells; lane 2, anti-CD3; lanes 3 to 6, LB7.1 cells alone at the DWT6.11:LB7.1 cell ratio 2:1, 4:1, 10:1, and 20:1, respectively; lanes 7 to 10, plus anti-CD3; lane 11 to 14, LB7.2 cells alone at the DWT6.11:LB7.2 cell ratio 2:1, 4:1, 10:1, and 20:1, respectively; and lanes 15 to 18, plus anti-CD3.
We investigated whether B7.1 and B7.2 regulate CD3-triggered cell death. As shown in Fig 7, LB7.1 cells reduced (yet not completely) cell death induced by CD3 triggering, which was associated with reduced DNA fragmentation (Fig 7B), in a dose-dependent manner (Fig 6), downregulation of FasL expression (Fig 7C), and upregulation of Bcl-XL.(Fig 7D).
B7.1-expressing cells can downregulate CD3-triggered cell death (A), DNA fragmentation (B), FasL mRNA induction (C), and BclXL upregulation (D). (A) DWT6.11 cells were seeded for 12 hours in a 24-well plate in the presence of MoAbs or in the presence of either L cells (L) or L cells expressing a transfected B7.1 cDNA (LB7.1), as indicated, with a 3:1 (DWT6.11:L) cell ratio. After this incubation period cells were collected and PI stained followed by cytofluorometry as described in the Materials and Methods. PI staining versus the number of nuclei is shown. Numbers above histograms indicate the percentage of apoptotic nuclei. Where indicated, the CTLA4Ig recombinant protein was added 15 minutes before treatment. (B) DNA was extracted from cells collected at 8 hours of incubation and analyzed on a 1% agarose gel containing ethidium bromide. (C) mRNA was extracted from cells collected at 6 hours of incubation, followed by reverse transcription and PCR using FasL, IL-2, or β2 microglobulin primer pairs as described in the Materials and Methods. Amplification products were analyzed on a 1% agarose gel, followed by ethidium bromide staining. (D) Proteins were extracted followed by SDS-PAGE fractionation and Western blotting with the indicated polyclonal serum. Results are representative of at least three independent experiments.
B7.1-expressing cells can downregulate CD3-triggered cell death (A), DNA fragmentation (B), FasL mRNA induction (C), and BclXL upregulation (D). (A) DWT6.11 cells were seeded for 12 hours in a 24-well plate in the presence of MoAbs or in the presence of either L cells (L) or L cells expressing a transfected B7.1 cDNA (LB7.1), as indicated, with a 3:1 (DWT6.11:L) cell ratio. After this incubation period cells were collected and PI stained followed by cytofluorometry as described in the Materials and Methods. PI staining versus the number of nuclei is shown. Numbers above histograms indicate the percentage of apoptotic nuclei. Where indicated, the CTLA4Ig recombinant protein was added 15 minutes before treatment. (B) DNA was extracted from cells collected at 8 hours of incubation and analyzed on a 1% agarose gel containing ethidium bromide. (C) mRNA was extracted from cells collected at 6 hours of incubation, followed by reverse transcription and PCR using FasL, IL-2, or β2 microglobulin primer pairs as described in the Materials and Methods. Amplification products were analyzed on a 1% agarose gel, followed by ethidium bromide staining. (D) Proteins were extracted followed by SDS-PAGE fractionation and Western blotting with the indicated polyclonal serum. Results are representative of at least three independent experiments.
Collectively, these data show that when provided as a single signal, B7.1 and B7.2 can trigger T-cell death through upregulation of FasL similarly to CD28 MoAb; but on costimulation, triggering of CD28 by its counter receptors augments cell survival factors, such as Bcl-XL, and downregulates both FasL expression and cell death. Also, the extent of cell death may depend on the level of CD28 engagement.
CD28 can induce FasL expression and cell death of previously activated normal peripheral T cells.
To confirm in normal peripheral T cells the observation that CD28 can promote a cell death signaling pathway, blood samples from normal donors were incubated in the presence of phytohemagglutinin (PHA) followed by stimulation with CD3 or CD28 IgM MoAbs. Cell death was evaluated at day 4 and day 5 post-PHA treatment. After a 48-hour stimulation, both the CD3 and the CD28 IgM antibodies induced a marked cell death of day-4 and day-5 PHA-activated cells (Fig 8A). Interestingly, only the CD3 antibody induced a significant cell death after a 24-hour stimulation. Aliquots from day 5 PHA-treated cells were further analyzed by Hoechst staining and confocal microscope visualization of dense and/or fragmented nuclei (Fig 8B, C, and D). Cell cultures stimulated for 48 hours with the CD3 (Fig 8C) or the CD28 IgM (Fig 8D) antibodies displayed typical apoptotic dense/fragmented nuclei compared with unstimulated day-5 PHA-treated cells (Fig 8B). Finally, we determined FasL expression in PHA-treated cell cultures by Western blotting. Compared with unstimulated cells, both the CD3 and the CD28 IgM antibodies markedly upregulated FasL expression following a 24-hour stimulation of day-5 PHA-activated cells (Fig 8E, compare lanes 4 and 5 with lane 1). Upregulation of FasL was also seen in day-3 PHA-treated cell cultures stimulated for 72 hours (Fig 8E). Together, these experiments show that CD28 can both upregulate FasL expression and trigger cell death in normal T-cell cultures.
Induction of cell death in normal peripheral human T cells. Normal T cells were cultured with PHA (1 μg/mL) for 3 to 5 days as indicated (D3, D4, and D5), followed by stimulation with the CD3 and the CD28 IgM antibodies. (A) PHA-treated cells were seeded for 24 and 48 hours in a 24-well plate in the presence of the indicated antibodies. After this incubation period, cells were collected and determined for cell death as described in the Materials and Methods. Day-5 PHA-treated cells (B), day-5 PHA-treated cells stimulated with the CD3 (C), or the CD28 IgM antibody (D) for 48 hours, were stained with Hoechst and fixed in 2% paraformaldehyde. Shown are representative confocal images. White arrows indicate condensed and fragmented nuclei. (E) Proteins were extracted from day-2 PHA-treated cells stimulated for 72 hours with CD3 (lane 2) or CD28 IgM (lane 3) antibodies, or from day-5 PHA-treated cells left unstimulated (lane 1) or stimulated for 24 hours with CD3 (lane 4) and CD28 IgM (lane 5) antibodies. Extracted proteins were fractionated by SDS-PAGE and immunoblotted with the FasL MoAb.
Induction of cell death in normal peripheral human T cells. Normal T cells were cultured with PHA (1 μg/mL) for 3 to 5 days as indicated (D3, D4, and D5), followed by stimulation with the CD3 and the CD28 IgM antibodies. (A) PHA-treated cells were seeded for 24 and 48 hours in a 24-well plate in the presence of the indicated antibodies. After this incubation period, cells were collected and determined for cell death as described in the Materials and Methods. Day-5 PHA-treated cells (B), day-5 PHA-treated cells stimulated with the CD3 (C), or the CD28 IgM antibody (D) for 48 hours, were stained with Hoechst and fixed in 2% paraformaldehyde. Shown are representative confocal images. White arrows indicate condensed and fragmented nuclei. (E) Proteins were extracted from day-2 PHA-treated cells stimulated for 72 hours with CD3 (lane 2) or CD28 IgM (lane 3) antibodies, or from day-5 PHA-treated cells left unstimulated (lane 1) or stimulated for 24 hours with CD3 (lane 4) and CD28 IgM (lane 5) antibodies. Extracted proteins were fractionated by SDS-PAGE and immunoblotted with the FasL MoAb.
DISCUSSION
The CD28 costimulatory molecule acts at multiple levels of T-cell differentiation, cell survival, proliferation, cytokine expression, and cytokine receptor expression.33-35 Its role in the regulation of cell survival has been recently emphasized.38Cell survival is of utmost importance for the regulation of thymocyte-negative selection as well as to maintain the number of lymphocytes throughout their life span. CD28 is one of the key regulators of these different functions. On one hand it plays an important role in the deletion of immature double positive thymocytes, thus participating in the negative selection process that will select mature T cells.36,37,54 On the other hand, in mature T cells, it regulates both the expansion of the antigen-activated T cells via cytokine-cytokine receptor expression and regulates cell survival by preventing cell death, at least at the initial stages of the immune response.38,55 The basis for these different functions are still unknown. Various candidates have been described that could explain its function in the regulation of cell survival and death. T-cell death in mature T cells is mainly induced by interaction between the TNF family such as Fas-L and TNF and TNFR family receptors.56 T-cell death in immature T cells is at least in part different. In this latter model, the events leading to cell death differ from mature T cells because Fas–Fas-L molecules are not involved and this apoptosis is resistant to PI3-K inhibitors as well as cyclosporine A.54
Signaling via the TCR regulates both the expression of Fas-L and TNF as well as the efficiency of Fas signaling in mediating apoptosis.57,58 CD28 costimulation prevents cell death during primary T-cell costimulation. This effect correlates with upregulation of two survival factors, BclXL and Bcl-2.38,55 CD28 costimulation regulates these two factors in two ways. On recruitment and activation of PI3-K it induces BclXL upregulation,43 and in addition, via the production of high quantities of IL-2 together with the upregulation of IL-2R α chain, CD28 contributes to the upregulation of Bcl-2.55 However, this initial resistance to apoptosis is followed by a subsequent sensitivity to AICD. At this stage CD28 directly, or after IL-2 production, could increase T-cell death via entry into cell cycle and increased susceptibility to Fas-dependent cell death.40 In the thymus, costimulation via CD28 is one of the most important pathways required for clonal deletion of immature CD4+CD8+ thymocytes.36,37,54 The understanding of the basis of the CD28 pathways involved in the prevention or induction of apoptosis are critical because this molecule is one of the candidate targets for cancer and acquired immunodeficiency syndrome (AIDS) immunotherapy.59,60Indeed, in some murine models of transplanted tumors B7.1 expression was shown as an efficient way to prevent tumor cell development through the induction of an immune response.61 62
In our report, we show that within the very same cell system we have identified at least two factors that can predict whether CD28 signaling will either prevent or induce apoptosis. As previously shown, one of the critical parameters for prevention of apoptosis is CD3-CD28 costimulation.38 Coengagement of CD3 and CD28 molecules with either MoAbs or LB7.1 cells (Figs 1 and 5) led to the inhibition of CD3-mediated apoptosis as shown by decreased quantities of subdiploid cells detected by propidium iodide labeling or degradation of one of caspase 3 substrates, namely PARP. These experiments suggest that one of the critical steps for inducing prevention of T-cell mediated apoptosis is the concurrent engagement of CD3-TCR and CD28 on the very same cell by their ligands on the antigen-presenting cells. Of note, in this model the B7.1 ligand is carried in trans by a transfected fibroblast. Coexpression of ligands binding to TCR and B7.1 on the same cell could even increase this function as reported in other models regarding costimulation of T-cell activation.63
By contrast, the sole engagement of CD3 or CD28 leads to apoptosis. These latter events are only triggered in this model at high levels of CD28-B7 interaction. We can conclude from the data reported previously that the induction of apoptosis mediated through CD28 depends on the amount of CD28 receptor engaged and the level of receptor crosslinking. Both CD28 major ligands, namely B7.1 and B7.2, elicit a dose-dependent apoptosis. The experiment shown in Fig 6 suggests that increasing the number of CD28 receptors engaged is important because the extent of apoptosis was detected in lanes 3 and 11, which correspond to the highest number of LB7.1 and LB7.2 cells added, respectively. However, this CD28 apoptosis is always less pronounced than CD3. Another argument regarding the role of CD28 aggregation is shown using CD28 MoAbs. CD28 IgG1 induces low levels of apoptosis on its own (Fig 2A) similar to the one induced by the ligands. However, crosslinking of the IgG1 MoAb or use of a CD28 IgM MoAb elicited a very robust apoptosis, which reached the same extent as the CD3 (Figs 2A, 8A, and 8D).
What are the likely effectors that are differently regulated after costimulation versus stimulation? Based on the current knowledge, the regulation of the amount of available molecules belonging to the ced-9/Bcl-2 family might be one of the key targets of CD28-mediated prevention of apoptosis. In fact CD28 costimulation induces BclXL upregulation, which is one of the major death suppressors.38 From our data we can first conclude that in this model BclXL upregulation is not sufficient to prevent apoptosis. Indeed, although CD3 and CD28 costimulation prevents apoptosis and induces BclXL expression (Fig 3D), CD28 IgM MoAb on its own induces apoptosis but at the same time induces robust expression of BclXL (Fig 3D). However, the role of Bcl-2 family in the prevention of cell death in the T-cell hybridoma is difficult to interpret because its apoptosis depends mostly on Fas–Fas-L molecules. In fact, the prevention of Fas-mediated apoptosis by the Bcl-2 family is supposed to depend on at least two factors. On one hand, it depends on the use by the Fas receptor of FADD or Daxx pathways,64 whereas only the latter is regulated by the Bcl-2 family. On the other hand, it was recently proposed that two types of cells exist where Fas-mediated apoptosis could be either inhibited (type II) or not (type I) by Bcl-2 family members (P. Krammer, personal communication, November 1997). Furthermore, we cannot exclude a direct role for CD28 in the prevention of Fas-mediated apoptosis because (1) CD28 costimulation prevents TNFR-2–triggered apoptosis41 and (2) post-transcriptional regulation of Fas-mediated signals via the TCR have recently been shown that suggest that other steps could be regulated, such as the molecules recruited by Fas intracytoplasmic domain.57 58
However, the only factor that we could find predictive of prevention versus induction of apoptosis in this model was Fas-L expression. As shown in Figs 2 and 4, stimulation by CD3 MoAbs, CD28 IgM MoAbs, or crosslinked CD28 IgG1 led to high levels of Fas-L expression. This was shown by the increased transcript levels as well as the prevention of apoptosis with blocking anti-Fas MoAbs in murine T-cell hybridoma, but also by Western blotting of normal human T-cell lysates (Fig 8E). In sharp contrast, costimulation with CD3 and CD28 MoAbs or LB7.1 cells led to both decreased apoptosis and decreased expression of Fas-L transcripts (Figs 4A, 5C, and 7C). Hence, at least two mechanisms could be used by CD28 costimulation to prevent apoptosis, the increase in survival factors such as BclXL (see above), and the decreased expression of TNF family members, such as Fas-L, that would inhibit the level of receptors required to induce Fas-dependent cell death. So far, three mechanisms have been described accounting for the regulation of Fas-L expression. Surface Fas-L is cleaved by metalloproteinases and released in the medium and binds to its cognate receptor to induce paracrine apoptosis.56 Alternatively, FasL may be trapped intracellularly, such as in herpes simplex virus type-2–infected cells.65 Another level of regulation is its increased transcription.66 Recent insights in the regulation of Fas-L expression and of the transcription factors involved in its regulation have been obtained. On stimulation of T lymphocytes via the TCR one can identify critical steps involved in its regulation that correspond to protein tyrosine kinases, small G proteins, and calcineurin. Hence, its expression is inhibited by immunosuppressive agents that regulate calcineurin activity such as cyclosporine A66 as well as tyrosine kinase inhibitors.66 p56Lck is one of the key protein tyrosine kinases regulating its expression because both its kinase and SH2 domain are required for optimal Fas-L expression in hybridoma cell lines and because mutant lacking Lck (Jcam-1) does not upregulate Fas-L on TCR triggering.67,68 The role of calcium in the upregulation of Fas-L expression has also been shown by cotransfection experiments with NFAT-c1 and by the identification of a functional NFAT site within its promoter.69 In addition, NFATp-deficient mice do not inducibly express Fas-L.70,71 Finally, a role for ras was shown in cotransfection experiments in which dominant negative ras inhibited TCR-induced Fas-L expression.69Other factors are likely regulating its expression because some non-T cells in immunoprivileged sites, such as the anterior eye chamber or testes, and some cancer cells constitutively express Fas-L.72 The basis for the prevention of Fas-L upregulation by CD28 costimulation are unknown so far. The functional dissection of its promoter will permit to monitor the in vivo modifications of the promoter occupancy after costimulation.
Hence, CD28 costimulation could downregulate Fas-L expression and thereby participate in the regulation of cell death. This function contrasts with the described costimulatory functions of CD28, which increases CD3-mediated cytokine and cytokine receptor expression.34,35,73 However, this is not the first example of receptors downmodulated on CD28 costimulation. Recently, three β chemokine receptors, CCR1, CCR2, and CCR5, were shown to be downmodulated on CD3 and CD28 costimulation.60 74
These data show that in the very same cell system one can determine opposite effects of CD28 stimulation. Whereas costimulation with CD3 protects from cell death, the sole ligation of CD28 by MoAbs or its ligands results in T-cell apoptosis. The basis of these opposite effects rely mostly on Fas-L regulation. Specifically, in this model CD28 costimulation prevents CD3-mediated Fas-L expression. The basis of this function is unknown but may be related to another group of receptors that are not upregulated by CD28 like cytokine receptors but, in sharp contrast, are downmodulated such as β chemokine receptors and receptors belonging to the TNF family members such as Fas-L.
ACKNOWLEDGMENT
The authors thank P. Golstein and C. Mawas for critical review of the manuscript and D. Isnardon for technical assistance.
Supported in part by the Institut National de la Santé et de la recherche Médicale (INSERM). Y.C. was a recipient of a fellowship from the EEC grant ERB-CHRX CT94-0537, and D.R. was a recipient from the Agence Nationale de Recherches sur le SIDA (ANRS).
Address reprint requests to Y. Collette, PhD, U119 INSERM, Université de Méditerranée, Bd Leı̈ Roure, 27, 13009 Marseille, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Flow cytometric analysis of a PI-stained CD28 transfected murine T-cell hybridoma. DWT6.11 cells were seeded for 8 hours in a 24-well plate in the presence of the indicated MoAbs as described in the Materials and Methods. (A) PI staining versus the number of nuclei in one representative experiment. Numbers above histograms indicate the percentage of apoptotic nuclei. (B) Results are presented as averaged percentage (±standard deviation [SD]) of subdiploid nuclei obtained from six independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1350/5/m_blod41624001x.jpeg?Expires=1766081297&Signature=ySD7CIFSsTZSjE-oXZd4BMIMDtv1YUDDvhARSwsDAaXUKveF26RjWaCfaCiDR3DwEuwIRdf6lvCdVQYPYPKxWLCNT7K0MPWsc~C~Rs8993sy3uNDBs3qlRC6SLJ8fJY1yhWB35wncHDe9ycuZRM7c2zfUj8Kk7inWye2fTnf~0J-uiSzJKcrrFGMyQ7YUjNzkvCzdLPvattyA2tMgkAWIab5IOVmSPkxebY1pjxhAmUdISL6fg1EeZ66JdHUQw-8N6GLoTzmBTrunsa6YpkQvvtQLY7v~VCv5Wj~FdoizIDibluElD3EiMXDOuY4ol7CqgbZ6qRY8U2DXL0p-OTHTQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 1. Flow cytometric analysis of a PI-stained CD28 transfected murine T-cell hybridoma. DWT6.11 cells were seeded for 8 hours in a 24-well plate in the presence of the indicated MoAbs as described in the Materials and Methods. (A) PI staining versus the number of nuclei in one representative experiment. Numbers above histograms indicate the percentage of apoptotic nuclei. (B) Results are presented as averaged percentage (±standard deviation [SD]) of subdiploid nuclei obtained from six independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1350/5/m_blod41624001x.jpeg?Expires=1766287985&Signature=1To5dHKNYf5Ap8EDYuu-BQXaN7OPTENAcJEiFJFw88hhrIsP2SzD1e0FYpHEW612hCDEnEXvxcSwxnY3nlod3zUrMN7Dpi6rSSPI0ylwO1C1vw4~dx3cczlGBwXHFHLmBe3k4elHA1ptJVEI3PJRQmLY5ymikuRnvdWozs0xackOTLBqRgIaaWwvERLLP3oItZR6Kl7-MoaRbpu9MHmxhzlT3HfrpV9s6InAePuxkVdqJ1yr6DXki10E1SZ-KxkXVVFNuj5SmnAa6w-98vF6XjktGd5tIS68iwRzN4HFufmLcEsJPL9NIPQoyJ58qV5VNd7vo1~JDpJNKpYviX1rHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)