Abstract
Thrombin, a central molecule in coagulation, is also involved in inflammation. Notably, thrombin induces endothelial neutrophil adhesion, P- and E-selectin expression, and chemokine production. We show here that thrombin induces expression of intercellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106) on human umbilical vein endothelial cells (HUVECs) associated with increased adhesion of monocytes. Thrombin increased mRNA steady-state levels and expression of ICAM-1 over 24 hours. Thrombin-induced VCAM-1 expression exhibited unusual kinetics, reaching maximum levels after 6 to 12 hours, but decreasing to near baseline after 24 hours. Thrombin activity on HUVECs was mediated through interaction with its specific receptor, because ICAM-1 and VCAM-1 expression were similarly induced by the 14-amino acid thrombin receptor-activating peptide. Thrombin-induced ICAM-1 and VCAM-1 expression was significantly inhibited by hirudin, but not by interleukin-1 receptor antagonist or anti-tumor necrosis factor monoclonal antibody (MoAb). Thrombin-activated HUVECs significantly increased greater numbers of adhering THP-1 macrophagic cells, peripheral blood mononuclear cells, or purified monocytes than unstimulated HUVECs. This adhesion was inhibited by anti-CD18 and anti-CD49d MoAb, demonstrating that thrombin-induced ICAM-1 and VCAM-1 were functional. These results show that, in addition to selectins, thrombin directly induces a cytokine-independent expression of adhesion molecules of the Ig superfamily on HUVECs that may support firm leukocyte attachment during inflammation.
© 1998 by The American Society of Hematology.
LEUKOCYTE-ENDOTHELIAL interactions play a central role in inflammation. Adhesion is mediated by molecules belonging to different families such as selectins, integrins, and Ig superfamily.1,2 Polymorphonuclear cell (PMN) adhesion to endothelial cells (ECs) is the main event in acute inflammation and follows several steps. Selectins, namely L-selectin (CD62L) on leukocytes, P-selectin (CD62P) on platelets and ECs, and E-selectin (CD62E) on ECs mediate weak reversible bonds between PMN and ECs under physiological flow conditions and are responsible for rolling, the first step in the adhesion cascade.3,4 Rolling is followed by PMN activation mediated by chemoattractants in the vicinity of endothelium glycocalix.5 PMN activation increases avidity of the β2 integrins (CD11a/CD18, LFA-1; CD11b/CD18, Mac1) for their ligands, inducing the firm later step of PMN-endothelium adhesion though interactions with molecules of the Ig superfamily, intercellular adhesion molecule-1 (ICAM-1; CD54), and ICAM-2 (CD102). ICAM-1, the counter-receptor for both CD11a/CD18 and CD11b/CD18, is a 5-Ig domain molecule expressed on various cells and constitutively present on EC.1,6 On EC, ICAM-1 expression is highly increased by inflammatory cytokines such as interleukin-1α/β (IL-1α/β), tumor necrosis factorα (TNFα), or interferon-γ (IFN-γ).1
The mechanisms of monocyte adhesion to ECs that characterize chronic inflammation are less well known, but may also follow several steps. Monocyte spontaneously adhere to ECs under static conditions.7 However, under physiological dynamic conditions, monocytes only adhere to cytokine-stimulated ECs.8 The first step of monocyte-endothelial adhesion induces a rolling motion due to the interaction of L-selectin on monocytes with its ligand on ECs.8,9 The central step of monocyte-endothelial adhesion is mediated by the interaction of the β1 integrin VLA-4 (CD49d) on monocytes with vascular cell adhesion molecule-1 (VCAM-1; CD106) on ECs.8 VCAM-1 is a 6- or 7-Ig domain molecule expressed on vascular and nonvascular tissues.10 Endothelial VCAM-1 expression is controlled by cytokines such as IL-1α/β, TNFα, or IL-4.8,10 The interaction of VLA-4 with VCAM-1 may induce rolling as well as firm arrest of monocytes on IL-4–stimulated human umbilical vein ECs (HUVECs).8,11,12 The next step of monocyte spreading is under the dependence of the interaction of β2 integrins with ICAM-1, whereas transendothelial migration may involve several molecules such as ICAM-1, VCAM-1, and platelet endothelial cell adhesion molecule-1 (PECAM-1).8 13
Both in vivo and vitro experiments have shown that the inflammatory and the coagulation cascades are linked.14-17 As an example, thrombin plays a central role in coagulation and in inflammation. This serine protease generated from prothrombin during the coagulation cascade is a potent platelet agonist and cleaves fibrinogen into fibrin, controlling the final step of clot formation.18Thrombin also exerts various proinflammatory functions on cells through interactions with a recently recognized specific receptor.19 Thrombin activates ECs to produce prostacyclin (PGI2) and platelet-activating factor (PAF) and to express P-selectin (CD62P).20-22 We have recently shown that thrombin also activates ECs in a more delayed and sustained way through protein synthesis, defining type II endothelial activation.23 Through this mode of activation, thrombin induces E-selectin (CD62E) expression as well as chemokine production in an IL-1α/β– and TNFα-independent way.23 24
Thrombin is important in acute inflammation, because it is chemotactic for PMN and has been shown to favor PMN-endothelial adhesion.23,25,26 In addition, thrombin may be an important stimulus in chronic inflammation, because it is known to be chemotactic for monocytes.27 However, whether thrombin increases monocyte adhesion to ECs is not known. In this study, we asked whether thrombin was able to induce endothelial Ig superfamily proteins ICAM-1 and VCAM-1 expression and to favor monocyte firm adhesion to ECs.
MATERIALS AND METHODS
Materials.
The following materials were purchased: M199 culture medium and fetal calf serum (FCS; BioWhittaker, Fontenay/Bois, France); human α-thrombin (1,000 U/mg; tested negative for contamination by plasmin, plasminogen, fibrin degradation products, human immunodeficiency virus [HIV], and hepatitis virus), recombinant hirudin, endothelial supplement growth factor from bovine pituitary gland, and polymyxin B sulfate (Sigma Chemical Co, Coger, Paris, France); BioMag goat antimouse IgG (BioAdvance, Emerainville, France) and sodium51chromate (ICN Pharmaceuticals, Orsay, France; 433 mCi/μg); calcein (Molecular Probes, Eugene, OR); rabbit antihuman TNFα polyclonal antibodies (Abs) and recombinant human IL-1β (Genzyme, Le Perray en Yvelines, France); mouse antihuman CD54 and CD106 monoclonal antibodies (MoAbs; IgG1), mouse antihuman CD2, antihuman CD8, antihuman CD20, antihuman CD18, antihuman CD49d and control IgG1, mouse antihuman HLA class I MoAbs, and fluorescein goat antimouse IgG F(ab’)2 (Immunotech, Marseille, France); thrombin receptor agonist peptide (TRAP-14, 42-55) consisting of Ser-Phe-Leu-Leu-Arg-Asn-Pro-Asn-Asp-Lys-Tyr-Glu-Pro-Phe and a control scrambled peptide consisting of Asn-Glu-Phe-Ser-Leu-Pro-Lys-Pro-Phe-Arg-Tyr-Leu-Asn-Asp (Neosystem Laboratories, Strasbourg, France).23 IL-1 receptor antagonist (IL-1Ra) was a gift of Dr D.E. Tracey (Upjohn Co, Kalamazoo, MI).
Cell cultures.
HUVECs were obtained as previously described17 and used on passage 2 or 3. Cells were grown until confluent in 25-cm2flasks coated with 1% gelatin. The cells were then cultured in 6- or 24-well plates in M199 supplemented with endothelial growth factor and containing 20% heat-inactivated FCS, 100 U/mL penicillin G, and 100 μg/mL streptomycin. Forty-eight hours before each experiment, endothelial growth factor was withheld and the cells were cultured in the same medium containing 10% FCS for the first 24 hours and then 5% FCS for the following 24 hours. Thrombin, TRAP-14, or control peptide was then added to HUVECs for various culture times. Thrombin at the concentration of 8 U/mL, as used in the main part of the following experiments, was not toxic for HUVECs, as stated by the trypan blue exclusion test, conserved expression of HLA class I antigens, and low amount of lacticodeshydrogenases release. In some experiments, either IL-1Ra (10 μg/mL), anti-TNFα (10 μg/mL), or hirudin (40 U/mL) was added to the culture. All experiments described in this report were performed in the presence of polymyxin B (7 μg/mL).
Peripheral blood mononuclear cells (PBMCs) from healthy volunteers were prepared from freshly drawn heparinized blood by Ficoll density gradient separation. Blood monocytes were purified from PBMCs by negative selection following a two-step procedure. First, PBMCs were depleted of CD2 cells by rosetting with 2-aminoethylisothiouronium bromide (AET) sheep erythrocytes. The resulting cells were then incubated with anti-CD3 MoAb (15 μg/107 cells), anti-CD8 MoAb (15 μg/107 cells), and anti-CD20 MoAb (30 μg/107 cells) for 30 minutes. After washing, BioMag goat antimouse IgG magnetic particles were added to the cell preparation for 30 minutes at 4°C, followed by cell magnetic separation, according to the manufacturer’s instructions. Contamination of the uncoated cells by CD3, CD8, or CD20 cells was less than 2%. Monocytes were greater than 95% pure by microscopic examination of Giemsa-stained centrifuge preparations.
Confocal microscopy.
Unstimulated, thrombin-stimulated, or IL-1β–stimulated HUVEC monolayers on coverslips were labeled with 2 μmol/L calcein for 30 minutes at 37°C or with an anti-HLA class I MoAb (20 μg/mL) for 30 minutes, followed by fluorescein goat antimouse IgG F(ab’)2 at room temperature. After washing, coverslips were inverted and deposited on a glass slide and then examined on a confocal laser scanning microscope (Leica, Heidelberg, Germany) operated under xy and xz modes to observe sections perpendicular to the monolayers. Images were then transferred to an IBM-compatible desk computer by real time digitization and processed, allowing us to determine the monolayer thickness, following a previously reported procedure.28
Fluorescence-activated cell sorter (FACS) analysis.
Unless otherwise indicated, HUVECs were stimulated with either thrombin (8 U/mL), IL-1β (50 pg/mL), or TRAP (100 μmol/L) or control scrambled peptide for 6, 12, or 24 hours. For surface expression of CD54, CD106, or HLA class I antigen, HUVECs were trypsinized and stained using the following sequence at 4°C: (1) unconjugated anti-CD54, anti-CD106, anti-HLA-class I MoAb, or an isotype control IgG1 (2 μg/105 cells); (2) fluorescein goat antimouse IgG F(ab’)2 fragments (1:200). Fluorescence was measured on a FACS analyzer (XL; Coultronics, Margency, France).
RNA extraction.
Unstimulated and thrombin-activated HUVECs in 25-cm2culture flasks were directly solubilized in RNA extraction solution (RNA-B; BioProbe Systems, Montreuil sous Bois, France). Total RNA was isolated using chloroform and precipitated with isopropanol. RNA was quantified by spectrophotometry at 260 nm.
Synthesis of the cDNA.
A 25-μL reverse transcription mixture in strand buffer (25 mmol/L Tris HCl pH 8.3, 37.5 mmol/L KCl, 1.5 mmol/L MgCl2) contained 4 μg RNA, 0.1 μg oligo (dT)12-18 (Pharmacia LKB, Biotechnology, France), 0.2 μmol/L dithiothreitol, 13 U RNase inhibitor (Eurogentec, Angers, France), 400 μmol/L dNTP (BioProbe Systems), and 100 U Moloney murine leukemia virus reverse transcriptase (Superscript RT; GIBCO BRL, Life Technology, Eragny, France) and was incubated at 37°C for 60 minutes.
Polymerase chain reaction (PCR).
PCR amplification of the cDNA using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers confirmed that equal amounts of RNA were reverse transcribed. For each condition, 3 different cDNA concentrations ranging from 32 to 6.4 ng RNA equivalent concentrations were amplified in 25 μL containing 250 mmol/L dNTP, 2 mmol/L MgCl2, 0.25 U Taq polymerase (Eurogentec), and 1 μmol/L of 5′ sense and 5′ antisense specific primers for ICAM-1 and VCAM-1 for 30 cycles in a modified version from.29 30Amplification consisted of 5 minutes of denaturation at 94°C, followed by 30 sequential cycles consisting of 1 minute at 55°C and 45 seconds at 72°C and then a final elongation cycle of 10 minutes at 72°C in a crocodile II thermal cycler (Appligen, Illkirch, France). Products of PCR (10 μL) were electrophoresed in a 2% agarose gel (Nusieve, Tebu, Le Perray en Yvelines, France) and then, after ethidium bromide coloration, were quantified using densitometry on a gel imager EASY Herolab (Osi, Elancourt, France). As predicted, the amplification product (amplicon) for ICAM-1 was 238 bp and for VCAM-1 was 260 bp.
Reverse transcription-PCR (RT-PCR)–specific primers.
Specific primers were 5′ TATGGCAACGACTCCTTCT 3′ sense and 5′ CATTCAGCGTCACCTTGG 3′ antisense for ICAM-1; 5′ ATGACATGCTTGAGCCAGG 3′ sense and 5′ GTGTCTCCTTCTTTGACACT 3′ antisense for VCAM-130; and 5′CCACCCATGGCAAATTCCATGGCA3′ sense and 5′TCTAGACCGCATCAGGTCAGGTCCACC3′ antisense for GAPDH (Genset, Paris, France).31
Adhesion assays.
THP-1 cells, PBMCs, or monocytes were labeled with 300 μL of51Cr for 45 minutes at 37°C and then washed 3 times in culture medium before counting. Before adhesion experiments, cells remained either untreated or were incubated at 4°C for 1 hour in the presence of either anti-CD18 MoAb, anti-CD49d MoAb, or control IgG1 at the concentration of 17 μg for 106 cells. After washing, 106/mL THP-1, 106/mL PBMCs, or 5 × 105/mL purified monocytes were deposited on unstimulated, IL-1β–stimulated, or thrombin-activated HUVECs in static conditions, under the volume of 500 μL of culture medium containing 5% FCS, for 1 hour at 37°C. Wells were then washed extensively to eliminate nonadherent cells, and then adherent cells were lysed with Triton X-100, collected, and counted in a gamma counter.
Statistical analysis.
Adhesion levels were expressed as the mean ± SEM of results obtained from 3 to 7 experiments performed in triplicate. The data were compared using the paired Student’s t-test.
RESULTS
Thrombin induces ICAM-1 (CD54) expression on HUVECs in a dose- and time-dependent manner: inhibition by hirudin.
HUVECs were cultured in the presence of thrombin and polymyxin B, and then ICAM-1 expression was studied by FACS analysis after various times. ICAM-1 was spontaneously expressed on HUVECs after 6 or 24 hours (Fig 1, top panels), but levels of expression were higher after stimulation with thrombin (Fig 1, middle panels). Induction was observed with 0.5 U/mL of thrombin and increased in a dose-dependent way (control, 31%; 0.5 U/mL, 35%; 2 U/mL, 45%; 8 U/mL, 50%, after 6 hours of thrombin stimulation; data not shown). Thrombin-induced ICAM-1 expression was higher after 24 hours than after 6 hours of stimulation (Fig 1 and Table 1), but was consistently less than after stimulation with IL-1β (Table1). The addition of the specific thrombin inhibitor hirudin inhibited thrombin-increased ICAM-1 expression on HUVECs (Fig 1, bottom panels).
Thrombin induces CD54 expression on HUVECs (FACS analysis). HUVECs were cultured for 6 hours (left side) and 24 hours (right side) in culture medium (upper panels), in the presence of 8 U/mL of thrombin (intermediate panels), or in the presence of thrombin with hirudin (lower panels). After cell trypsinization, CD54 expression was studied by FACS analysis (1 representative experiment among 5).
Thrombin induces CD54 expression on HUVECs (FACS analysis). HUVECs were cultured for 6 hours (left side) and 24 hours (right side) in culture medium (upper panels), in the presence of 8 U/mL of thrombin (intermediate panels), or in the presence of thrombin with hirudin (lower panels). After cell trypsinization, CD54 expression was studied by FACS analysis (1 representative experiment among 5).
Endothelial VCAM-1 (CD106) expression is induced by thrombin in a dose- and time-dependent fashion and inhibited by hirudin.
Thrombin induced significant VCAM-1 expression compared with HUVECs grown in culture medium (Fig 2, top and middle panels). VCAM-1 was consistently expressed after 6 hours of stimulation with thrombin, reached maximum levels between 6 and 12 hours, and then markedly decreased 24 hours after stimulation (Fig 2, middle panels, and Table 2). Thrombin induced VCAM-1 expression in a dose-dependent way (control, 3%; 0.5 U/mL, 9%; 2 U/mL, 17%; 8 U/mL, 36%, after 6 hours of thrombin stimulation; data not shown). IL-1β–stimulated levels of VCAM-1 were consistently higher than after thrombin stimulation and, in contrast to thrombin, remained elevated after 24 hours of stimulation (Table 2). When added to the culture, hirudin 90% inhibited the effects of thrombin on VCAM-1 expression (Fig 2, bottom panels).
Thrombin induces CD106 expression on HUVECs (FACS analysis). HUVECs were cultured for 6 hours (left side) and 24 hours (right side) in culture medium (upper panels), in the presence of 8 U/mL of thrombin (intermediate panels), or in the presence of thrombin with hirudin (lower panels). After cell trypsinization, CD106 expression was studied by FACS analysis (1 representative experiment among 5).
Thrombin induces CD106 expression on HUVECs (FACS analysis). HUVECs were cultured for 6 hours (left side) and 24 hours (right side) in culture medium (upper panels), in the presence of 8 U/mL of thrombin (intermediate panels), or in the presence of thrombin with hirudin (lower panels). After cell trypsinization, CD106 expression was studied by FACS analysis (1 representative experiment among 5).
Thrombin induces ICAM-1 and VCAM-1 expression through interaction with its specific receptor.
To establish whether thrombin was acting through interaction with its specific receptor, a 14 AA peptide (TRAP-14) reproducing the active NH2 terminal part of the thrombin receptor was tested for ICAM-1 and VCAM-1 induction. TRAP-14 induced similar ICAM-1 expression than thrombin itself (Table 3). In contrast, a 14-AA control scrambled peptide did not induce significant ICAM-1 expression compared with control (Table 3). TRAP-14 also induced VCAM-1 expression in a similar fashion and kinetics than thrombin itself (Table 4), whereas the control peptide did not induce significant VCAM-1 expression compared with control (Table 4).
Thrombin increases steady-state levels of ICAM-1 and VCAM-1 mRNA.
Using RT-PCR, ICAM-1 mRNA was detected in unstimulated HUVECs, but mRNA steady-state levels clearly increased 2 hours after thrombin stimulation, reached maximal levels after 10 hours of stimulation, and remained elevated after 24 hours of stimulation (Fig 3). VCAM-1 mRNA was present in unstimulated cells, increased after 2 hours of stimulation, reached a maximum between 4 and 6 hours, and then started to decrease until 24 hours of stimulation (Fig 3).
Increased CD54 and CD106 mRNA steady-state levels in thrombin-activated HUVECs. PCR products of RNA prepared from HUVECs stimulated by 8 U/mL of thrombin for different times were deposited on a 2% agarose gel. ICAM-1 mRNA was detected in unstimulated cells but increased after 2 hours and remained high after 24 hours of thrombin stimulation. VCAM-1 mRNA was detected in unstimulated HUVECs, raised to a maximum level between 4 and 6 hours and then decreased until 24 hours.
Increased CD54 and CD106 mRNA steady-state levels in thrombin-activated HUVECs. PCR products of RNA prepared from HUVECs stimulated by 8 U/mL of thrombin for different times were deposited on a 2% agarose gel. ICAM-1 mRNA was detected in unstimulated cells but increased after 2 hours and remained high after 24 hours of thrombin stimulation. VCAM-1 mRNA was detected in unstimulated HUVECs, raised to a maximum level between 4 and 6 hours and then decreased until 24 hours.
Thrombin-induced ICAM-1 and VCAM-1 expression is not mediated by IL-1α/β or TNFα.
Thrombin-induced ICAM-1 or VCAM-1 expression was not significantly decreased by saturating concentrations of IL-1Ra (10 μg/mL), whereas at these same concentrations, IL-1Ra nearly completely inhibited IL-1β–induced ICAM-1 or VCAM-1 expression (Table 5). Similarly, in 2 experiments, anti-TNFα MoAb did not significantly decrease thrombin-induced ICAM-1 and VCAM-1 (Table 6) expression.
Thrombin stimulation of HUVECs increases monocyte adhesion.
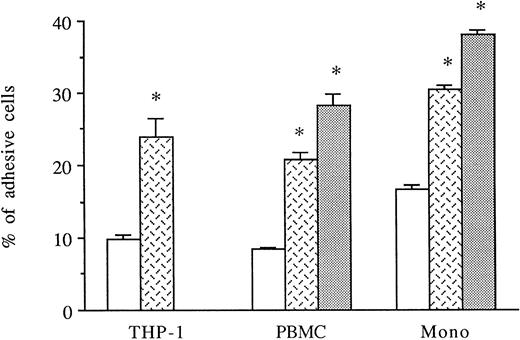
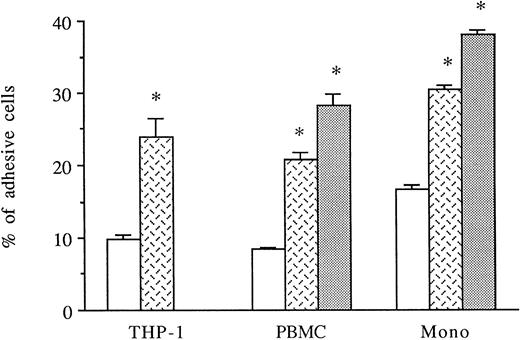
HUVECs were activated for 6 to 8 hours with thrombin. THP-1 cell line, PBMCs, or purified monocytes were used as sources of monocytes. We previously observed that THP-1 cells spontaneously expressed both CD18 and CD49d on their membranes by FACS analysis (data not shown). Labeled THP-1 cells, PBMCs, or monocytes were then added to unstimulated, IL-1β–stimulated, or 6-hour thrombin-activated HUVECs and, after 1 hour, adherence of mononuclear cells was measured. As shown in Fig 4, thrombin significantly increased the levels of THP-1, PBMCs, or purified monocyte adhesion to HUVECs (250%, 249%, and 180% increase, respectively) compared with HUVECs cultured in medium alone. However, the amount of mononuclear cell adhesion on 8 U/mL thrombin-activated HUVECs was consistently lower than on 50 pg/mL IL-1β–stimulated endothelial cells (Fig 4).
Thrombin induces increased monocyte adhesion to HUVECs. Radiolabeled THP-1 cells, PBMCs, or monocytes were deposited on untreated or 6-hour treated thrombin- (8 U/mL) or IL-1β– (50 pg/mL) activated HUVECs and allowed to adhere for 1 hour. After cell lysis, radioactivity was counted and expressed for each condition as the percentage of adhesive cells compared with 100% deposited cells (*P < .02 compared with adhesion of respective cells on unstimulated HUVECs, n = 4). (□) Medium; () thrombin; (▧) IL-1.
Thrombin induces increased monocyte adhesion to HUVECs. Radiolabeled THP-1 cells, PBMCs, or monocytes were deposited on untreated or 6-hour treated thrombin- (8 U/mL) or IL-1β– (50 pg/mL) activated HUVECs and allowed to adhere for 1 hour. After cell lysis, radioactivity was counted and expressed for each condition as the percentage of adhesive cells compared with 100% deposited cells (*P < .02 compared with adhesion of respective cells on unstimulated HUVECs, n = 4). (□) Medium; () thrombin; (▧) IL-1.
Inhibition of thrombin-induced mononuclear cell adhesion by anti-CD18 and anti-CD49d MoAb.
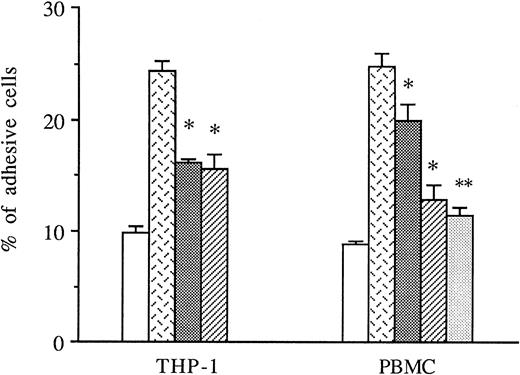
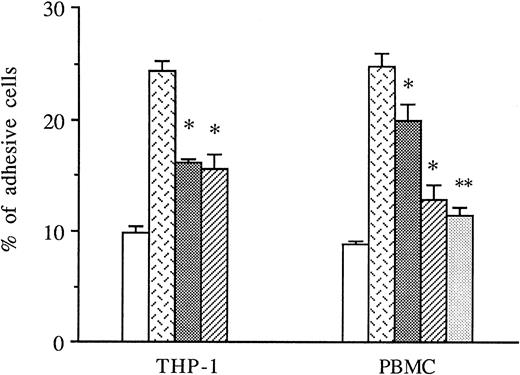
THP-1 cells or PBMCs were treated with anti-CD49d MoAb, anti-CD18 MoAb, or a control IgG1 before the addition to thrombin-activated HUVECs, and the amount of adhesive cells was measured in these conditions. As shown in Fig 5, both anti-CD49d MoAb and anti-CD18 MoAb alone or in combination significantly decreased thrombin-induced THP-1 and PBMC adhesion to thrombin-activated HUVECs, whereas the control IgG1 did not.
Inhibitory effects of anti-CD18 and anti-CD49d MoAb on mononuclear cells adhesion to thrombin-activated HUVECs. In some experiments, THP-1 cells or PBMCs were previously treated with either anti-CD49d MoAb, anti-CD18 MoAb, or a control IgG1 and then deposited on HUVECs stimulated with 8 U/mL of thrombin for 6 hours. After cell lysis, radioactivity was counted and expressed for each condition as the percentage of adhesive cells compared with 100% deposited cells (*P < .05, **P < .01 compared with adhesive respective cells on thrombin-activated HUVECs in the presence of control IgG1, n = 3). (□) Medium; () thrombin + IgG1; (░) thrombin + anti-CD18 MoAb; (▨) thrombin + anti-CD49d MoAb; (▧) thrombin + anti-CD18 + anti-CD49d.
Inhibitory effects of anti-CD18 and anti-CD49d MoAb on mononuclear cells adhesion to thrombin-activated HUVECs. In some experiments, THP-1 cells or PBMCs were previously treated with either anti-CD49d MoAb, anti-CD18 MoAb, or a control IgG1 and then deposited on HUVECs stimulated with 8 U/mL of thrombin for 6 hours. After cell lysis, radioactivity was counted and expressed for each condition as the percentage of adhesive cells compared with 100% deposited cells (*P < .05, **P < .01 compared with adhesive respective cells on thrombin-activated HUVECs in the presence of control IgG1, n = 3). (□) Medium; () thrombin + IgG1; (░) thrombin + anti-CD18 MoAb; (▨) thrombin + anti-CD49d MoAb; (▧) thrombin + anti-CD18 + anti-CD49d.
Mononuclear cell adhesion on thrombin-activated HUVECs is not due to HUVEC retraction.
Because thrombin has been reported to induce HUVEC retraction after 30 minutes of stimulation,32 we wanted to eliminate thrombin-induced HUVEC retraction and leukocyte adhesion on gelatin covering plastic dishes in our experiments. We used a previously reported confocal microscopy method that allowed us to measure HUVEC thickness.28 Whereas thrombin-activated HUVECs showed an increased thickness after 15 minutes of stimulation (medium, 4.5 ± 0.3 μm; thrombin, 5.9 ± 0.5 μm), after 6 hours of thrombin stimulation, no difference was observed (medium, 4.5 ± 0.2 μm; thrombin, 4.45 ± 0.4 μm; mean of 10 determinations for each experiment, 3 different experiments; data not shown), showing that long-term HUVEC stimulation by thrombin did not induce significant HUVEC retraction. In addition, THP-1 cells, PBMCs, and monocytes did not adhere to plastic covered by gelatin alone (1% to 3% adhesion; data not shown).
DISCUSSION
Thrombin not only is involved in the coagulation cascade, but also appears to play an important role in inflammation through cell activation properties such as prostaglandin, PAF, or chemokine secretion and selectin expression.20-27 Inflammation is characterized by a multistep adhesive interaction between leukocytes and endothelium mediated by molecules belonging to different families such as selectins, integrins, and the Ig superfamily. In vitro, thrombin is able to induce P- and E-selectin expression on HUVECs and may thus control the first step of adhesion.22,23 Thrombin also induces chemokine production by HUVECs23,24 and thus may favor leukocyte activation, the second step of the adhesive cascade.5 In this study, we showed that thrombin was also involved in the last step of leukocyte-endothelium adhesion, because thrombin was able to induce expression of functional forms of both ICAM-1 and VCAM-1 on endothelial cells.
First, we observed that thrombin stimulated significant increases in mRNA steady-state levels and membrane expression of ICAM-1 in HUVECs following the usual kinetics. Thrombin-increased expression of ICAM-1 was always at a lower level than after IL-1β stimulation. Thrombin also increased mRNA steady-state levels of VCAM-1, with a maximum between 4 and 6 hours, followed by a sharp decrease and weakly detectable levels after 24 hours. VCAM-1 gene transcription is under the control of NF-κB,33 and some functions of thrombin such as vascular smooth muscle cells proliferation have been shown to also involve NF-κB.34 VCAM-1 expression on HUVECs was consistent with mRNA concentrations, being significant after 6 hours of thrombin stimulation, with a maximum after 12 hours and weakly detectable after 24 hours. This kinetic of VCAM-1 expression appears rather unusual, because VCAM-1 expression has been usually reported to be sustained for almost 48 hours after IL-1α/β or TNFα stimulation.1,10 However, a recent report showed that VCAM-1 expression on HUVECs after IL-1β stimulation is maximum after 6 to 12 hours and decreased rapidly thereafter.35Nevertheless, in our hands, despite low concentrations of IL-1β (50 pg/mL), VCAM-1 labeling after IL-1β stimulation was sustained over 24 hours, at a time that it was weakly detectable after thrombin stimulation. IL-1β–induced VCAM-1 expression was also stronger than after thrombin stimulation. Therefore, we can conclude that thrombin is a less potent and more transient HUVEC activator than IL-1β for ICAM-1 and VCAM-1 expression.
A previous report had shown that thrombin induced ICAM-1 expression on HUVECs.36 Surprisingly, thrombin was reported to induce ICAM-1 on HUVECs after a few minutes of stimulation, without the need of protein synthesis and independently of the thrombin receptor pathway. ICAM-1–increased expression is known to depend on mRNA transcription and protein synthesis, thus requiring several hours.1,5 Our data are consistent with this finding and not with the previous report. Moreover, we found that thrombin induction of ICAM-1 and VCAM-1 was due to an interaction of thrombin with its specific receptor. This recently discovered receptor belongs to a new family of protease-activated receptors.19 Thrombin cleaves the extracellular NH2-terminal part of the thrombin receptor and shows a new NH2-terminal part, which then tethers the receptor itself.19 We used a 14-amino acid thrombin receptor activating peptide, TRAP-14, identical to the newly shown NH2-terminal part of the trombin receptor, and found that activation of HUVECs by this peptide was able to induce both ICAM-1 and VCAM-1 in a way similar to that of thrombin itself.
Inducers of ICAM-1 and VCAM-1 include endotoxin, IL-1α/β, and TNFα.1,5,10 In this study, we prevented the effects of possible endotoxin contamination of the thrombin preparation by adding polymyxin B to HUVECs in all experiments. In addition, thrombin action appears specific, because it was inhibited by the specific thrombin inhibitor, hirudin, and reproduced by TRAP-14. Moreover, if endotoxin contamination was responsible for the effects of thrombin in these experiments, thrombin would have induced IL-1α/β and TNFα, which would have likely mediated ICAM-1 and VCAM-1 expression. In agreement with our previous report,23 we found no significant inhibitory effects of either IL-1Ra or anti-TNFα MoAb on thrombin-induced ICAM-1 and VCAM-1 expression. Therefore, thrombin has direct proinflammatory properties on HUVECs without a requirement for intermediate autocrine or juxtacrine actions of either IL-1α/β or TNFα.37 38
Thrombin is known to favor PMN adhesion to HUVECs and to be chemotactic for neutrophils in vitro and thus to be potentially important in acute inflammation in vivo.25,26 Thrombin has been shown to be also chemotactic for monocytes in vitro.27 In this report, we found that thombin induced significant monocyte adhesion to HUVECs in vitro that could be significantly decreased by anti-CD18 and anti-CD49d MoAb. Increased adhesion of monocytes on thrombin-activated HUVECs could not be due to thrombin-induced HUVEC retraction32 and adhesion to gelatin, because we eliminated HUVEC retraction using a confocal microscopy method that allowed us to measure HUVEC thickness and we observed no significant mononuclear cell adhesion on gelatin alone. Adhesion of THP-1 on thrombin-activated HUVECs appeared to be more affected by anti-CD18 MoAb than that of PBMCs. This result may be the consequence of different levels of CD11/CD18 avidity on THP-1 compared with PBMCs. Therefore, thrombin is likely to play an important role in chronic inflammatory situations characterized by tissular monocyte infiltration, such as rheumatoid arthritis, atherogenesis, and chronic allograft rejection.
In rheumatoid arthritis, for example, thrombin is present in the synovial fluid and has been shown to mediate both direct cartilage degradation39 and synovial cell proliferation.40 Rheumatoid joints are characterized by monocyte and T-lymphocyte migration from the blood compartment.41 Memory T-lymphocyte adhesion to endothelium involves E-selectin as well as ICAM-1.42 In experimental models, monocyte migration into the joints can be blocked by a combination of anti-CD11a/CD18, anti CD11b/CD18, and anti-CD49d MoAbs, demonstrating the important role of these molecules in the pathogenesis of inflammatory arthritis.43 Because thrombin directly induces endothelial E-selectin expression23 and both ICAM-1 and VCAM-1, as shown in this report, thrombin in rheumatoid arthritis fluid may participate in mononuclear cell adhesion. In addition, thrombin induces endothelial chemokine production, such as IL-8 and MCP-1, that have been found at high concentrations in rheumatoid arthritis joint fluids.41 Thus, thrombin may participate to both leukocyte migration and adhesion into rheumatoid joints.
Thrombin induction of VCAM-1 expression may also be important in atherogenesis, because the first steps of the atherosclerotic lesions are characterized by monocytes adhesion to ECs.44 VCAM-1 has been shown to be a critical molecule in this phenomenon, because it is expressed in close association with foam cells and its endothelial expression is induced by lipids.45 ICAM-1 is also present in the atheromatous plaque and may participate in monocyte adhesion. Thrombin has been suggested to be an important player in atherogenesis, because the thrombin receptor has been found widely expressed in the atheromatous plaque, in close association with macrophages and vascular smooth muscle cells.46 Thrombin is indeed a potent smooth muscle cell mitogen47 and induces MCP-1 production by HUVECs, a chemokine active on monocytes that is highly expressed in the plaque.48 Thrombin ability to induce both ICAM-1 and VCAM-1 adds important clue to understand its role in atherogenesis.
Chronic allograft rejection is also characterized by mononuclear cell infiltration, and ICAM-1 and VCAM-1 are both highly expressed on ECs during allograft rejection.49,50 Thrombin is also present in rejected tissues, because infiltrating monocytes express tissue factor, fibrin deposition is intense, and thrombomodulin, a thrombin inhibitor, is decreased.51 Thrombin ability to induce both ICAM-1 and VCAM-1 and to favor endothelial monocyte adhesion may therefore participate in the rejection cascade.
In conclusion, we showed that thrombin is able to directly induce not only endothelial selectin expression and, therefore, low-affinity adhesive interaction between leukocyte and endothelium, but also molecules of the Ig superfamily, such as ICAM-1 and VCAM-1, which mediate firm adhesion of mononuclear cells to endothelium and may be important during chronic inflammation.
Address reprint requests to Gilles Kaplanski, MD, PhD, INSERM U387, Hôpital Sainte-Marguerite, 270 Boulevard Sainte-Marguerite, 13009 Marseille, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.