Abstract
We have developed a multiplex reverse transcription-polymerase chain reaction (RT-PCR) reaction, which enables us to detect 29 translocations/chromosomal aberrations in patients with acute lymphoid leukemia (ALL) and acute myeloid leukemia (AML). Through the construction and optimization of specific primers for each translocation, we have been able to reduce the set-up to 8 parallel multiplex PCR reactions, thus greatly decreasing the amount of work and reagents. We show the value of our set-up in a retrospective analysis on cryopreserved material from 102 AML and 62 ALL patients. The multiplex RT-PCR detected a hybrid mRNA resulting from a structural chromosomal aberration in 45 of 102 (44%) of the AML and in 28 of 62 (45%) of the pediatric ALL cases. Importantly, in 33% of AML and in 47% of the ALL cases with cytogenetic data, submicroscopic chromosomal aberrations or masked translocations were shown that were not detected in the cytogenetic analysis either for structural reasons or because of an insufficient number of metaphases obtained. This multiplex RT-PCR system, which can handle up to 10 patients with a response time of 2 working days, is thus an important tool that complements cytogenetic analysis in the up-front screening of acute leukemia patients and should provide a rapid and efficient characterization of leukemia cells, even in situations with sparse patient material.
THE DIAGNOSIS OF acute leukemia is multidisciplinary, with histology, immunology, and cytogenetics as the most often used methodologies. Neither immunophenotyping nor histology provides tools for prognosticating patients, whereas cytogenetic evaluation has been shown to delineate patients with a defined prognosis.1 The value of cytogenetics as a prognostic tool in cancer is based on the existence of a number of balanced chromosomal translocations. At present, more than 50 different consistently occurring translocations have been described, many of which have been found to be specific for particular subtypes of leukemia or lymphoma (for a recent review, see Look2).
Molecular studies of these rearrangements have provided important insights into the mechanisms of tumorigenesis. Thus, many genes involved in translocations are transcription factors that appear to have a direct role in hematopoiesis. Translocations may alter the functions or activities of cellular proto-oncogenes located at or near the breakpoint by at least two mechanisms, either (1) by juxtaposition of a cellular proto-oncogene to the regulatory element of a tissue-specific gene, eg, Ig or T-cell receptor genes in leukemia, leading to inappropriate expression of the oncogene,3,4 or (2) by creating fusion genes coding for chimeric proteins with functional features different from the two parental proteins, eg, t(1;19)(q23;p13) and t(8;21)(q22;q22).5 6
A translocational breakpoint gene may have several fusion partners, the most promiscuous example being the MLL gene (also calledALL1, HTRX1, and HRX) at chromosome band 11q23, for which more than 40 different fusion partners together with an internal duplication have been described.7-15 Thus, depending on the fusion partner, the MLL gene can contribute to the pathogenesis of lymphoid and myeloid malignancies. TheMLL/AF4 fusion gene, detected in t(4;11)(q21;q23) translocations, is observed in acute lymphoid leukemia (ALL) only,14,16-18 whereas the MLL/AF9 fusion gene detected in t(9;11)(p22;q23) translocations is found primarily in acute myeloid leukemia (AML) patients.14,19 The dupMLLhas been described in both ALL and AML patients.15,20 21
Because cytogenetic analysis is time-consuming and yields sufficient metaphases only in 60% to 80% of the bone marrow samples, efforts to design polymerase chain reaction (PCR) reactions for translocations have been ongoing for some time.22 23 The availability of cDNA sequence information for an increasing number of fusion genes has resulted in PCR protocols for individual translocations. PCR analysis does not require much patient material, can be performed on resting cells, and is very sensitive in detecting rare abnormal cells. Thus, it should be of great potential benefit to bring the PCR methodology up-front in the diagnosis of acute leukemia. However, considering the great number of fusion genes and breakpoint variants presently characterized, more than 50 separate PCR reactions are needed for the screening of a patient with a standard procedure, which is at best labor-intensive and material-demanding and probably not practically feasible.
We describe here our efforts to establish a multiplex reverse transcription-PCR (RT-PCR) analysis system that facilitates the detection in 8 parallel PCR reactions of 29 translocations/chromosomal aberrations, including more than 80 mRNA breakpoint or splice variants.
MATERIALS AND METHODS
Patient samples and cell lines.
For the combined purpose of optimizing the PCR primers and obtaining unlimited amounts of material for positive controls for the multiplex assay, we used cell lines as the RNA source. For the t(1;19)(q23;p13), we used 697; for t(2;5)(p23;q35), Karpas-299; for t(4;11)(q21;q23), RS4;11 and MV-4-11; for t(6;11)(q27;q23), ML-2; for t(9;11)(p22;q23), Mono-Mac-6; for t(15;17)(q21;q22), NB4; for t(17;19)(q22;p13), HAL-01; and for TALD, RPMI8402. In addition, we used RNA from patients positive for dupMLL(11q23), inv(16)(p13q22), t(6;9)(p23;q34), t(8;21)(q22;q22), t(9;22)(q34;q11), t(10;11)(p12;q23), t(11;19)(q23;p13.3), and t(11;19)(q23;p13.1) that were identified during the study.
Leukemic cell samples from patients admitted to the Departments of Hematology and Pediatrics, Aarhus University Hospital (Aarhus, Denmark) were subjected to Isopaque-Ficoll sedimentation and the mononuclear cell suspensions used for routine immunophenotyping purposes. In cases in which more than 5 × 106 cells were available, these were cryopreserved in 10% fetal calf serum and 10% dimethyl sulfoxide according to standard techniques. All cell collection was performed according to protocols approved by the Local Ethical Committee for the County of Aarhus. Likewise, the Biobase containing cell material from the patients has been approved by the Danish Data Protection Agency (Registertilsynet).
The cell lines Karpas-299, ML-2, Mono-Mac-6, NB-4, 697, JOSK-M, JOSK-I, NALM-6, and RPMI8402 were obtained from DSM-Deutsche Sammlung von Mikroor ganismen und Zellkulturen (Braunschweig, Germany). The cell lines RS4;11 and MV-4-11 were obtained from the American Type Culture Collection (Manassas, VA). The cell line HAL-01 was kindly provided by Dr Kazuma Ohyashiki (Tokyo Medical College, Tokyo, Japan). All cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum and antibiotics. The medium for the cell line Mono-Mac-6 was supplemented with 9 μg/mL bovine insulin. An overview of the characteristics of most of these lines can be found in Drexler et al.24
Cytogenetic analysis.
Bone marrow cells were cultured at 37°C in an atmosphere of 5% CO2 in air for approximately 24 hours. After 3 to 5 hours, methotrexate (10−5 mol/L) was added, and 17 hours later the S-phase block was released with thymidine (10−3 mol/L). Colchicine was added 10 minutes before termination of the culture period.25 Cells were harvested with conventional methods and slides were prepared. The slides were aged by heating at 60°C for 17 hours before banding. Giemsa bands were produced with Wright's stain as described elsewhere.26
RNA preparation.
Cells were thawed out and washed twice in phosphate-buffered saline. Total RNA was prepared either by the guanidinium thiocyanate-phenol chloroform method27 or by using a RNeasy Kit (Quiagen Gmbh, Hilden, Germany) according to the manufacturer's recommendations. The RNA solution was subsequently treated with 0.1 U/μL RNase-free DNase (Boehringer Mannheim, Mannheim, Germany) in 50 mmol/L Tris-HCl, pH 8.0, 10 mmol/L MgCl2 at 37°C for 30 minutes. After DNase treatment, EDTA (pH 8.0) was added to a final concentration of 10 mmol/L and the RNA solution was extracted once in phenol/chloroform 1:1. Sodium acetate was added to a final concentration of 200 mmol/L and RNA was precipitated with 1 vol of isopropanol. RNA was pelleted in an Eppendorf centrifuge at 13,000 rpm for 30 minutes, washed with 80% ethanol, and resuspended in 25 μL diethylpyrocarbonate ddH2O. Five microliters were withdrawn for quantification on a GeneQuant RNA/DNA calculator (Pharmacia Biotech, Sollentuna, Sweden). Subsequently, RNA was diluted to 0.1 μg/μL in DEP ddH2O and stored until use at −80°C in 10-μL aliquots.
The multiplex PCR setup.
We have designed a multiplex RT-PCR strategy to detect the transcripts of chromosomal translocations/rearrangements found in leukemic patients. Taking advantage of the fact that a number of the genes involved in translocations can have different fusion partners, we combined such genes in the assay, thus reducing the number of primers. In 8 multiplex PCR reactions this assay tests for 29 translocations or chromosomal rearrangements that may result in the generation of more than 80 fusion gene variants because of heterogeneity of breakpoints and/or alternative splicing. The translocations and the resulting transcripts tested for in the multiplex PCR assay are shown in Table 1. Table 1 also includes a number MLL gene fusion variants (marked T), which in theory may be expected to occur, but have to our knowledge, not yet been described.
Internal positive control.
Translocation specific cDNA primers.
The amount of patient RNA can be a limiting factor, and efficient cDNA synthesis is therefore a critical step in RT-PCR. To increase the sensitivity of cDNA synthesis and to reduce the background from irrelevant RNA, we opted not to use random hexamer primers, but instead designed a number of translocation-specific cDNA primers shown in Table 2. The cDNA primers were 11 to 13 nucleotides (nt) long and located 10 to 100 nt downstream of the most 3′ PCR primer. The melting temperature (Tm) of the cDNA primers was approximately 40°C, which is sufficiently high to ensure efficient cDNA priming and low enough to ensure that these primers would not interfere with the subsequent PCR reaction that was performed without purification of the cDNA.
Construction of primers.
All PCR oligonucleotide primers were designed with the primer analysis software OLIGO version 5.0 (National Biosciences Inc, Plymouth, MN) and published sequence data from the EMBL DNA database. Oligonucleotide primers were purchased high-performance liquid chromatography-purified from DNA Technology (Science Park, Aarhus, Denmark).
RT-PCR.
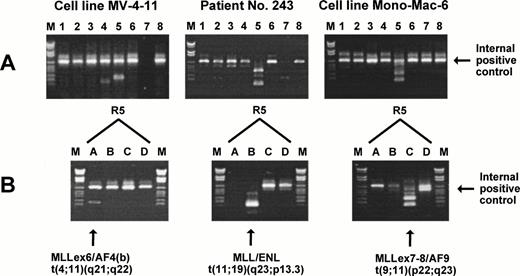
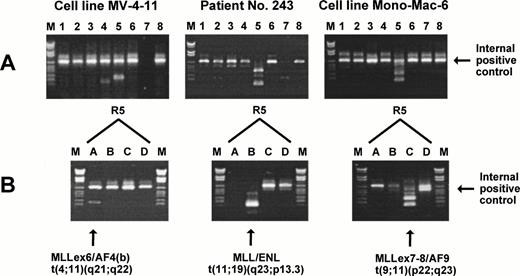
To achieve maximal sensitivity, a nested PCR protocol was used and, to minimize the risk of contamination, filter-tips and four different laboratory rooms with indigenous pipettes were used for (1) preparation of stock solutions; (2) RNA preparation and cDNA synthesis/setup of first PCR; (3) the first to second PCR transfer; and (4) gel electrophoresis. One microgram of total RNA was incubated at 65°C for 5 minutes with a mixture of translocation-specific cDNA primers (2.5 pmol of each) and then reverse transcribed by incubation at 37°C for 45 minutes in a total volume of 25 μL containing 20 U RNase inhibitor (Boehringer), 1 mmol/L of each dNTP, 10 mmol/L dithiothreitol, 50 mmol/L Tris-HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2, and 400 U Moloney murine leukemia virus reverse transcriptase (BRL, Bethesda, MD). After the incubation, the cDNA reaction mixture was diluted with ddH2O to 50 μL. PCR amplification was performed as 8 parallel nested (2-round) multiplex reactions in a Perkin Elmer 9600 thermocycler (Roche Molecular Systems, Branchburg, NJ). Five microliters of diluted cDNA reaction was added to each of 8 20-μL multiplex mixtures that contained 11 mmol/L Tris-HCl, pH 8.3, 55 mmol/L KCl, 1.65 mmol/L MgCl2, 0.2 mmol/L of each dNTP, a mixture of oligonucleotide primers (5 pmol of each primer), and 1.5 U AmpliTaq-Gold polymerase (Perkin Elmer). The first PCR consisted of an initial activation of the polymerase at 95°C for 15 minutes, followed by 25 cycles of PCR amplification (annealing at 58°C for 30 seconds, elongation at 72°C for 1 minute, and denaturation at 95°C for 30 seconds). After the first PCR, 1-μL aliquots from each of the 8 PCR reactions were transferred to 8 24-μL second-round multiplex mixtures that contained 10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.2 mmol/L of each dNTP, 5 to 12.5 pmol of each primer, and 1.5 U AmpliTaq-Gold polymerase. The second PCR consisted of an initial activation of the polymerase at 95°C for 15 minutes, followed by 20 cycles of PCR amplification (annealing at 58°C for 30 seconds, elongation at 72°C for 1 minute, and denaturation at 95°C for 30 seconds), and finally by 10 minutes of extension at 72°C. Fifteen microliters of each PCR reaction was electrophoresed in a 1.5% agarose gel for 60 minutes at 100 V and stained with ethidium bromide as shown in Fig 1A. Negative controls without DNA template were included for all PCR reaction mixtures.
Multiplex RT-PCR and split-out analysis. (A) The two cell lines MV-4-11 and Mono-Mac-6 and patient no. 243 were positive for a translocation in multiplex reaction R5 (see Table 3). (B) To determine the translocation, a split-out PCR analysis was performed using the individual primer sets R5A, R5B, R5C, and R5D. M, DNA molecular weight marker VI (Boehringer).
Multiplex RT-PCR and split-out analysis. (A) The two cell lines MV-4-11 and Mono-Mac-6 and patient no. 243 were positive for a translocation in multiplex reaction R5 (see Table 3). (B) To determine the translocation, a split-out PCR analysis was performed using the individual primer sets R5A, R5B, R5C, and R5D. M, DNA molecular weight marker VI (Boehringer).
Split-out PCR and DNA sequence analysis.
Because each multiplex reaction identifies a number of translocations, many of which may be found in several variants, the number of possible translocation-positive PCR fragments is large (Table 1). To determine and verify a fusion gene in a positive multiplex reaction, we therefore performed split-out analysis using individual primer sets, as outlined in Fig 1B and detailed in Table 3. The split-out was performed using the same reaction conditions as for the multiplex PCR, except that only 1 U/reaction of AmpliTaq-Gold polymerase was used. Split-out of samples with a limiting amount of RNA was performed with 1 μL from the first round multiplex PCR as template and the second-round individual PCR primer sets. These analyses were performed with and without the internal positive control primers. Negative controls without DNA template were included for all PCR reaction mixtures. The presence of translocations were confirmed by determination of the sequence of the translocation specific DNA fragment. The DNA sequencing was performed using agarose gel-purified PCR fragments as a template and a Taq DyeDeoxy Terminator Sequencing kit (Perkin Elmer). The product was analyzed using an automated 373A DNA sequencer (Applied Biosystems, Foster City, CA).
RESULTS
Optimization of multiplex RT-PCR conditions.
The aim of the multiplex RT-PCR procedure is to detect and define translocation-specific mRNA related to leukemia. To set up the procedure, we first defined primers with a binding sequence near putative breakpoints of the corresponding mRNA sequence. Primers were designed to allow identical conditions in all PCR reactions. For cDNA synthesis, we elected to use primers specific for the recombined mRNA rather than random or poly-dT primers. Use of specific primers improved sensitivity 25- to 125-fold relative to random hexamer primers. When cell lines or patient material were available with known translocations that lead to recombination-specific mRNA, we tested the constructed primer pairs in PCR reactions before and after combining the different primer sets into the multiplex PCR reaction. If not working properly, the primers were redesigned. Finally, the sensitivity of the multiplex PCR assay was evaluated by limiting dilution experiments in which fivefold dilutions of RNA from translocation-positive cell lines were mixed with RNA from the NALM-6 cell line. Several series of multiplex RT-PCR experiments consistently showed that the translocation positive band could be readily detected in the 3,000- or 15,000-fold dilutions, indicating that the multiplex assay, at least for the cell lines tested, may detect 1 malignant cell out of 5,000 normal cells.
Translocations detected in AML and ALL patients by the multiplex PCR analysis.
To verify the value of the multiplex PCR system, we reproduced cytogenetic findings by multiplex PCR with material from cell lines and patients with known translocations. To evaluate the multiplex RT-PCR as a potential diagnostic tool used in up-front leukemia diagnosis, we next applied the multiplex PCR assay in a retrospective analysis of RNA purified from cryopreserved mononuclear blood or bone marrow samples of 102 AML and 62 pediatric ALL patients. RNA quality was evaluated by inspection of the 8 internal positive control bands in the multiplex PCR. In 8 of 102 of the AML and in 6 of 62 of the ALL cases, the internal positive control band had a weak and scattered appearance or was absent. This could be ascribed to insufficient amount or quality of RNA due to low cell number or cell lysis.
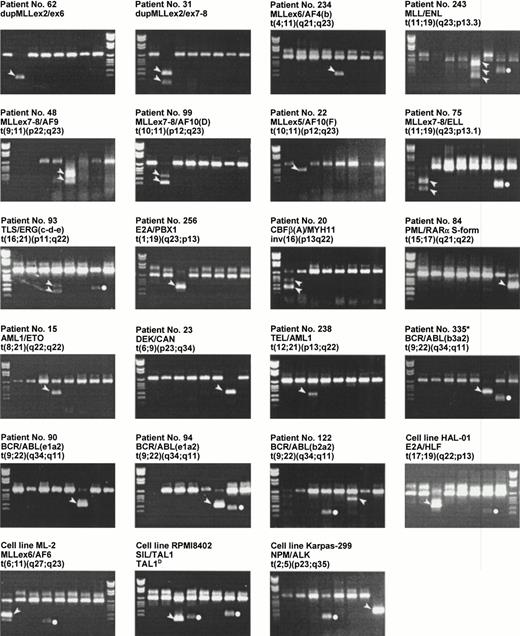
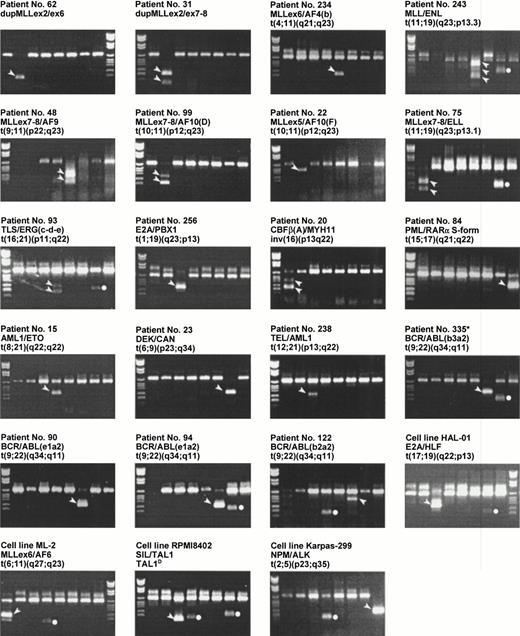
As seen in Tables 4 and 5, the multiplex PCR analysis detected a fusion gene in 45 of 102 of the AML and in 28 of 62 of the ALL cases. The frequencies and distributions between the two patient groups of the 16 different chromosomal aberrations resulting in a fusion gene that could be detected in the multiplex PCR are compared with cytogenetic data in Table 6. Examples of various aberrations detected by the multiplex RT-PCR are shown in Fig 2. The value of the described multiplex PCR analysis is demonstrated by the finding of 3 previously undescribed fusion gene variants: (1) a duplication of the MLL gene in which exon 5 was fused to exon 2 (patient no. 44), (2) a new breakpoint of the AF10 gene in a t(10;11)(p12;q23) (patient no. 22),30 and (3) a new breakpoint in the TLS gene in a t(16;21)(p11;q22) (patient no. 93).
Examples on chromosomal aberrations found by the multiplex RT-PCR. The loading order and DNA molecular weight marker are as in Fig 1. The band specific for the translocation is indicated by an arrowhead beside the band. The dots indicate activation of HOX11 (lane 4) or EVI1 (lane 7). Because HOX11 and EVI1 represent activation of native gene products and not of chimerical products, these are not further discussed in this presentation. *Patient no. 335 was a chronic myeloid leukemia case not included in this study.
Examples on chromosomal aberrations found by the multiplex RT-PCR. The loading order and DNA molecular weight marker are as in Fig 1. The band specific for the translocation is indicated by an arrowhead beside the band. The dots indicate activation of HOX11 (lane 4) or EVI1 (lane 7). Because HOX11 and EVI1 represent activation of native gene products and not of chimerical products, these are not further discussed in this presentation. *Patient no. 335 was a chronic myeloid leukemia case not included in this study.
In this material, we did not identify patients positive for the following translocations: TAL1D, t(X;11)(q13;q23), t(1;11)(q21;q23), t(1;11)(p32;q23), t(3;5)(q25.1;q34), t(3;21)(q26;q22), t(5;12)(q33;p13), t(5;17)(q35;q22), ?t(9;9), t(9;12)(q34;p13), t(11;17)(q23;q21), and t(17;19)(q22;p13). These translocations should be detected by the PCR primers used and their absence in our material can be ascribed to the low frequency of these chromosomal aberrations. Thus, the t(X;11)(q13;q23), t(5;17)(q35;q22), ?t(9;9), and t(9;12)(q34;p13) have, to our knowledge, been described only in single cases, and only a few cases of the t(1;11)(p32;q23), t(1;11)(q21;q23), t(3;5)(q25.1;q34), t(5;12)(q33;p13), t(11;17)(q23;q21), and t(17;19)(q22;p13) have been reported. The t(3;21) has been described primarily in therapy-related AML, and only recently in 1 de novo AML patient.31 TheTAL1D, with the fusion gene SIL/TAL1, has been found in 25% of T-ALL cases.32 Because only 6 T-ALL patients were included in this study, the absence of patients with theSIL/TAL1 fusion-gene may be ascribed to statistical variation. However, we cannot exclude that one or more of the PCR primers for these translocations may be incompatible with the multiplex PCR conditions, with the exception of the t(17;19)(q22;p13) andTAL1D, for which we have positive controls. This issue awaits the availability of positive patients, cell lines, or in vitro synthesized control RNA.
Comparison between the multiplex PCR and the cytogenetic analysis.
Cytogenetic data were available for 66 AML cases. In 62 of the cases, metaphase cells were obtained, but cytogenetic aberrations were detected in only 28 cases. The corresponding numbers for ALL were in total 45 cases, 34 with available metaphase cells and 17 with chromosomal aberrations. When the cytogenetic analysis showed one of the translocations included in the multiplex PCR, the corresponding fusion gene mRNA was detected, except in 1 case in which the multiplex PCR could not be performed because of insufficient RNA. The chromosomal rearrangements dupMLL, TALD, t(12;21)(p13;q22), and t(6;11)(q27;q23) cannot be detected (or are easily overlooked) by classical cytogenetic analysis. In the multiplex PCR analysis, 14 of 102 AML and 15 of 62 ALL cases were positive for this group of aberrations. A new finding in this work is the high frequency of dupMLL in ALL. Thus, in 6 AML and in 2 ALL cases with an adequate cytogenetic analysis (10 or more metaphases obtained at presentation), a fusion gene was detected by PCR that was not detected by the cytogenetic analysis. Masked translocations, which are not apparent in cytogenetic analysis but detectable by Southern blotting or RT-PCR, have previously been observed.33 34 In contrast, numerical aberrations, which cannot be detected by PCR, were found by cytogenetic analysis in 17 of 66 of the AML and in 11 of 45 of the ALL cases. The combined results from multiplex PCR and cytogenetic analysis are presented in Table 6. Taken together, the two methods uncovered chromosomal aberrations in 42 of 66 of the AML and in 27 of 45 of the ALL cases and supplemented each other in detecting chromosomal aberrations.
DISCUSSION
During the last decade, the multidisciplinary diagnosis of acute leukemia has been expanded by the description of an ever-increasing number of balanced translocations that are amenable to detection by classical karyotype analysis and by PCR analysis when sequence information is available for the designing of PCR primers.
Multiplex PCR has been used previously for characterization of individual or small groups of translocations found in leukemic cells.22 23 We have scaled up this method of analysis to cover most of the published translocations and describe here our experience with a multiplex PCR procedure on cryopreserved material from more than 160 patients diagnosed and treated at Aarhus University Hospital. We emphasize that the material is not necessarily representative as an unselected material, because cryopreservation of cells in the Biobase at the Laboratory of Immunohematology, Aarhus University Hospital, was performed only if the sample contained at least 5 million cells after immunophenotyping. Consequently, patients with scarce cell material at diagnosis were not included. Moreover, in 8 of 102 of the AML and 6 of 62 of the ALL cases, too small amounts of RNA to warrant PCR analysis were obtained from cryopreserved cells because of cell lysis. In fresh material, the fraction of patients with insufficient RNA should therefore, in theory, be lower, a supposition that is substantiated by our recent results from applying the method prospectively (Hoklandet al, unpublished data). Our cell material was cryopreserved over a period of 14 years; therefore, we caution against a close comparison between cytogenetics and PCR results. Banding techniques have improved considerably over the years, and the percentage of translocation positive cases has, in our hands, increased over time. Also, cytogenetic analysis on patients dating back to the 1980s was performed on only 10 metaphase cells per sample.
Given these limitations, we believe that the data presented here clearly prove the usefulness of the multiplex PCR concept. First, the assay can be performed (including the split-out phase) within 2 to 3 days and is amenable to the analysis of up to 10 samples simultaneously by 1 person. Second, the reaction clearly increases the number of translocation-positive patients relative to cytogenetic analysis, especially in cases of material with sufficient numbers of high-quality metaphase cells. This is exemplified by the demonstration of 22 of 66 AML and 21 of 45 ALL patients in whom additional chromosomal rearrangements were found by the multiplex PCR. Importantly, these translocations were not restricted to submicroscopical chromosomal aberrations, which are difficult, if not impossible, to detect by cytogenetic analysis. Rather, we found a wide range of translocations, including both those that are frequent [eg, t(8;21) and t(15;17)] and those that are infrequent [eg, t(11;19)]. Of equal importance, the additional translocations were found in both AML and ALL patients.
The multiplex PCR detects the expression, on the RNA level, of fusion genes generated by chromosomal rearrangements. It does not detect rearrangements in which native oncogenes are deregulated, as described in, eg, t(8;14), t(11;14), and t(1;14). Such rearrangements may be detected by PCR on DNA level. Similarly, Ig and T-cell receptor gene rearrangements35 cannot be detected by the proposed multiplex system. Although not generally considered to be of independent prognostic significance, these aberrations can be very important in the detection of minimal residual disease. Finally, the relative contribution of this reaction and fluorescent in situ hybridization (FISH) techniques36 cannot presently be directly evaluated, but (as with karyotypic analysis) we would expect these methodologies to be complementary.
For at least two reasons, the PCR methodology cannot fully replace karyotypic analysis. First, numerical aberrations and abnormalities other than balanced translocations cannot be detected. Second, unknown balanced translocations are obviously not detected. Cytogenetic analysis will thus form an important platform for molecular characterization of new genetic aberrations.
Because of its versatility and sensitivity, we believe that this novel multiplex PCR procedure holds promises as a screening tool for the initial diagnostic phase of acute leukemia. In addition, moving the PCR methodology up-front will allow its use for remission evaluation, when bone marrow material is often scarce. Here, the very high sensitivity of the PCR reaction may yield information in cases in which the translocation found at diagnosis would also be detected at remission. Our retrospective data (patients no. 70, 90, 108, 259, and 265) and the preliminary clinical experience clearly support the notion given above, because we have positive multiplex reaction in several marrow preparations at remission for the corresponding translocation identified originally by cytogenetic analysis at diagnosis.
It might be argued that the multiplex concept is weakened by the decrease in sensitivity relative to single PCR reactions, particularly when the multiplex reaction is used for detection of minimal residual diseases (eg, in Ph+ patients). In acute leukemia at diagnosis, the cell source used for RNA preparations is usually greater than 90% leukemic blasts. However, as demonstrated in this report, the sensitivity of our reactions is comparable to single-pair reactions probably because of the use of two (nested) primer sets. Moreover, the split-out primer sets will have a sensitivity comparable to the individual multiplex reactions.
We believe that the multiplex PCR assay is clinically useful as an efficient and fast procedure for the detection of genetic changes in acute leukemia and that it complements the cytogenetic analysis in a fruitful manner. Clearly, this novel approach for addressing the multitude of genetic changes in acute leukemia is open for addition of new primer sets as information of novel translocations accumulates. However, in our hands, this reaction has already yielded new information in the retrospective setting as well as in the initial diagnosis. Finally, our approach can be extended to the detection of minimal residual leukemia, because the split-out reaction has a sensitivity equivalent to that of single PCR assays. Thus, the multiplex approach would be of significance not only at diagnosis, but also for subsequent clinical decision-making.
ACKNOWLEDGMENT
The authors thank Dr Niels Clausen (Aarhus University Hospital) for supplying the pediatric ALL samples and Dr Kazuma Ohyashiki (Tokyo Medical Collage) for the HAL-01 cell line. We are indebted to Dr K. Paludan for critical reading of the manuscript.
Supported by Research Grants from the Danish Cancer Society and the Karen Elise Jensen Foundation.
Address reprint requests to Poul Jørgensen, PhD, Institute of Molecular and Structural Biology, Aarhus University, C.F. Møllers Allé Bld. 130, DK-8000 Aarhus C, Denmark; e-mail:pj@mbio.aau.dk.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.