Abstract
It is now well known that the initial phase of graft-versus-host disease (GVHD) involves cytokine release during preconditioning of the recipient of an allogeneic bone marrow transplant (BMT). Tumor necrosis factor (TNF), in particular, has been implicated in pathological damage and is released pretransplant due to irradiation and cytotoxic preconditioning regimens. Interleukin-10 (IL-10), a natural immunosuppressant of TNF , may be involved in downregulation of these responses, which may be an individual patient-specific effect. In this study, we determined the genotype for polymorphisms associated with TNF and IL-10 in 80 potential allo-BMT recipients and correlated the genotype with the severity of GVHD in 49 patients for whom clinical data relating to GVHD was available. The widely studied TNF −308 polymorphism does not show any significant associations, but the d3 homozygous allele of the TNFd microsatellite is preferentially associated with grade III/IV GVHD (7 of 11 patients) compared with its occurrence in 8 of 38 patients with grade 0/II GVHD (P = .006). Alleles of the IL-10 −1064 promoter region microsatellite polymorphism that possess greater numbers of dinucleotide (CA) repeats also significantly associate with more severe GVHD. This region has been demonstrated to be important in the regulation of the IL-10 promoter. Eighteen of 38 patients with grade 0-II GVHD possessed alleles with greater numbers (12 or more) of dinucleotide repeats, compared with 9 of 11 cases with grade III-IV GVHD (P < .02). Of the 38 patients with grade 0-II GVHD, 3 of 38 had a both TNFd3/d3 and IL-10 (12-15) genotype, compared with 6 of 11 patients with grade III-IV GVHD (P < .001). There was no association of either the TNFd or IL-10 microsatellite polymorphisms with mortality (P = .43 and .51, respectively). Our results suggest that patient cytokine gene polymorphism genotypes may influence GVHD outcome by affecting cytokine activation during the pretransplant conditioning regimens, and these results are the first to suggest a genetic predisposition to this important transplant-related complication.
THE PROINFLAMMATORY cytokines and their related receptors and inhibitors (interleukin-1 [IL-1], IL-1r, IL-1ra, IL-2, IL-6, tumor necrosis factor α [TNFα], and IL-10) have been implicated in a number of immunological diseases (briefly reviewed in Daser et al1), including graft-versus-host disease (GVHD) after allogeneic bone marrow transplantation (BMT). In GVHD, an initial insult during preconditioning provokes an inflammatory response and release of IL-1 and TNFα. The subsequent cascade of cytokine production initiates tissue damage, which is compounded by a second phase involving the activation of alloreactive T cells from the donor marrow.2,3 Raised TNFα levels have been reported during the initial phase of pretransplant conditioning for allo-BMT and have been shown to correlate with severe complications and mortality after BMT.3,4 A recent clinical phase II study using monoclonal antibodies to TNFα as part of the conditioning regimen led to a delay in the onset and severity of the GVHD.5 These studies suggest a role of recipient TNFα production in the subsequent development of GVHD.
The gene encoding the proinflammatory cytokine TNFα is located within the major histocompatibility complex (MHC) locus on chromosome 6, which may account, in part, for the association between HLA type and some immune diseases. Inducibility of TNFα has been found to be low in HLA DR2 genotypes and high in HLA DR3 and DR4 genotypes,6 suggesting the existence of inherently high or low TNFα producers in the population. A polymorphism in the promoter region of the TNFα gene, the −308 (G/A) polymorphism of an AP-2 transcription factor binding site, has been widely studied and is associated with the HLA haplotype (A1, B8, DR3),7-9 with increased in vitro TNFα expression being associated with the uncommon TNF2 (A) allele. It has been suggested that high levels of TNFα secretion after mitogen stimulation of peripheral blood lymphocytes from BMT recipients may be associated with this TNF2 (A) allele.4
A number of microsatellite repeat sequence elements have been mapped around the TNFα gene locus.10 One of these highly informative dinucleotide (GA) microsatellites, TNFd, has been mapped downstream of the TNFα gene and is located within an intron of the recently described LST1 gene.11 The d3 allele of this polymorphism has been associated with higher in vitro TNFα production in cells derived from immune-suppressed heart transplant recipients.12 Interestingly, this association is not evident when cells derived from normal, non–immune-suppressed subjects are stimulated to measure TNFα expression, possibly indicating that the proposed associations may only be manifested after conventional immunosuppression.
IL-10 is a potent immunosuppressant produced by monocytes and a strong inhibitor of TNFα (for review see Roncarolo13 and Korholz et al14). Low levels of IL-10 production have been correlated with increased incidence of both acute and chronic GVHD.3 The ability of donor as well as host cells to produce either high or low levels of TNFα and IL-10 may influence transplant outcome. In this regard, heart transplant patients were shown to be more susceptible to rejection episodes if they produced high levels of TNFα and low levels of IL-10.15,16 A number of polymorphisms of the IL-10 gene have been described, including single base (G/A) polymorphism (−1082) associated with altered levels of in vitro IL-10 expression16 and microsatellite polymorphism (−1064) mapping near candidate IL-10 gene control elements.17 Recent findings suggest a role for some of these polymorphisms in determining rejection in heart transplant recipients.15 16
In this present study we have determined the BMT recipient TNFα and IL-10 genotype for gene polymorphisms associated with either reported cytokine productivity or candidate gene control regions and correlated results with HLA phenotype and clinical outcome, including the incidence and severity of posttransplant GVHD.
MATERIALS AND METHODS
Patients and normal controls for polymorphism studies.
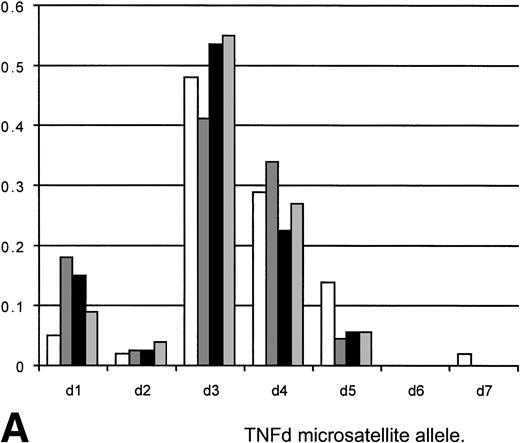
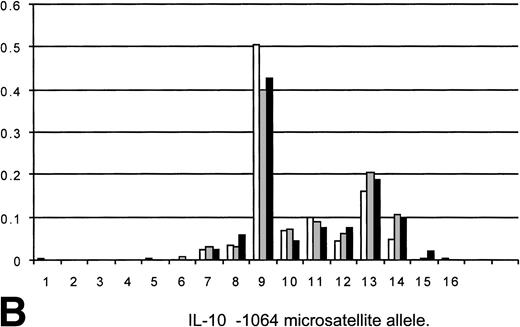
Eighty patients being considered for transplant with underlying hematological malignancies predominantly acute leukemia (ALL) or chronic granulocytic leukemia (CGL) were tested for TNFα and IL-10 polymorphism allele frequencies and genotype. Twenty-eight normals (laboratory personnel) were also tested for TNFα and IL-10 polymorphism allele frequencies and genotype. The results are included in Fig 1A and B.
(A) Allele frequencies for the TNFd microsatellite polymorphism as measured in this study (80 Allo BMT recipients, 160 alleles) and two other published reports (Udalova et al10and Turner et al12). Results on a panel of 28 unselected normals (56 alleles) are also shown. The observed allele frequencies show similar frequencies to those reported elsewhere, indicating that the appropriate allele designations/identifications were being made. The frequency of predicted d3/d3 homozygotes of 23.1 (predicted from the allele frequency by Hardy-Weinberg equilibrium) compared well with the observed frequency of 26. (□) HT panel (210); (▧) heart transplants (44); (▪) BMT (160); (▧) 28 normals (56). (B) Allele frequencies for the IL-10 −1064 microsatellite as measured in this study (80 allo-BMT recipients, 160 alleles) plus frequencies from two other studies (Eskdale and Gallagher19and Worthington et al, personal communication, 1998). The observed allele frequencies show similar frequencies to those reported elsewhere, indicating that the appropriate allele designations/identifications were being made. The frequency of genotypes predicted from the allele frequencies by Hardy-Weinberg equilibrium compare well with the observed frequencies (i9/9 predicted 14.45, observed 15; i9/13 predicted 12.75, observed 13). (□) Gallagher (204); (▧) Worthington; (▪) BMT (160).
(A) Allele frequencies for the TNFd microsatellite polymorphism as measured in this study (80 Allo BMT recipients, 160 alleles) and two other published reports (Udalova et al10and Turner et al12). Results on a panel of 28 unselected normals (56 alleles) are also shown. The observed allele frequencies show similar frequencies to those reported elsewhere, indicating that the appropriate allele designations/identifications were being made. The frequency of predicted d3/d3 homozygotes of 23.1 (predicted from the allele frequency by Hardy-Weinberg equilibrium) compared well with the observed frequency of 26. (□) HT panel (210); (▧) heart transplants (44); (▪) BMT (160); (▧) 28 normals (56). (B) Allele frequencies for the IL-10 −1064 microsatellite as measured in this study (80 allo-BMT recipients, 160 alleles) plus frequencies from two other studies (Eskdale and Gallagher19and Worthington et al, personal communication, 1998). The observed allele frequencies show similar frequencies to those reported elsewhere, indicating that the appropriate allele designations/identifications were being made. The frequency of genotypes predicted from the allele frequencies by Hardy-Weinberg equilibrium compare well with the observed frequencies (i9/9 predicted 14.45, observed 15; i9/13 predicted 12.75, observed 13). (□) Gallagher (204); (▧) Worthington; (▪) BMT (160).
Cytokine gene polymorphism studies were performed on all patients, including the 49 of 80 patients who subsequently underwent BMT from an HLA– and HLA-DR–matched, mixed lymphocyte culture-negative, sibling donor. The TNFα and IL-10 polymorphism results from these patients were correlated with incidence and severity of GVHD. These patients included 32 males and 17 females (median age, 33; age range, 14 to 47 years). All 49 patients received non–T-cell–depleted marrow grafts. The underlying diseases were CGL (n = 12), acute myeloid leukemia (AML; n = 18), ALL (n = 15), Hodgkin’s disease (HD; n = 1), non-Hodgkin’s lymphoma (NHL; n = 1), and myelodysplastic syndrome (MDS; n = 2).
Conditioning regimens and GVHD prophylaxis.
Before January 1991, standard conditioning for the patients with acute leukemia was with cyclophosphamide (120 mg/kg) and total body irradiation (TBI; 12 Gy in 6 fractions in 3 days at 25 cGy/min). Since January 1991, the TBI dose has remained the same, but melphalan (3 mg/kg) has been substituted for the cyclophosphamide. Patients with MDS were conditioned with cyclophosphamide alone. Patients with CGL were usually conditioned with busulphan (4 mg/kg/d for 4 days) and cyclophosphamide.
The majority of patients were treated with either cyclophosphamide or melphalan plus TBI (Table 1). The number of patients treated with these two types of conditioning regimes were equally distributed between the cohorts of patients developing GVHD grades 0-II and GVHD III-IV (30 of 38 and 9 of 11, respectively; χ2 = .043; df = 1; P = .835; Tables 1and 2).
GVHD prophylaxis and therapy.
GVHD prophylaxis was with cyclosporin alone, administered intraveneously at a dose of 5 mg/kg until oral treatment could be tolerated, when the same dose was administered by mouth. Once clinical GVHD was diagnosed, standard therapy was administered with high-dose steroids; if there was no immediate response, this was followed by increased cyclosporin (5 to 10 mg/kg).
Ethics committee approval and informed consent.
This study was approved by the local ethics committee and informed consent was obtained from all patients and normal controls under study.
HLA typing.
All patients in this study were tested by conventional serology for HLA A and B alleles along with low resolution molecular typing of DRB1 using polymerase chain reaction (PCR) sequence-specific primer.18 All tissue typing was routinely performed at the Northern Blood Transfusion Service (Newcastle, UK).
TNFα and IL-10 genotypes.
Patient genotypes were determined for the TNFα−308, TNFd microsatellite, and IL-10 −1064 microsatellite polymorphisms essentially as described.7,10,19 PCR products were separated by gel electrophoresis on polyacrylamide gels (10%, 19:1 acryl:bis) and visualized by silver staining. All PCR products were compared with a control of known genotype (TNF−308 1/2, TNFd1/d3, IL-10 i9/13) to ensure accurate interpretation. Allele designations, eg, d1/d3, 9/13 etc, are as described in the original reports of these polymorphisms.7,10 19
Statistical analyses.
Comparisons between patient groups were made using the χ2test for heterogeneity.
RESULTS
Incidence and severity of GVHD.
GVHD was diagnosed and graded according to previously published criteria.20 Of the 49 patients transplanted, 9 showed no evidence of acute GVHD. Fourteen patients developed grade I GVHD: 10 with skin alone, 3 with skin plus gastrointestinal involvement, and 1 with gastrointestinal involvement alone. Fifteen patients developed grade II GVHD; all had skin involvement, 4 had hepatic involvement (2 with gastrointestinal disease), and 3 had skin and gastrointestinal involvement with no evidence of liver disease. Eleven of 49 patients developed severe (grade III-IV) GVHD. All 9 patients with grade III disease had skin and gastrointestinal GVHD, together with liver involvement in 4 patients. In this whole cohort, only 2 patients died of GVHD (Tables 1 and 2).
TNFα−308 polymorphism.
Of the 80 patients tested, 3 were homozygous for the uncommon TNF2 allele (the allele frequencies would predict 2.1 homozygotes) and 20 cases were heterozygous (TNF1/2). The allele frequencies of TNF1 and TNF2, respectively, were 0.838 and 0.162. These results were comparable with those quoted for a normal Caucasian population (0.84 and 0.16, respectively, measured on 40 individuals [80 alleles]7). In common with other studies, a strong association of the TNF2 allele with HLA haplotypes containing DR3, and a weaker association with DR4 was observed. (All but 4 of the observed TNF2 alleles were carried by individuals possessing a DR3 or a DR4 haplotype.)
The rare allele TNF2 did not associate with incidence or severity of GVHD. Thirteen of 38 patients with GVHD grade 0-II and 2 of 11 patients with grade III/IV GVHD possessed a TNF2 allele. There was no association of GVHD with HLA type.
Allele frequencies of the TNFd and IL-10 microsatellite polymorphisms.
The allele frequencies of TNFα and IL-10 microsatellites found in the 80 patients tested, along with those previously reported in other studies, and a panel of 28 unselected normals are shown in Fig 1A and B. The observed allele frequencies were similar to those reported elsewhere, indicating that the appropriate allele designations were made for patient and normal control cohorts. The frequency of predicted homozygotes (as predicted by Hardy-Weinberg equilibrium) for the more common alleles compare with the observed frequencies, suggesting that homozygous genotypes were being appropriately assigned in the study group (TNFd3/d3 predicted v observed; 23.1 v 26; IL-10 i9/9 predicted v observed; 14.45 v 15; i9/13 predictedv observed; 12.75 v 13).
TNFd and IL-10 microsatellite polymorphisms and association with GVHD.
The d3 allele of the TNFd microsatellite was preferentially associated with grade III/IV GVHD, with 7 of 11 patients possessing the d3/d3 genotype, compared with its occurrence in 8 of 38 patients with grade 0-II (χ2 = 7.598; df = 1; P < .006; Tables 1and 2).
Alleles of the IL-10 −1064 promoter region microsatellite polymorphism that possessed greater numbers of dinucleotide repeats (alleles 12, 13, 14, or 15, as described by Eskdale and Gallagher19) also significantly associated with more severe GVHD. Eighteen of 38 patients with grades 0-II GVHD possessed high repeat number alleles (12-15; Table 1), compared with 9 of 11 patients with grades III-IV GVHD only (χ2 = 5.443; df = 1; P < .02; Table 2).
If both TNFd and IL-10 genotypes were considered together, there appears to be a retrospective association with GVHD severity and occurrence. Of the 38 patients with grade 0-II GVHD, 3 of 38 had a TNFd3/d3/IL-10 (12-15) genotype, compared with 6 of 11 patients with grade III-IV GVHD (χ2 = 14.11; df = 1; P < .001).
TNFd and IL-10 microsatellite polymorphisms and association with death and relapse.
The TNFd3/d3 genotype was not associated with increased mortality; 8 of 15 patients with the homozygous genotype died, compared with 14 of 34 patients who did not have this allele (χ2 = .62; df = 1;P = .43). Possession of an IL-10 genotype with alleles containing greater numbers of dinucleotide repeats (alleles 12, 13, 14, or 15) was not associated with mortality; χ2 analysis of its association failed to reach significance (χ2 = .42; df = 1; P = .51). Of the 9 patients who died from disease relapse, 8 had grade 0-I GVHD, compared with 1 relapse from the group of patients with grade II-III-IV GVHD (χ2 = 7.79; df = 1;P < .006).
DISCUSSION
The importance of the cytokine cascade in both the initial preconditioning and posttransplant phases of GVHD is well established. TNFα secretion is of particular importance during the irradiation and cytotoxic treatment of the recipient before transplant giving rise to initial endothelial cell damage and aiding T-cell activation by upregulation of class I, class II, and adhesion molecules. Several reports have dealt with the measurement of serum TNFα and its role in predicting GVHD. One problem that arises that is independent of the problems associated with serum measurements of cytokines is that high levels of TNFα are also associated with other transplant-related complications, including infection and veno-occlusive disease.3
In this present study, the d3 allele of the TNFd polymorphism was found to be associated with severe GVHD grades III/IV but not with overall clinical survival. This result suggests that the effect seen in the d3/d3 TNFd genotype may be susceptible to immunosuppression as suggested by Turner et al12 in studies on heart transplant recipients in which the effect was related to the degree of immunosuppression. The increased incidence of GVHD in the homozygous d3/d3 cohort was associated with increased GVHD, but also this subpopulation was possibly more responsive to the GVHD therapy in the form of increased cyclosporin and methotrexate and therefore did not succumb to fatal GVHD. A similar effect may be the explanation for the lack of correlation with GVHD mortality and IL-10 (12-15) allele polymorphisms. This hypothesis can only be tested in larger cohorts of patients with and without increased GVHD prophylaxis and altered therapy. Our studies have also shown that the −308 polymorphism was not associated with GVHD severity. Mayer et al4 have recently completed a study on 53 CML allograft recipients indicating a possible role of the −308 polymorphism in severe GVHD. That study was confined to CML patients and included matched unrelated donor transplants as well as HLA-identical siblings (personal communication, 1998) and therefore may not be directly compared with this present study. Our study is also too small to correlate TNF−308 polymorphism with incidence or severity of GVHD in particular cohorts of leukemia patients.
A large number of studies have looked unsuccessfully for associations between TNF2 genotype and disease incidence, severity, or susceptibility in a range of immunoregulatory disorders. These include studies on rheumatoid arthritis,21,22 systemic lupus erythematosus,23 inflammatory bowel disease,24lichen sclerosus,25 and ankylosing spondylitis.26 An association between the TNF2 allele and the incidence of insulin-dependent diabetes mellitus has been reported, but this association is not independent of the HLA type and probably reflects linkage disequilibrium between the TNF2 polymorphism and the ancestral HLA haplotype.27 A similar association has been seen in rheumatoid arthritis patients with systemic lupus erythematosus.28 An association between disease incidence and the TNF2 allele that is independent of the HLA type has been reported in studies on asthma.29 Taken together, these findings indicate that the TNF2 genotype may not be important in immunoregulatory disorders and that other candidate loci or genetic elements must be considered.
A proven association of cytokine gene polymorphisms with GVHD would undoubtedly enable the clinician to modify therapy in those patients predicted to develop GVHD by virtue of their underlying cytokine genotype. The study reported here documents the possible role of IL-10 gene polymorphism in GVHD. Analysis of both the TNFα and IL-10 polymorphisms and GVHD severity was based on knowledge that grade 0-II GVHD is more susceptible to therapeutic intervention than grades III-IV, which are often fatal.18 This increase in risk of severe GVHD if possessing risk-associated alleles for both the TNFα d3/d3 and IL-10 (12, 13, 14, and 15) genotype was highly significant (P < .001). However, possession of either genotype alone was also significant. These results suggest both an interacting role for TNFα and IL-10 and a role for both as independent indicators of severity of GVHD. This further suggests that other factors undoubtedly regulate TNFα and IL-10 production and play a role in the ultimate degree of GVHD.
The extension of these studies to include other indicators/predictors of GVHD in HLA-identical siblings, such as minor histocompatibility testing,30 HTLp frequency analysis,31 cytokine production,32 and/or predicting GVHD using a skin explant model,33 may allow the development of an individual risk index for GVHD. Such a risk index would allow for improved management for GVHD, which is still the major cause of morbidity and mortality after allogeneic BMT.
Recent approaches to therapy include engineering a shift in cytokine profiles before BMT as a way to attenuate inflammatory processes of GVHD.3 5 These approaches include the possible use of anti-TNFα antibodies and recombinant IL-10, a strong inhibitor of TNFα. The finding of 8 of 9 deaths from disease relapse in the grade 0-I GVHD group suggests that prior knowledge of a patient’s risk status for cytokine production might be of value not only in attenuating the effects of GVHD in high-risk individuals, but also in potentially upregulating the response in GVHD low-risk individuals to manipulate the graft-versus-leukemia (GVL) effect. Such management options would only become reality if accurate GVHD/GVL predictions for an individual patient could be made.
ACKNOWLEDGMENT
The authors thank the Blood Transfusion Service (Barrack Road, Newcastle, UK) for HLA typing data and Jane Worthington (ARC Epidemiology Research Unit, Manchester, UK) for advice on the IL-10 microsatellite. We also thank Jim Cavet for critical reading of the manuscript.
Supported by a Tyneside Leukaemia Research Association Programme Grant.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Peter G. Middleton, PhD, LRF Laboratory, Catherine Cookson Building, University of Newcastle, Medical Molecular Biology Group, Floor 4, The Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, UK.