Abstract
Flavopiridol has been reported to induce apoptosis in lymphoid cell lines via downregulation of bcl-2. The in vitro activity of flavopiridol against human chronic lymphocytic leukemia (CLL) cells and potential mechanisms of action for inducing cytotoxicity were studied. The in vitro viability of mononuclear cells from CLL patients (n = 11) was reduced by 50% at 4 hours, 24 hours, and 4 days at a flavopiridol concentration of 1.15 μmol/L (95% confidence interval [CI] ±0.31), 0.18 μmol/L (95% CI ±0.04), and 0.16 μmol/L (95% CI ±0.04), respectively. Loss of viability in human CLL cells correlated with early induction of apoptosis. Exposure of CLL cells to 0.18 μmol/L of flavopiridol resulted in both decreased expression of p53 protein and cleavage of the caspase-3 zymogen 32-kD protein with the appearance of its 20-kD subunit. Contrasting observations of others in tumor cell lines, flavopiridol cytotoxicity in CLL cells did not correlate with changes in bcl-2 protein expression alterations. We evaluated flavopiridol’s dependence on intact p53 by exposing splenocytes from wild-type (p53+/+) and p53 null (p53−/−) mice that demonstrated no preferential cytotoxicity as compared with a marked differential with F-ara-a and radiation. Incubation of CLL cells with antiapoptotic cytokine interleukin-4 (IL-4) did not alter the LC50 of flavopiridol, as compared with a marked elevation noted with F-ara-a in the majority of patients tested. These data demonstrate that flavopiridol has significant in vitro activity against human CLL cells through activation of caspase-3, which appears to occur independently of bcl-2 modulation, the presence of IL-4, or p53 status. Such findings strongly support the early introduction of flavopiridol into clinical trials for patients with B-CLL.
B-CELL CHRONIC lymphocytic leukemia (CLL) is the most common leukemia in the Western hemisphere, with approximately 10,000 new cases diagnosed each year.1Relative to other forms of leukemia, the overall prognosis of CLL is good, with even the most advanced stage patients having a median survival of 3 years.2 However, unlike most of the other forms of acute and chronic leukemia, substantial therapeutic progress has not been made over the past 40 years in either prolonging survival or introducing curative therapy. The addition of fludarabine as initial therapy for symptomatic CLL patients has led to a higher rate of complete responses (27% v 3%) and duration of progression-free survival (33 v 17 months) as compared with previously used alkylator-based therapies.3 Although attaining a complete clinical response after therapy is the initial step toward improving survival in CLL, the majority of patients either do not attain complete remission or fail to respond to fludarabine. Furthermore, all patients with CLL treated with fludarabine eventually relapse, making its role as a single agent purely palliative. Therefore, identifying new agents with novel mechanisms of action that complement fludarabine’s cytotoxicity and abrogate the resistance induced by intrinsic CLL drug-resistance factors will be necessary if further advances in the therapy of this disease are to be realized.
Several biologic factors, including abnormal p53 function, overexpression of bcl-2, and incubation of tumor cells with interleukin-4 (IL-4), have been associated with either in vitro or in vivo drug resistance in CLL.4-12 Of these, the most extensively studied, uniformly predictive factor for poor response to therapy and inferior survival in CLL patients is aberrant p53 function, as characterized by point mutations or chromosome 17p13 deletions.4-7 Indeed, virtually no responses to either alkylator or purine analog therapy have been documented in multiple single institution case series for those CLL patients with abnormal p53 function.4-7 Introduction of a therapeutic agent that has the ability to overcome the drug resistance associated with p53 mutation in CLL would potentially be a major advance for the treatment of the disease. We describe here that flavopiridol, a novel synthetic flavone currently entering phase II trials, demonstrates marked in vitro cytotoxicity toward human CLL cells and may circumvent either IL-4–induced resistance or that incurred by a p53 mutation.
MATERIALS AND METHODS
Patients, Cell Separation, and Culture Conditions
Written, informed consent was obtained to procure cells from patients with previously diagnosed B-CLL as defined by the modified NCI criteria13 and normal healthy volunteers. Clinical data provided in Table 2 includes modified Rai stage, previous treatment, and fludarabine response status at the time of cell acquisition. Criteria for being considered fludarabine refractory included lack of partial or complete response to treatment with this agent or relapse within 6 months of last fludarabine treatment. Response was judged according to the modified NCI criteria.13 Mononuclear cells were isolated from the peripheral blood using density gradient centrifugation (Ficoll-Paque Plus; Pharmacia Biotech, Piscataway, NJ). Cells were immediately cultured (1 × 107 cells/mL) in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin-G, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine (Life Technologies, Grand Island, NY) and incubated at 37°C in a 5% CO2 incubator.
Treatment of Mice and Harvested Cells
Homozygous typed p53-deficient mice (C57BL/GJ-Trp53tm1Tyj;p53−/−; The Jackson Laboratory, Bar Harbor, ME) and wild-type mice (C57BL/6NCR; p53+/+; Frederick Cancer Research and Development Center, Frederick, MD) were obtained. Cells from both spleens and thymus glands were harvested from the mice and were immediately cultured (5 × 106 cells/mL) in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin-G, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine (Life Technologies) and incubated at 37°C in a 5% CO2incubator. Flavopiridol (NSC649890) and F-ara-a (Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute) and recombinant IL-4 (Promega Corp, Madison, WI) were obtained for in vitro studies described later in the text.
Viability Assays
Viability assays of isolated mononuclear cells from CLL patients were performed using the MTT assay. Cells (1 × 106 per well) in a volume of 100 μL were placed in a 96-well flat-bottom plate and the test drug (100 μL per well at 2× final concentration) or medium alone was added to the plates. All human experiments were performed in quadruplicate. Cells were incubated for fixed times (4 hours, 24 hours, and 96 hours). After the incubation, 50 μL of MTT (Sigma Chemical Co, St Louis, MO) at a concentration of 2 mg/mL was added to each well. Cells were incubated for 24 hours and centrifuged at 300g for 5 minutes, and 150 μL of supernatant was removed. A total of 200 μL of protamine sulfate (Sigma) in phosphate-buffered saline (PBS) at 25 mg/mL was added to each well. Centrifugation was repeated, followed by the replacement of 200 μL of protamine sulfate. Plates were centrifuged and 200 μL of protamine sulfate was removed. Plates were then allowed to air dry. The precipitated MTT formazan was solubilized with 150 μL of dimethyl sulfoxide (DMSO) with constant agitation for 4 hours. After this procedure, the optical density at 540 nm was obtained using an Anthos Reader 2001 (Anthos Labtec Inc, Frederick, MD) with a Biolise-Windows program. Cell viability was expressed as the ratio of absorption between drugged cells/control sample.
Viability assays on isolated cells from the p53−/− and p53+/+ mice were performed using the trypan blue exclusion assay. All experiments were performed in duplicate. Cells were counted with a hemocytometer and viability was expressed as the percentage of alive equal to alive/(alive + dead) × 100. The relative effects of flavopiridol and F-ara-a exposure after 24 hours of exposure were expressed as the viability with drug/viability in medium.
Apoptosis Assays
After 4 and 24 hours of incubation with 0.18 μmol/L flavopiridol in supplemented RPMI and 10% FBS, apoptosis studies were performed using the following techniques.
TdT/PI.
Cells (5 × 105) were added to cold 1% buffered formaldehyde (10% Formadehyde [methanol free, ultrapure grade; Polysciences, Inc, Warrington, PA], prepared in Dulbecco’s phosphate-buffered saline without CaCl2 and MgCl2 [PBS]) for 15 minutes on ice. Cells were then washed with PBS and resuspended in 70% methanol and stored at −20°C. To complete the assay, cells were washed with PBS, resuspended in 50 μL of Cacodylate/biotin dUTP reaction buffer (0.2 mol/L potassium cacodylate, 25 mmol/L Tris-HCl [pH 6.6], 2.5 mmol/L cobalt chloride [CoCl2], 0.25 mg/mL BSA, 10 U of TdT, and 0.5 nmol biotinylated deoxyuridine triphosphate) with a TdT-negative control for each sample (all supplies from Boehringer Mannheim, Mannheim, Germany) and incubated for 60 minutes at 37°C. Cells were then washed twice with 2 mL of rinsing buffer (0.1% Triton X-100 and 5 mg/mL bovine serum albumin [BSA] in PBS), resuspended in 100 μL of fluorescein isothiocyanate (FITC)-Avidin solution (4× saline sodium citrate buffer; 0.1% Triton X-100; 5% nonfat dry milk, and 2.5 μg/mL of fluoresceinated avidin [Boehringer Mannheim]), and incubated for 30 minutes in a light-protected area. Cells were subsequently washed twice with rinsing buffer, resuspended in 0.25 mL of PI/RNAse solution (100 μg/mL propidium iodine [Sigma] and 7.5 μg/mL RNAse-DNAse free [Boehringer Mannheim] in PBS), and incubated for 30 minutes. Samples were analyzed on a FACScan (Becton Dickinson, San Jose, CA) illuminated at 488 nm and measuring green fluorescence (detecting FITC levels) at 530 nm using an exponential scale. Red fluorescence (measuring PI content of the cells) was measured on a linear scale at greater than 600 nm. All samples were analyzed in duplicate or triplicate fashion.
Annexin/PI.
After incubation with 0.18 μmol/L of flavopiridol or medium for 4 or 24 hours, 5 × 105 cells were washed with PBS and then resuspended in binding buffer (10 mmol/L HEPES/NaOH, pH 7.4, 150 mmol/L NaCl 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2) containing 2 μL of Annexin V-FITC stock (BioWhittaker, Inc, Walkersville, MD) and 10 μL of 20 μg/mL PI (Sigma). After incubation for 10 minutes at room temperature in a light-protected area, the specimens were quantified by flow cytometry on a FACScan (Becton Dickinson).
Protein Extraction and Western Blot Analysis
The bcl-2, bax, p53, p27, and CPP32 protein expressions were analyzed by Western blot after incubation in either medium or two concentrations of flavopiridol (0.18 and 0.33 μmol/L) for 1, 4, and 24 hours. Whole-cell lysates were prepared by pelleting 1.25 × 108 PBS-washed mononuclear cells in a microcentrifuge, aspirating the supernatant, and adding 0.5 mL of cold lysis buffer, as described previously.14 This cell suspension was incubated at 4°C for 40 minutes with constant agitation and then centrifuged for 15 minutes at 14,000 rpm at 4°C. The supernatant was recovered, alliquoted, and frozen at −80°C.
Protein was quantified in each supernatant by the BCA method (Pierce, Rockford, IL). A varied amount (2 to 100 μg) of each sample was used for each protein studied based on varied expression of protein in human CLL cells. Once identified, a single loading concentration was used for each protein examined. Samples were diluted with Tris-buffered saline to a volume of 25 μL and then added to 25 μL of 2× Laemmli’s Sample Buffer, as previously described.15 The samples were boiled for 4 minutes. Rainbow-colored protein molecular weight markers (Amersham Life Science, Arlington Heights, IL) and samples were loaded onto 10% to 14% polyacrylamide gels and electrophoresed. The proteins were transferred to a 0.45-μm nitrocellulose membrane (Schleicher and Schueel, Keene, NH) using an electroblot apparatus (Hoefer, San Francisco, CA). The transfer buffer consisted of 0.1 mol/L Tris-HCl, pH 8.8, 0.192 mol/L glycine, and 10% methanol.
After transfer of the proteins, the nitrocellulose membranes were blocked for 1 hour in TBS-T (Tris-buffered saline-0.05% Tween; JT Baker, Phillipsburg, NJ) containing 5% skim milk for 24 hours. The membranes were incubated with either 1 μg/mL of monoclonal mouse antihuman bcl-2 antibody clone 124 (Dako, Carpinteria, CA), 1 μg/mL of polyclonal goat antihuman CPP32 p20 (N-19) antibody (Santa Cruz Biotechnology, Santa Cruz, CA), 2 μg/mL polyclonal rabbit antihuman bax (Santa Cruz Biotechnology), 1 μg/mL polyclonal rabbit antihuman p27 (Oncogene Research Products, Cambridge, MA), or 0.1 μg/mL of monoclonal p53 (Ab6; Oncogene Research Products) diluted in TBS-T with 5% skim milk for 24 hours at 4°C or for 1 hour at room temperature with constant agitation. After washing 4 times with TBS-T, the blots were incubated with horseradish peroxidase-conjugated antimouse IgG (H and L chains; Pierce), horseradish peroxidase-conjugated antigoat IgG (Santa Cruz Biotechnology), or horseradish peroxidase-conjugated antirabbit IgG (Caltag Laboratories, Burlingame, CA) diluted 1:5,000 with 5% skim milk in PBS for 1 hour at room temperature with constant agitation. The blots were washed 4 times with TBS-Tween and shown with chemiluminescent substrate (Pierce Super-Signal chemiluminescent; Pierce) for 1.5 minutes. Autoradiography was performed with x-ray film (Kodak, Rochester, NY). Gel-loading equivalence was confirmed either by reprobing with 1 μg/mL polyclonal goat antihuman actin (I-19; Santa Cruz Biotechnology) or by exposing the nitrocellulose gel to Fast Green Stain to show total protein banding pattern. Protein bands were quantified by computer densitometry.
Polymerase Chain Reaction (PCR) of Murine Splenocytes
The genotypes of the mice were verified (1) by lification of the p53 exon 6/intron 6/exon 7 fragment in the p53 wild-type mouse and demonstration of the lack of product in the p53 knock out mouse and (2) by amplification of a neo/p53 exon 7 hybrid product in the p53 knock-out mouse and the lack of hybrid product in the p53 wild-type mouse. The amplification methods used have been previously described.16
PCRs were performed with 100 ng of genomic p53 wild-type and p53 knock-out DNA using 20 pmol of each primer in a 50 μL reaction with 1× Expand buffer (Boehringer Mannheim) containing 1.5 mmol/L MgCl2, 37.5 μmol/L of each dNTP, and 2.5 U DNA Taq polymerase (Qiagen, Valencia, CA). The PCR program consisted of 35 cycles at 94°C for 1 minute, 65°C for 1 minute, and 72°C for 1 minute. Murine-specific p53 exon 6 forward primers (W5′) and exon 7 reverse primers (W3′) were used to demonstrate the presence of an uninterrupted p53 gene in the p53 wild-type mice.17 The knock-out mice produced no p53 PCR fragment with this primer pair. Verification of the knock-out genotype was accomplished by PCR amplification of a hybrid neo/p53 product using forward neo primers (M5′) and p53 exon 7 reverse primers (W3′). Wild-type control mice lacked the inserted neo gene and therefore did not produce the hybrid neo/p53 PCR fragment. These primer sequences have been previously described.17
Fluorescent In Situ Hybridization for 17p13 Deletions
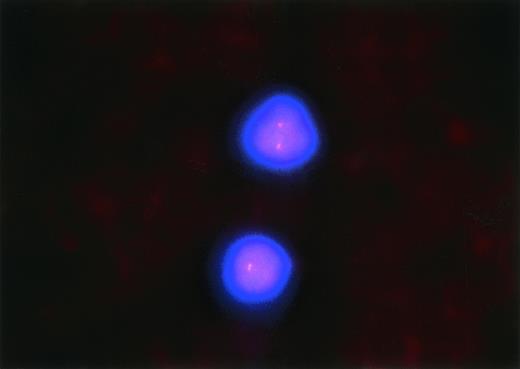
From peripheral blood that had been collected in EDTA collection tubes, smears were prepared from the patient 28a described in Table 2 and Fig3 and air-dried at ambient temperatures for 1 to 7 days. Before hybridization, the slides were denatured in 70% formamine/2× SCC (0.3 mol/L sodium chloride in 0.03 mol/L citric acid, pH 7.0) at 74°C ± 1°C for 5 minutes, followed by serial dehydration in ethanol. The slides were then drained and maintained on a 45°C to 50°C slide warmer for approximately 2 minutes during the time of probe denaturation. One microliter of the probe LSI p53 (17p13.1), labeled with Spectrum Orange (Vysis, Downers Grove, IL), was mixed with 2 μL purified H2O and 7 μL of Vysis’ LSI Hybridization Buffer. The probe mixture was next denatured in a 74°C ± 1°C water bath for 5 minutes. The denatured probe mixture was applied to the denatured specimen target (∼10 μL probe mixture for a 22 mm × 22 mm target area). A glass coverslip was applied to the target area and sealed with rubber cement. Hybridization was performed in a humidified chamber at 37°C for 14 to 18 hours. Posthybridization washes consisted of a 2-minute wash in 4× SSC/0.3% NP-40 at 74°C ± 1°C followed by 1 minute in 2× SSC/0.1% NP-40 (Sigma) at room temperature. The slides were then air-dried in the dark, counter-stained with Vysis’ DAPI in Antifade, cover-slipped, and examined with a Zeiss Standard Fluorescent Microscope (Olympus Optical Co, Tokyo, Japan) equipped with epifluorescence optics and dual bandpass for DAPI/Spectrum Orange. One hundred to 200 cells were counted, and the percentages of 0, 1, or 2 signals were determined. A diagnosis of monoallelic deletion of p53 was established when the percentage of cells with 1 signal exceeded the controls’ mean by greater than 3 standard deviations. The same procedure outlined above was applied to 16 blood smears that had been obtained from patients who had undergone bone marrow biopsy for staging of breast cancer and who had no evidence of metastatic breast cancer or other malignancy in the blood or bone marrow. For these 16 cases, the mean and standard deviation of the percentage of cells with 1 hybridization signal (±3 SD) was 10.3% (±20.3%). Patient 28a had 35% of cells showing 1 hybridization signal and 65% demonstrating 2 hybridization signals.
Statistics
Groups of data were compared using paired or nonpaired Student’st-tests (two-sided) as appropriate. Nonparametric data were analyzed using the Wilcoxan signed-rank test. JMP Statistics software (SAS Institute, Trumbull, CT) or Quatropro software (Novell Inc, Orem, UT) was used to perform these analyses.
RESULTS
Flavopiridol Is Cytotoxic Toward CLL Cells With an Optimal Exposure Period of 24 Hours
Peripheral mononuclear cells from 11 consecutive patients with CLL studied in the laboratory (UPN 1a, 2a, 3a, 5a, 6a, 7a, 9a, 10a, 1b, 2b, 3b, and 4b; see Table 2 for clinical data) were exposed to either medium or varying (0.01, 0.033, 0.1, 0.33, 1, 3.3, 10, 33, and 100 μmol/L) concentrations of flavopiridol. Cells were incubated either in drug or medium as follows: 4 hours and then developed; 4 hours and then washed of drug and developed after in vitro incubation in fresh medium for 4 days; 24 hours and then developed; 24 hours and then washed of drug and developed after in vitro incubation in fresh medium for 4 days; and developed after 4 days of continuous exposure to the agent. No loss of viability was noted immediately after 4 hours of flavopiridol exposure (data not shown). The viability of CLL cells at each other time point and concentration are depicted in Table 1. All of the patients with CLL demonstrated in vitro response to flavopiridol, including 5 patients with fludarabine-refractory CLL. The average concentration of flavopiridol required to produce 50% cytotoxicity (LC50) was 1.15 μmol/L (range, 0.52 to 1.93 μmol/L; 95% confidence interval [CI] ±0.31 μmol/L) after 4 hours of exposure to this agent followed by incubation in fresh medium until 4 days. After 24 hours of exposure to flavopiridol with immediate addition of MTT or incubation in fresh medium until 4 days, the LC50 decreased to 0.18 μmol/L (range, 0.09 to 0.33 μmol/L; 95% CI ±0.04 μmol/L) and 0.19 μmol/L (range, 0.1 to 0.26 μmol/L; 95% CI ±0.04 μmol/L), respectively. After continuous 96-hour exposure to flavopiridol, the LC50 declined to 0.16 μmol/L (range, 0.04 to 0.24 μmol/L; 95% CI ±0.05 μmol/L). Comparing these time points, the LC50 was significantly lower for both the 24-hour (P = .02) and the 96-hour (P= .02) flavopiridol exposures as compared with the 4-hour incubation. The LC50 was not significantly different (P = .57) between CLL cells exposed to flavopiridol for 24 versus 96 hours. Therefore, these data support a 24-hour treatment administration schedule for phase II studies of flavopiridol in patients with B-CLL.
Flavopiridol Is Equivalently Cytotoxic to Untreated and Previously Treated B-CLL
Because in vitro drug resistance is more frequently observed in previously treated patients, we next assessed if the degree of flavopiridol cytotoxicity varied by pretreatment status. A cohort of 27 patients with either untreated (n = 16) or previously treated (n = 11) CLL were identified from our institutions. The clinical history of these patients at the time of phlebotomy is described in Table 2. Of the previously treated CLL patients, 5 had fludarabine refractory disease. Mononuclear cells isolated from these patients were exposed to varying (0.01, 0.033, 0.1, 0.33, 1, 3.3, 10, 33, and 100 μmol/L) concentrations of flavopiridol for 4 days. Patients with untreated B-CLL had a mean LC50of 0.12 (range, 0.02 to 0.22 μmol/L; 95% CI ±0.03 μmol/L) after 4 days of exposure to flavopiridol. A cohort of previously treated B-CLL had a similar mean LC50 of 0.15 μmol/L (range, 0.03 to 0.24 μmol/L; 95% CI ±0.05 μmol/L) after 4 days of exposure to flavopiridol that was not significantly different (P = .77) from the untreated patients. Even for the 6 patients who were clinically fludarabine refractory, the LC50 was 0.17 μmol/L (range, 0.09 to 0.21 μmol/L; 95% CI ±0.05 μmol/L), suggesting that in vitro sensitivity to flavopiridol is not altered by prior treatment and more advanced disease.
Flavopiridol Is Also Cytotoxic Toward Normal Mononuclear Cells
To assess the tumor selectivity of flavopiridol against CLL cells, we exposed mononuclear cell isolates from 9 healthy volunteers to varying (0.01, 0.033, 0.1, 0.33, 1, 3.3, 10, 33, and 100 μmol/L) concentrations of flavopiridol. Cells were incubated under identical conditions to those of CLL cells, as described previously. No cytotoxicity was observed immediately after 4 hours of incubation. The LC50 after 4 hours of agent exposure followed by incubation in fresh medium until 4 days was 1.21 μmol/L (range, 0.60 to 1.90 μmol/L; 95% CI ±0.61 μmol/L) for normal cells. After 24 hours of exposure followed by incubation for 96 hours or a continuous 96-hour exposure to flavopiridol, the LC50 declined to 0.24 μmol/L (range, 0.15 to 0.27 μmol/L; 95% CI ±0.03 μmol/L) and 0.18 μmol/L (range, 0.02 to 0.21 μmol/L; 95% CI ±0.05 μmol/L), respectively. As shown in Fig 1, the LC50 was not significantly different (P = .57) between the mononuclear cells obtained either from volunteers or patients with CLL exposed to flavopiridol for 24 versus 96 hours, respectively. In fact, comparison of the therapeutic index between B-CLL cells and normal mononuclear cells demonstrated no significant difference at 4 (P = .16), 24 (P = .70), and 96 (P = .82) hours, respectively.
The in vitro cytotoxicity achieved after exposure of CLL cells to flavopiridol for different times. (□) Human CLL cells (n = 11 patients); (▨) normal mononuclear cells (n = 9 patients). Cytotoxicity is expressed as the LC50 (drug concentration required to reduce viability by 50%).
The in vitro cytotoxicity achieved after exposure of CLL cells to flavopiridol for different times. (□) Human CLL cells (n = 11 patients); (▨) normal mononuclear cells (n = 9 patients). Cytotoxicity is expressed as the LC50 (drug concentration required to reduce viability by 50%).
Flavopiridol Induces Apoptosis in CLL Cells
In an attempt to characterize if the cytotoxicity induced by flavopiridol was due to apoptosis, mononuclear cells from CLL patients (n = 7) were incubated for either 4 or 24 hours in medium or 0.18 μmol/L of flavopiridol. Unlike fludarabine, which induces apoptosis only after 1 day of exposure (data not shown), flavopiridol produced apoptosis as early as 4 hours postincubation. Indeed, the mean spontaneous apoptosis rate in medium incubated CLL cells at 4 hours was 12.8% (range, 4.4% to 17.7%; 95% CI ±3.6%), which increased significantly (P = .01) to 25.0% (range, 11.8% to 37%; 95% CI ±6.5%) with flavopiridol exposure. At 24 hours, the mean spontaneous apoptosis rate in medium was 20% (range, 10.2% to 34.1%; 95% CIs ±7.8%) that increased significantly (P = .02) to 73.4% (range, 60.7% to 87.4%; 95% CI ±11.0%). A representative tunnel assay of CLL cells (patient UPN 9a) incubated in medium or 0.18 μmol/L of flavopiridol assessing apoptosis concurrently with cycle status is shown in Fig 2. Noteworthy is the fact that flavopiridol appears to be inducing apoptosis in G0-1 arrested cells. Because necrotic cells sometimes show uptake of TdT, a separate assessment of apoptosis was performed using annexin-V and propidium iodine. Figure 3demonstrates a representative annexin-V/PI assay with CLL cells (patient UPN 28a) incubated in medium or 0.18 μmol/L of flavopiridol for 4 and 24 hours. This demonstrates a definitive population of cells with the altered annexin-V phospholipid observed with apoptosis, but lacking PI staining. Similar apoptosis was observed with the tunnel assay outlined above (data not shown). These data support the observation of others that flavopiridol is inducing cytotoxicity at least in part through the pathway of apoptosis.
Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.
Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.
Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.
Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.
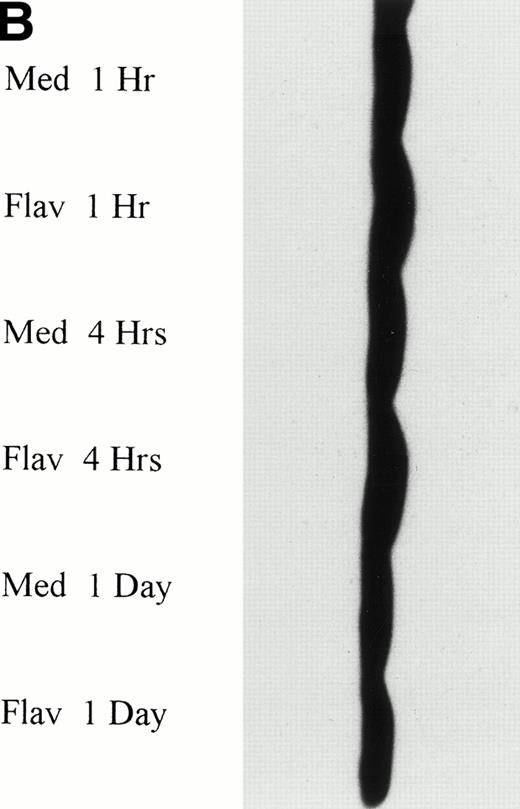
Flavopiridol Cytotoxicity Does Not Correlate With Changes in bcl-2 or bax Protein Expression
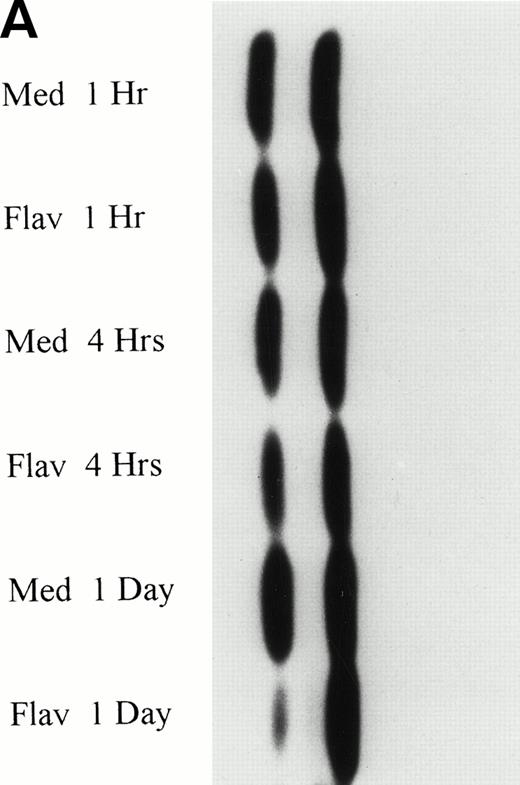
To determine if flavopiridol was inducing early apoptosis either through decreased expression of bcl-2 or increased bax protein, we incubated mononuclear cells from CLL patients with 0.18 μmol/L flavopiridol or medium with subsequent assessment of bcl-2 protein (n = 7) or bax (n = 3) protein expression at 4 and 24 hours. Figure 4A depicts bcl-2 expression in 3 patient samples at the 0.18 μmol/L flavopiridol concentration, demonstrating minimal change in bcl-2 protein expression as compared with medium. Quantification of protein banding by densitometry in these seven experiments demonstrated similar or slightly increased expression of bcl-2 expression with 0.18 μmol/L of flavopiridol as compared with medium at 4 and 24 hours, respectively. Mononuclear cells from 2 additional CLL patients were incubated with a higher concentration of flavopiridol (0.33 μmol/L), which induced a high rate of apoptosis (85% and 88%) as measured by the TdT/PI at 1 day, but similarly failed to demonstrate a change in bcl-2 protein expression. The protein expression of bax was also not altered with 0.18 μmol/L flavopiridol incubation as compared with medium-matched control (data not shown). These data suggest that flavopiridol-induced apoptosis in human CLL cells does not occur as a consequence of bcl-2 protein modulation.
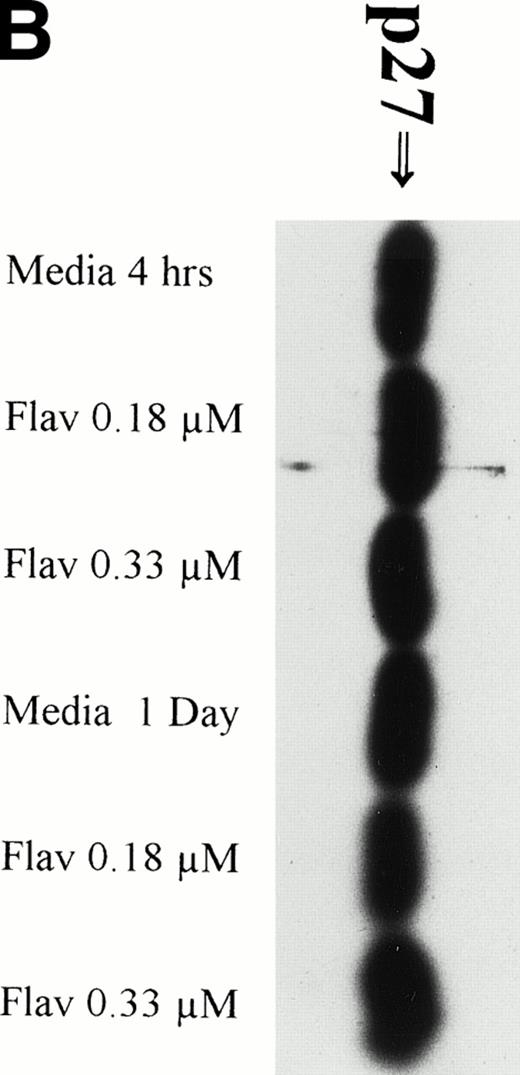
(A) Expression of bcl-2 protein in human CLL cells at 4 and 24 hours after incubation with medium or 0.18 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium or flavopiridol (0.18 μmol/L). Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Two micrograms of protein/lane from the CLL cell lysates was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and electrophoresed. Bcl-2 protein was detected using an anti–bcl-2 monoclonal antibody (Dako). Lane equivalent loading was certified by assessment with Fast Green Staining (not shown). (B) Expression of p27 protein in human CLL cells at 4 and 24 hours after incubation with medium or 0.18 and 0.33 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium or flavopiridol (0.18 or 0.33 μmol/L). Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Five micrograms of protein/lane from the CLL cell lysates was loaded on a 10% SDS-PAGE gel and electrophoresed. The p27 protein was detected using an anti-p27 polyclonal antibody (Oncogene). Lane equivalent loading was certified by assessment with Fast Green Staining (not shown).
(A) Expression of bcl-2 protein in human CLL cells at 4 and 24 hours after incubation with medium or 0.18 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium or flavopiridol (0.18 μmol/L). Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Two micrograms of protein/lane from the CLL cell lysates was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and electrophoresed. Bcl-2 protein was detected using an anti–bcl-2 monoclonal antibody (Dako). Lane equivalent loading was certified by assessment with Fast Green Staining (not shown). (B) Expression of p27 protein in human CLL cells at 4 and 24 hours after incubation with medium or 0.18 and 0.33 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium or flavopiridol (0.18 or 0.33 μmol/L). Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Five micrograms of protein/lane from the CLL cell lysates was loaded on a 10% SDS-PAGE gel and electrophoresed. The p27 protein was detected using an anti-p27 polyclonal antibody (Oncogene). Lane equivalent loading was certified by assessment with Fast Green Staining (not shown).
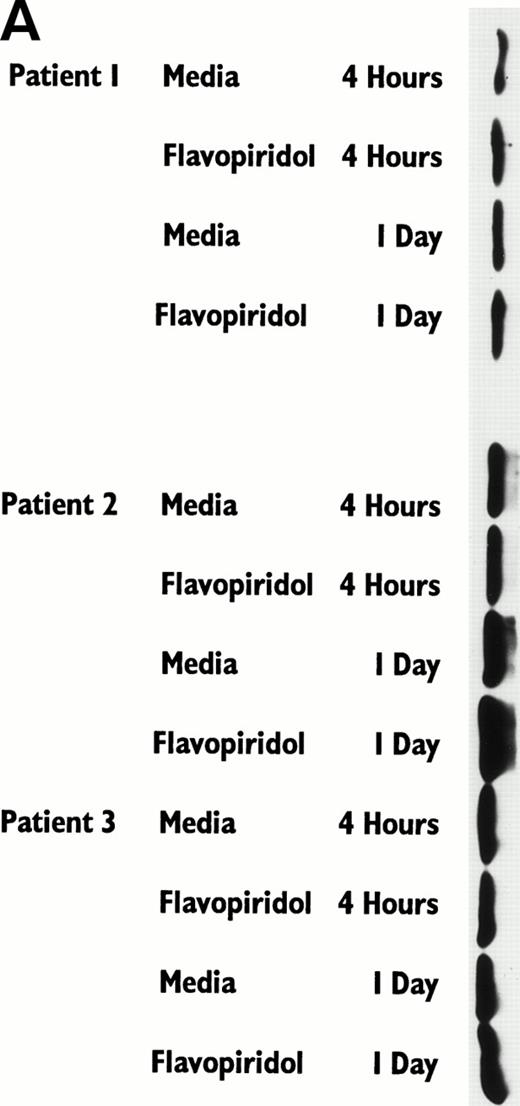
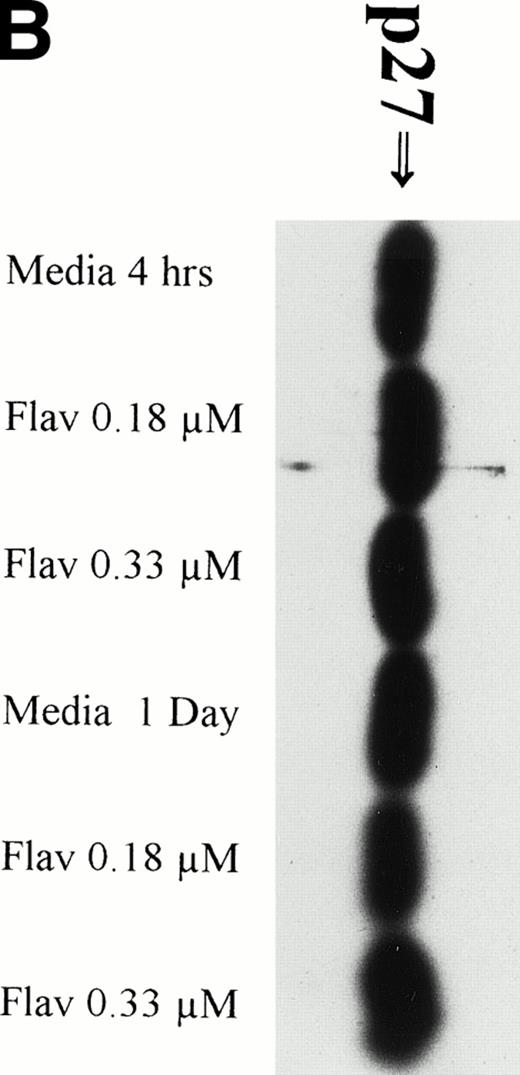
Flavopiridol Cytotoxicity Does Not Correlate With Changes in Expression of p27
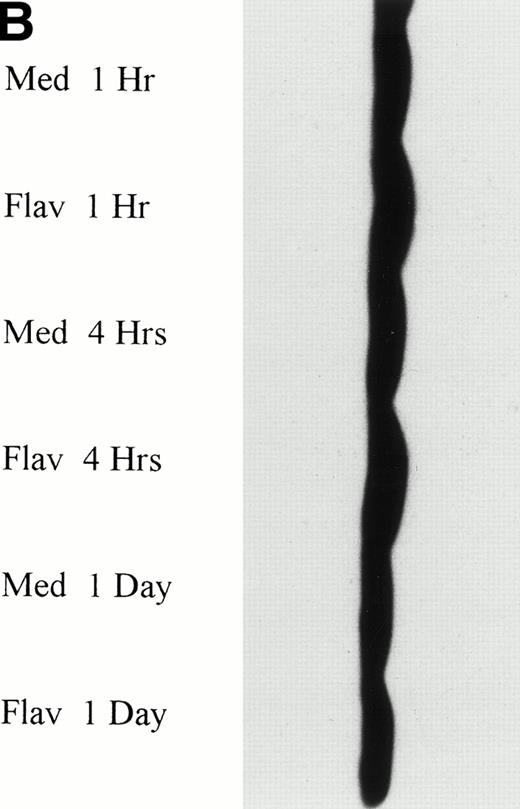
It has recently been preliminarily identified that the cell cycle inhibitor p27 is variably overexpressed in CLL lymphocytes, is induced by IL-4 incubation, and may have a role in suppressing apoptosis. Because p27 binds to CDK2 and flavopiridol inhibits this kinase, we sought to determine if flavopiridol was modulating expression of this protein. We incubated mononuclear cells from 3 CLL patients in medium or flavopiridol (0.18 or 0.33 μmol/L), which induced a high rate of apoptosis as measured by the TdT/PI at 1 day, but failed to demonstrate a change in p27 protein expression, as shown in Fig 4B.
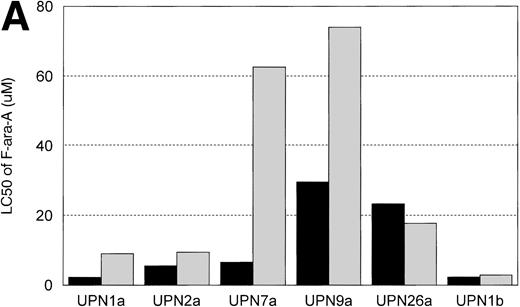
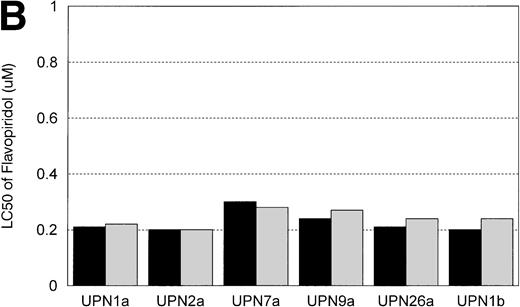
IL-4 Induces Resistance to F-ara-A, But Not Flavopiridol
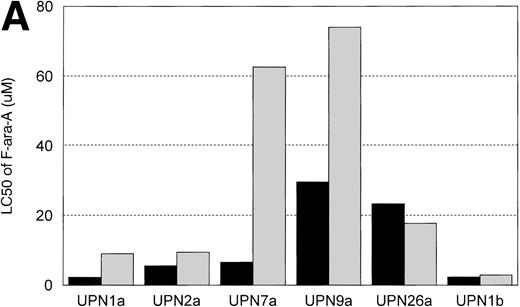
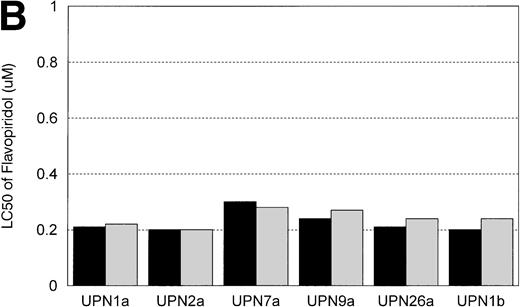
In vitro incubation of B-CLL cells with IL-4 has been demonstrated to induce drug resistance toward chlorambucil and prednisone. We sought to determine if another agent currently used in the treatment of CLL (ie, F-ara-a) and flavopiridol were affected by IL-4 incubation. Cells from 6 patients with B-CLL were exposed to varying concentrations (0.01, 0.033, 0.1, 0.33, 1, 3.3, 10, 33, and 100 μmol/L) of F-ara-a and flavopiridol with or without 10 ng/mL of IL-4. As demonstrated in Fig 5A, incubation with IL-4 for 4 days increased the LC50 of F-ara-a in 5 of 6 CLL cell samples tested from a mean of 11.6 μmol/L (range, 2.2 to 29.6 μmol/L; 95% CI ±9.4 μmol/L) to 29.3 μmol/L (range, 2.89 to 74.0 μmol/L; 95% CI ±24.6 μmol/L). The magnitude of this change was not equivalent between patients, with only 3 having significant increases over the respective controls. In contrast, as shown in Fig 5B for the same 6 CLL patients, the LC50 of cells exposed in medium and flavopiridol was 0.23 μmol/L (range, 0.2 to 0.3 μmol/L; 95% CI ±0.03 μmol/L) versus 0.24 μmol/L (range, 0.2 to 0.28 μmol/L; 95% CI ±0.02 μmol/L) for medium and IL-4 and flavopiridol. These data suggest that IL-4 incubation does not increase the in vitro drug resistance to human CLL cells exposed to flavopiridol.
(A) The in vitro effect of IL-4 incubation on in vitro drug resistance to F-ara-a in human CLL cells. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 1 × 106/mL in medium (control) and varying concentrations of F-ara-A (0.01 to 100 μmol/L) in the absence (▪) or presence (▧) of IL-4 (10 ng/mL). After 4 days of incubation, the viability was assessed using the MTT test and is expressed for each patient as the LC50 (drug concentration required to reduce viability by 50%). (B) The in vitro effect of IL-4 incubation on in vitro drug resistance to flavopiridol in human CLL cells. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 1 × 106/mL in medium (control) and varying concentrations of flavopiridol (0.01 to 100 μmol/L) in the absence (▪) or presence () of IL-4 (10 ng/mL). After 4 days of incubation, the viability was assessed using the MTT test and is expressed for each patient as the LC50 (drug concentration required to reduce viability by 50%).
(A) The in vitro effect of IL-4 incubation on in vitro drug resistance to F-ara-a in human CLL cells. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 1 × 106/mL in medium (control) and varying concentrations of F-ara-A (0.01 to 100 μmol/L) in the absence (▪) or presence (▧) of IL-4 (10 ng/mL). After 4 days of incubation, the viability was assessed using the MTT test and is expressed for each patient as the LC50 (drug concentration required to reduce viability by 50%). (B) The in vitro effect of IL-4 incubation on in vitro drug resistance to flavopiridol in human CLL cells. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 1 × 106/mL in medium (control) and varying concentrations of flavopiridol (0.01 to 100 μmol/L) in the absence (▪) or presence () of IL-4 (10 ng/mL). After 4 days of incubation, the viability was assessed using the MTT test and is expressed for each patient as the LC50 (drug concentration required to reduce viability by 50%).
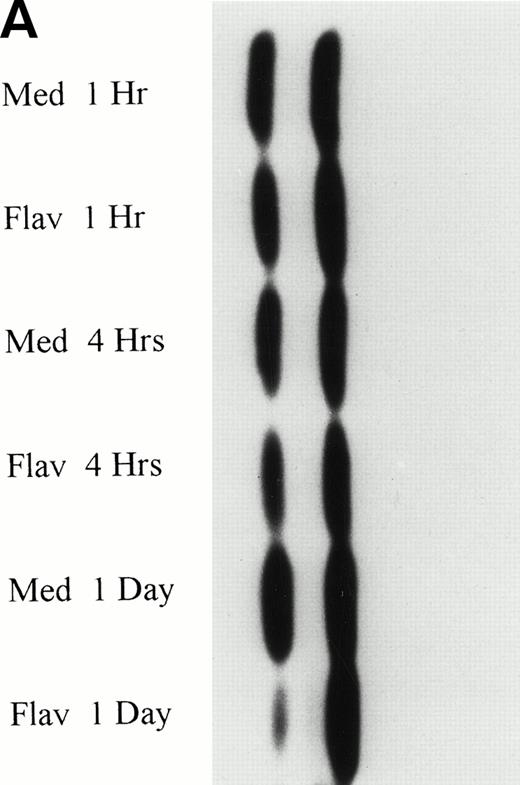
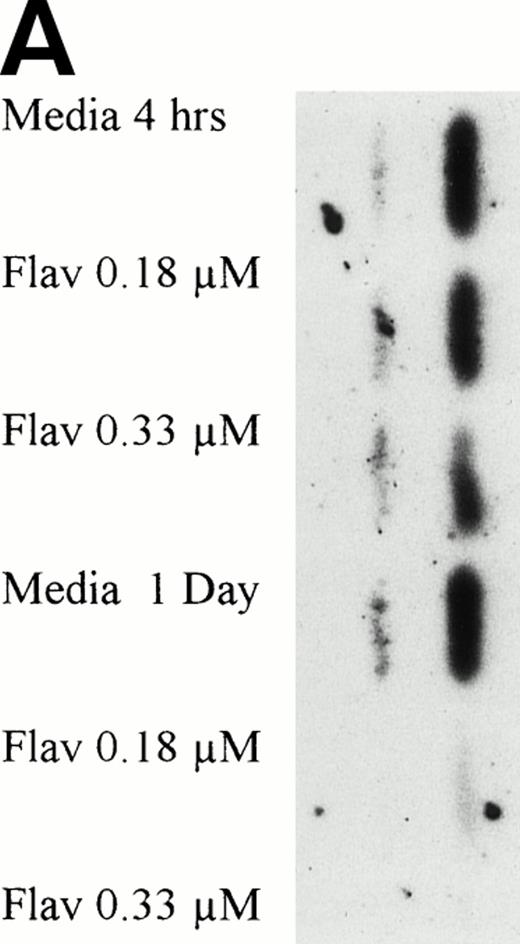
Flavopiridol Causes a Decrease in p53 Protein Expression
The normal CLL cellular response to DNA damage induced by alkylator and/or purine analog exposure is an increase of p53 protein expression. To determine if flavopiridol was inducing apoptosis via a similar pathway, we incubated mononuclear CLL cells from 5 patients with 0.18 μmol/L of flavopiridol or medium with subsequent assessment of p53 protein expression at 1, 4, and 24 hours. Figure 6A depicts a representative Western blot demonstrating a decrease in p53 protein expression with flavopiridol exposure at 4 and 24 hours as compared with medium-only control samples. Figure 6B depicts a reprobing experiment with actin, demonstrating equivalent protein loading among each of the samples. Fast Green Staining demonstrated similar results (data not shown).
(A) Expression of p53 protein in human CLL cells at 1, 4, and 24 hours after incubation with medium or 0.18 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium or 0.18 μmol/L of flavopiridol. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Twenty-five micrograms of protein/lane from the CLL cell lysates was loaded onto a 10% SDS-PAGE gel and electrophoresed. The p53 protein (identified by an arrow) was detected using an anti-p53 monoclonal antibody (Oncogene). (B) To assess for equal lane loading, gel 6A was reprobed with antiactin polyclonal antibody (Santa Cruz), as depicted. Equal lane loading was also confirmed using Fast Green Staining (not shown).
(A) Expression of p53 protein in human CLL cells at 1, 4, and 24 hours after incubation with medium or 0.18 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium or 0.18 μmol/L of flavopiridol. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Twenty-five micrograms of protein/lane from the CLL cell lysates was loaded onto a 10% SDS-PAGE gel and electrophoresed. The p53 protein (identified by an arrow) was detected using an anti-p53 monoclonal antibody (Oncogene). (B) To assess for equal lane loading, gel 6A was reprobed with antiactin polyclonal antibody (Santa Cruz), as depicted. Equal lane loading was also confirmed using Fast Green Staining (not shown).
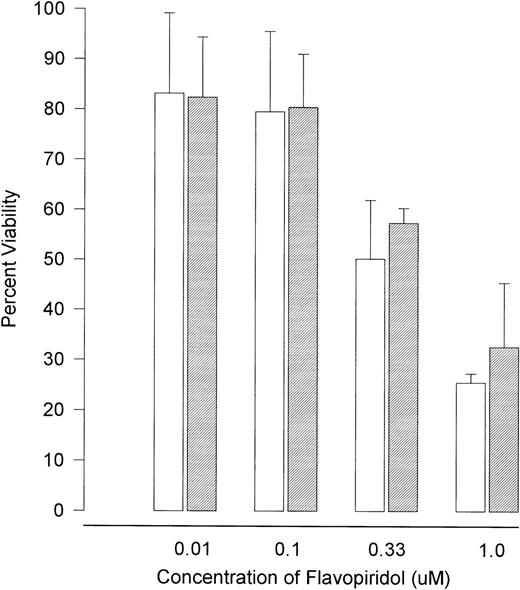
Flavopiridol Induces Apoptosis Independent of p53 Status
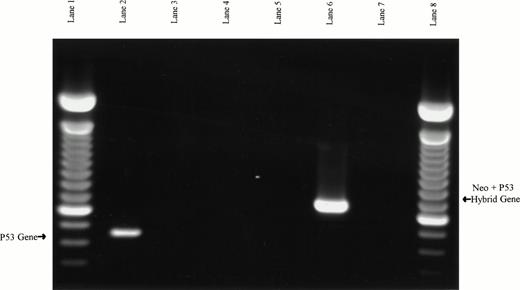
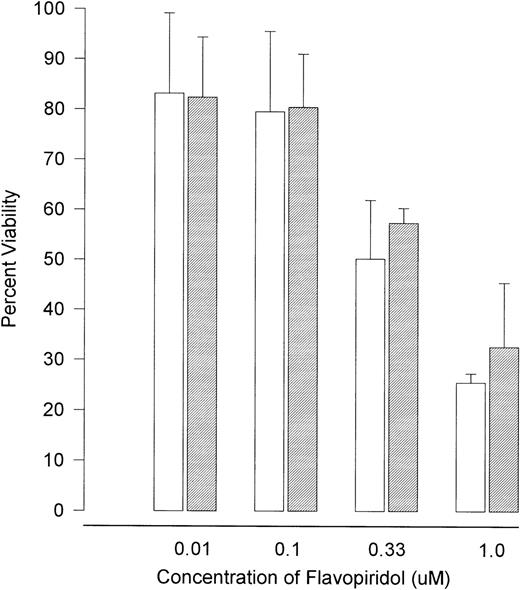
The observation that p53 protein expression was actually decreased in response to flavopiridol exposure suggested that this agent might be operating in a unique, p53-independent fashion. To test this hypothesis, we explored whether the observed effects of flavopiridol were dependent on intact p53. Splenocytes from 4 wild-type and 3 typed p53 null type mice were exposed to varying (0.01, 0.1, 0.33, and 1 μmol/L) concentrations of flavopiridol. The absolute molecular status of the wild-type and p53 null type mice were secondarily confirmed by identifying the presence or absence of the p53 gene and neogene as previously described16 17 and is shown in Fig 7. The cytotoxicity as assayed by trypan blue after 24 hours of incubation at each concentration of flavopiridol is summarized in Fig 8. Viability of the p53 null (mean, 81%; 95% CI ±4.3%) and wild-type (mean, 90%; 95% CI ±2%) mice splenocytes after 24 hours of incubation in medium was similar. A noticeable decline in splenocyte viability was noted with incremental increases in flavopiridol concentrations without preferential cytotoxicity to p53+/+ as compared with the p53−/−splenocytes. This nonpreferential toxicity was also observed in the thymocytes (data not shown). In contrast to this, the viability of the p53−/− splenocytes (89%) was affected minimally by 500 cGy of irradiation as compared with almost complete loss of viability (22%) in the p53+/+ splenocytes. Incubation with F-ara-a at varying concentrations yielded a result similar to that observed after irradiation. After 24 hours of incubation with F-ara-a, the p53−/− splenocytes had a LC50 of greater than 100 μmol/L, contrasting with that of p53+/+ cells in which the LC50 was only 3.95 μmol/L. The possible clinical relevance of these murine experiments to B-CLL is exemplified by the in vitro response of UPN28a to flavopiridol. This patient had a 17p13 deletion, as shown in Fig 9. In vitro drug incubation with fludarabine and flavopiridol demonstrated a LC50 of greater than 100 μmol/L for F-ara-a and 0.22 μmol/L for flavopiridol. This is further demonstrated by Fig 3 illustrating marked apoptosis in CLL cells from this same patient after 24 hours of 0.18 μmol/L of flavopiridol exposure.
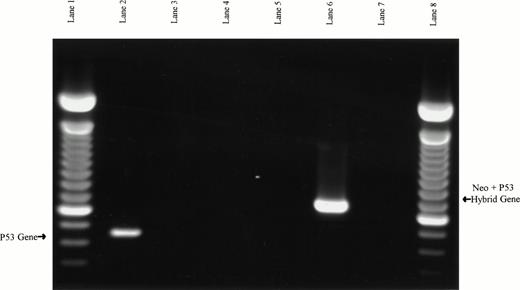
PCR-based verification of murine splenocyte genotypes. Samples in lanes 2, 3, and 4 used the p53 exon 5 (W5′) and exon 7 (W3′) primers to assay for the presence of wild-type p53 PCR product. Samples in lanes 5, 6, and 7 used neo primers (M5′) and the p53 exon 7 reverse primers (W3′) to assay for a hybrid neo/p53 product expected in the p53 null mice. Murine wild-type DNA was used as PCR template in lanes 2 and 5, murine p53 null DNA was used in lanes 3 and 6, and no DNA templates were used in lanes 4 and 7 (negative controls). The expected p53 product from wild-type DNA is present in lane 2, and the expected neo/p53 hybrid product from p53 null DNA is present in lane 6. Lanes 1 and 8 are 100-bp ladders.
PCR-based verification of murine splenocyte genotypes. Samples in lanes 2, 3, and 4 used the p53 exon 5 (W5′) and exon 7 (W3′) primers to assay for the presence of wild-type p53 PCR product. Samples in lanes 5, 6, and 7 used neo primers (M5′) and the p53 exon 7 reverse primers (W3′) to assay for a hybrid neo/p53 product expected in the p53 null mice. Murine wild-type DNA was used as PCR template in lanes 2 and 5, murine p53 null DNA was used in lanes 3 and 6, and no DNA templates were used in lanes 4 and 7 (negative controls). The expected p53 product from wild-type DNA is present in lane 2, and the expected neo/p53 hybrid product from p53 null DNA is present in lane 6. Lanes 1 and 8 are 100-bp ladders.
The viability of splenocytes from (□) wild-type (p53+/+) and (▧) null (p53−/−) murine splenocytes after 24 hours of incubation with varying concentrations of flavopiridol. Error bars indicate 95% CIs. The cells were obtained from spleens of typed and PCR confirmed p53+/+ and p53−/− mice. These cells were cultured at 1 × 106/mL in medium (control) and varying concentrations of flavopiridol (0.01 to 1 μmol/L). After 1 day of incubation, the viability was assessed using the Trypan blue exclusion test.
The viability of splenocytes from (□) wild-type (p53+/+) and (▧) null (p53−/−) murine splenocytes after 24 hours of incubation with varying concentrations of flavopiridol. Error bars indicate 95% CIs. The cells were obtained from spleens of typed and PCR confirmed p53+/+ and p53−/− mice. These cells were cultured at 1 × 106/mL in medium (control) and varying concentrations of flavopiridol (0.01 to 1 μmol/L). After 1 day of incubation, the viability was assessed using the Trypan blue exclusion test.
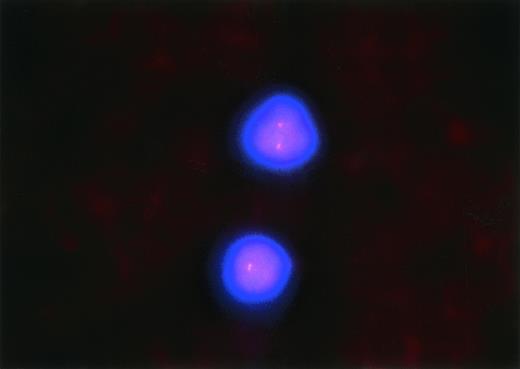
Fluorescence in situ hybridization detection of the monoallelic deletion of 17p13.1 in patient 28a. Two lymphocytes from this patient’s blood smear are shown, each with only one detectable signal for 17p13.1. This same pattern was observed in 35% of the blood leukocytes and constituted the diagnosis of monoallelic deletion of 17p13.1 (original magnification × 1,000).
Fluorescence in situ hybridization detection of the monoallelic deletion of 17p13.1 in patient 28a. Two lymphocytes from this patient’s blood smear are shown, each with only one detectable signal for 17p13.1. This same pattern was observed in 35% of the blood leukocytes and constituted the diagnosis of monoallelic deletion of 17p13.1 (original magnification × 1,000).
Flavopiridol Exposure Results in Activation of Caspase 3
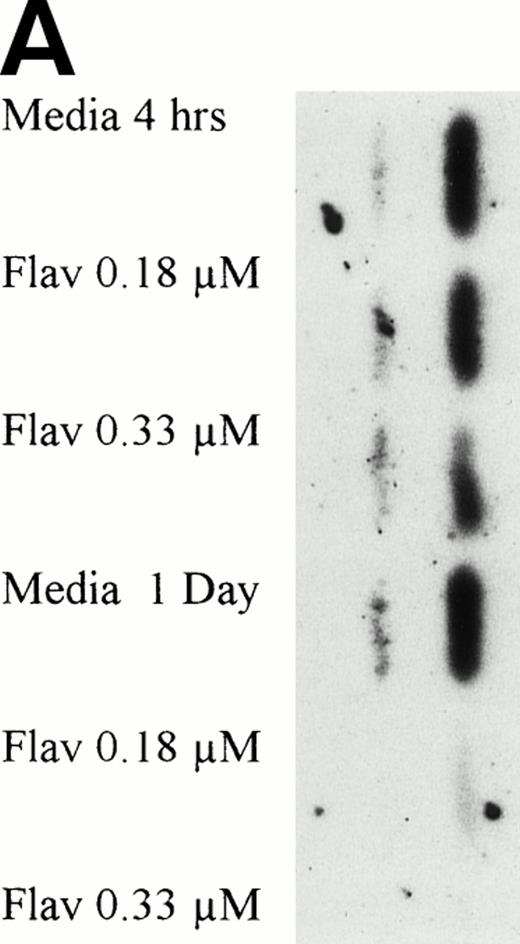
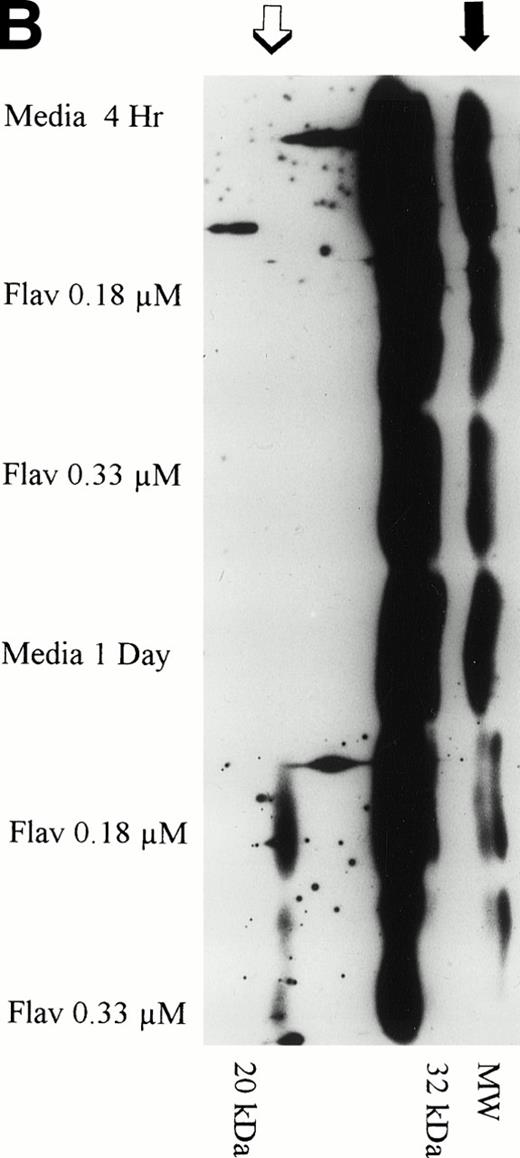
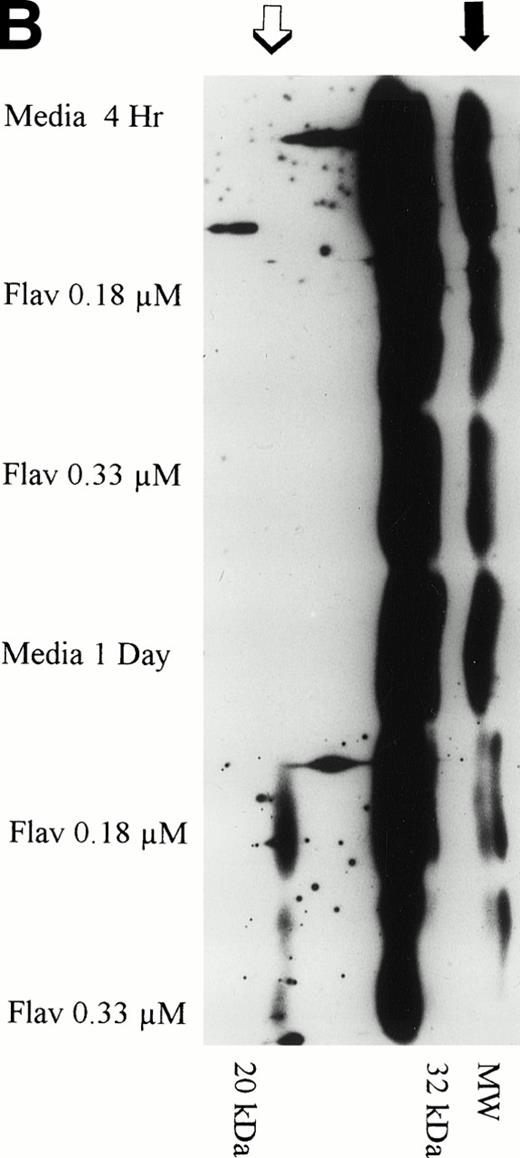
The protein caspase 3 exists in zymogen form, is overexpressed in B-CLL, and when cleaved to its 17- to 20-kD and 10-kD fragments cleaves the DNA repair enzyme poly(ADP-ribose) polymerase (PARP) that commits the cell to apoptosis. Because caspase is activated at a point in the apoptotic pathway distal to p53, we sought to determine if flavopiridol incubation resulted in cleavage of this protein. We incubated mononuclear CLL cells from 3 patients with 0.18 μmol/L of flavopiridol or medium with subsequent assessment of caspase 3 protein expression and its fragments at 1, 4, and 24 hours. Figure 10A depicts a representative Western blot demonstrating a decrease in the zymogen caspase-3 32-kD protein. Detection of the subsequent appearance of the caspase-3 protein cleavage 20-kD fragment at 24 hours of flavopiridol exposure as compared with medium control is demonstrated in Fig 10B.
(A) Expression of caspase-3 zymogen protein (32 kD) in human CLL cells at 4 and 24 hours after incubation with medium alone or 0.18 or 0.33 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium or flavopiridol (0.18 or 0.33 μmol/L). Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Fifteen micrograms of protein/lane from the CLL cell lysates was loaded onto a 14% SDS-PAGE gel and electrophoresed. The caspase-3 protein was detected using an anti–caspase-3 polyclonal antibody (Santa Cruz). Lane equivalent loading was certified by assessment with Fast Green Staining (not shown). (B) Expression of caspase-3 zymogen protein (32 kD; identified by a solid arrow) and cleavage product (20 kD; identified by an open arrow) in human CLL cells at 4 and 24 hours after incubation with medium alone or 0.18 or 0.33 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium alone or 0.18 or 0.33 μmol/L of flavopiridol. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Seventy-five micrograms of protein/lane from the CLL cell lysates was loaded onto a 14% SDS-PAGE gel and electrophoresed. The caspase-3 protein was detected using an anti–caspase-3 polyclonal antibody (Santa Cruz). Overexposure of the nitrocellulose gel was necessary due to the instability of the cleavage product. Lane equivalent loading was certified by assessment with Fast Green Staining (not shown).
(A) Expression of caspase-3 zymogen protein (32 kD) in human CLL cells at 4 and 24 hours after incubation with medium alone or 0.18 or 0.33 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium or flavopiridol (0.18 or 0.33 μmol/L). Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Fifteen micrograms of protein/lane from the CLL cell lysates was loaded onto a 14% SDS-PAGE gel and electrophoresed. The caspase-3 protein was detected using an anti–caspase-3 polyclonal antibody (Santa Cruz). Lane equivalent loading was certified by assessment with Fast Green Staining (not shown). (B) Expression of caspase-3 zymogen protein (32 kD; identified by a solid arrow) and cleavage product (20 kD; identified by an open arrow) in human CLL cells at 4 and 24 hours after incubation with medium alone or 0.18 or 0.33 μmol/L of flavopiridol. The cells were obtained from CLL patients after obtaining informed consent, isolated, and cultured at 5 × 106/mL in medium alone or 0.18 or 0.33 μmol/L of flavopiridol. Cell lysates were prepared and protein concentration was quantified using the BCA method (Pierce). Seventy-five micrograms of protein/lane from the CLL cell lysates was loaded onto a 14% SDS-PAGE gel and electrophoresed. The caspase-3 protein was detected using an anti–caspase-3 polyclonal antibody (Santa Cruz). Overexposure of the nitrocellulose gel was necessary due to the instability of the cleavage product. Lane equivalent loading was certified by assessment with Fast Green Staining (not shown).
DISCUSSION
Whereas Senderowicz et al17 preliminarily reported the observation of in vitro apoptosis in a single patient with human B-CLL, this manuscript evaluates the effect of flavopiridol in multiple patients with this disease. Furthermore, we show that this agent has marked activity against B-cell CLL, irrespective of previous treatment, purine analog refractoriness, the presence of a 17p13 deletion, or IL-4 incubation. In a recently completed phase I study administering flavopiridol as a 72-hour infusion, a median plasma concentration of 0.44 μmol/L was attained at the recommended phase II starting dose.18 At this concentration, hypotension and diarrhea were noted, but not myelosuppression. These attainable plasma drug levels and dose-limiting toxicities have been noted by a second group.19 This mean attainable concentration of flavopiridol represents 2.4 times the LC50 of human B-cell CLL cells in vitro. The apoptosis/cytotoxicity observed with flavopiridol in CLL cells was observed early at 4 to 24 hours as compared with fludarabine, in which significant apoptosis/cytotoxicity is not noted before 4 days. In contrast, the maximal flavopiridol drug effect at this low, clinically attainable concentration requires 24 hours of continuous exposure. Incubation with flavopiridol beyond 24 hours produced an insignificant change in the LC50 of CLL cells.
Our data also demonstrate that flavopiridol has almost an identical dose response when comparing the LC50 of human CLL cells and normal mononuclear cells. This finding suggests that flavopiridol might produce adverse effects on normal lymphocytes; consequently, studies of lymphocyte subsets should be incorporated into planned phase II studies. Additionally, our data provide support for inclusion of CLL patients on phase I/II studies of flavopiridol and subsequent phase II studies in this disease using a 24-hour continuous infusion schedule. Additionally, a heightened awareness for possible opportunistic infections should be present in these studies.
The mechanism by which flavopiridol produces cytotoxicity in tumor cell lines or fresh human tumor cells is currently unknown. Initial preliminary investigations of flavopiridol in breast and lung cancer cell lines demonstrated that flavopiridol-induced cell cycle arrest in both G1 and G2. These studies also demonstrated that flavopiridol was cytostatic to exponentially growing cells with little cytotoxic effect demonstrable in stationary nonproliferating cells.20 A subsequent study using MCF-7 breast cancer cell lines has demonstrated flavopiridol inhibits G1 progression by a reduction of activity of CDK2 and CDK4.16 In these studies, G1 inhibition of cell cycle progression occurred independent of functional p53 and Rb status. Similar inhibition of G2 progression occurs through flavopiridol-inhibition of CDC2 activity with selective loss of phosphotyrosine content.21-23 Two recent reports challenged the notion that flavopiridol is cytostatic to proliferating cells by demonstrating overt cytotoxicity with drug incubation in several tumor cell lines independent of growth phase status.24,25 Finally, König et al26 reported that flavopiridol induced growth inhibition and apoptosis in CLL cell lines with concomitant downregulation of bcl-2 protein and mRNA expression. Because bcl-2 overexpression inhibits apoptosis, the investigators suggested that modulation of bcl-2 might in part explain how flavopiridol induces cytotoxicity. This observation of bcl-2 protein downregulation had previously been observed by Schwartz et al27 in solid tumor cell lines but only in one of the two lymphoid cell lines recently examined by Parker et al.28
Our data from human CLL cells demonstrate that flavopiridol is overtly cytotoxic to cells that are in G0-1. Others have observed that cytotoxicity both at G0-1 and other cell cycle positions can occur both through or in the absence of apoptosis depending upon the specific type of tumor cell line.24,25The combined results of the tunnel and annexin studies on mononuclear CLL cell isolates suggest that flavopiridol induces apoptosis, although the assays used cannot exclude a component of direct cell necrosis, as has been observed in certain cell lines.24 Unlike König et al26 or Schwartz et al,27 we were unable to demonstrate quantitative decreases of bcl-2 expression after incubation of human CLL cells with flavopiridol. One reason for this discordant result might be that a higher concentration of flavopiridol was used by this group, as compared with the 0.18 to 0.33 μmol/L used in this study. However, it is notable that, in the absence of alteration of bcl-2 protein expression, prompt apoptosis was noted in all of the human CLL cell samples assessed in our laboratory. Additionally, all of the previous studies examining bcl-2 expression have included dividing cell lines that are not biologically reflective of the G0-1 arrested human CLL cells used in this study. Whereas bcl-2 downregulation may contribute to the therapeutic efficacy of flavopiridol in solid and lymphoid cell lines, it does not appear to be necessary for induction of apoptosis in human CLL cells. What does appear to correlate with flavopiridol-induced apoptosis in human CLL cells is cleavage of caspase-3. Caspase-3 is a cystein protease that exists as an inactive zymogen in cells and has recently been identified as being overexpressed in CLL.29 Activation of this protein is believed to occur as a response to cytochrome c release from the mitochondria into the cytosol.30,31 Activation of this enzyme results in poly (ADP-ribose) polymerase (PARP) cleavage and morphologic changes consistent with apoptosis. Our results demonstrate that caspase-3 is cleaved in conjunction with notable flavopiridol-induced apoptosis, which complements the observation of Chandra et al32 that caspase-3 activation is present in human CLL cells after fludarabine and prednisone-induced apoptosis. Whether flavopiridol is initially triggering apoptosis upstream from caspase-3 or directly promoting its activation is currently unknown and remains a major focus of our laboratory efforts.
Given the heterogenous nature of CLL and other related low-grade lymphoproliferative disorders, our laboratory’s initial preclinical evaluation includes an assessment of efficacy against molecular aberrations such as p53 mutations or incubation with IL-4, both of which predict a poor prognosis or induce in vitro drug resistance in CLL.4-7,12 Our data demonstrate that, unlike F-ara-a, IL-4 incubation only minimally alters the LC50 of flavopiridol. The in vivo relevance of this observation is uncertain. However, it has been recently observed that T-cells from patients with CLL have overexpression of IL-4.33 If this overexpression of IL-4 is demonstrated to be linked to in vivo drug resistance in CLL, our data suggest that flavopiridol appears to induce cytotoxicity independent of the presence of this cytokine. Our in vitro studies also suggest that this agent would be effective in patients with p53 deficient leukemia cells. Whereas others28 have inferred this from cytotoxicity observed in cell lines known to have p53 mutations, we used typed p53 null mice whose status was subsequently confirmed by PCR and observed no difference in flavopiridol dose response or apoptosis frequency as compared with wild-type mice. We chose to use nontransformed murine splenocytes with aberrations of p53, because it closely approximates the biologic pattern of a nonproliferating CLL cell as compared with a transformed cell line in which cell proliferation occurs and other molecular aberrations are likely to exist. Additionally, we have demonstrated significant in vitro apoptosis with flavopiridol from a patient whose CLL cells had a 17p13 deletion. Based on the data presented, we believe that flavopiridol represents an ideal agent for targeted therapy for patients with CLL, including those with p53 mutations.
In summary, we conclude that flavopiridol has marked in vitro activity against human CLL cells at concentrations that are readily attainable in the clinic with the optimum time of drug exposure of approximately 1 day. Contrary to previous reports, flavopiridol exposure does not appear to modulate bcl-2 expression in human CLL cells but does result in activation of caspase-3. Unlike other effective agents currently used in the treatment of CLL, flavopiridol induces cytotoxicity independent of p53 status or IL-4 incubation, making it an ideal agent for targeted therapy toward high-risk subsets of patients with this disease. These findings justify early introduction of previously treated patients with CLL into phase I studies of flavopiridol using a 24-hour infusion schedule.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John C. Byrd, MD, Hematology-Oncology Service, ward 78, Walter Reed Army Medical Center, Washington, DC 20307.


![Fig. 2. Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236002ax.jpeg?Expires=1768857839&Signature=JYVyXcGblxM5bHIH~CEdkqEAiAnAM7uZzSHssNj9B-Jt-KWXXY9BTPxJkKljgiHHmiNnWhNYd29UHKS~s8V6oIhY1e2-bAlIryriom7Li-SJXFcHxrBXH1bPzUJexPVvaGjREQ91zdYpWZGc4QCoURj7zgM64M6wNnpcLTYRORXDt0V8~lq1gueBKudDGP5NlM~QhjAsYdPhXLR3KHhK27G6B8hM9QuqA1s-Ty46b-Y8LpO0uX6DuI5w6Qb~oDCSzcwKKSBW6lbgwNCHN9A752gmZZzDXPXRDzfG7zf9jbJ7qmVT8fM7PCoG~WJl9S6YLUW9yB7VXOujK57zJ33NVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236002bx.jpeg?Expires=1768857839&Signature=BVTVmI7LeCcju8nhivo97vHvpryb9fFKnJdNNUuftJ3NZ8Emc0cnSIbxsfD8qu428kpkzeqFulj4HV2cDVI~A8vyf36mZWslt-v8sV7j8IaQGiMJgQ5nu0ffBkjKOHMqUC-gHUBBE~Y396yFejqk9IaBq4XwgpQfALsOnSwEO3L3hYTBPfFYH~UDATVDGHQGOiFFxZHpbnrHEIilreJ-mT9BK6rBDkKPY-n2M-LmVDAo5QyZJd1WXlTmVgjDzLqFkD1ddylpDtDy8c32L9qjOQE1yBioA9~yvmwEBIW~oo~r9fbiXOFU2rh7WDM0jNst8V2ArtJK-2PBvxaOEZbjpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236002cx.jpeg?Expires=1768857839&Signature=vPm8UlHtT5JX46OLMQX5Mim9CaFillufPQwmpco~Z2bIJMPfgvJPcbt6RRIkYdq1FVQouim1rLFNy28gsiIhjEWJb0bQpZPQbFAFmSMgMq8n1l9qScI2feCqC4KnmP7ZeOW6ezBlzlUfjOlJy3EDKpZtHK7GL5TGcSXYlQ99YKmytOs31ufgOl2Hiaiwk0heMM8951Hs-OWePg6zynRYI4ucrl4kadV4z9jmGiRa7eTUC~TjrTB8Phf-02GoVp3ip4kLSz2FLDOvTp2rvVYaDKeS9Nl9BEN7aTdAw3-KdUtgZqxGI32~i5Fe56nXdb9s0iUByTuFDURoNNxbfFwKkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236002dx.jpeg?Expires=1768857839&Signature=hgsdHNRnehM3ttCIwagw6U1wpUcE1qgjRhjPHQW-w1erp-DR2Mgx3kp1u4avyYtmr9zocZw4~yIy31tWfF~V-yE1WGoxAtaUwXdQ3aNg-2gQFvUB8nTsV70DkYv368DBwnskm-lBumz3MXPEas1Smj-PjroFYr4eTcUjWvSqcu5P22gfqBnpK9zmbyLSHY-cjj-CUnkwhM50nF-hQ51FMnvinnN5QzdyLz0hs-8sJWK9fmPkaU8JBhpZNYMIsd1aWUeJRMoD4c841P3JFd9RkRPpOAImuR0FCmcyhwTJ9t7Lvszid850FQCoii9WM3ENgs8NbNDJaIoI82eRebNV1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236003ax.jpeg?Expires=1768857839&Signature=PpfuGRiwt19KcL64BzNQRQBPRHQd1NGovx2LbSvizxDd5BRA6Xnjg7Nkn8egofJWqNFU7K1wDpTNkKx3M7-bB2Kp-EY8YUtHa2ZU7KQLQhRC6bgdqJ6Xbas82e30dEpE8lnbNCpBLH8aKNx8VMSbM2V~XN8gDvNe8mUhyL4f~zAMmdv562ko9qswjtzpAIQIK3EHYInX6ts82VSmg3JminocPkZ801jCuQgBwKvAl1gH9kkuasfpePMFUq8vxss9yOMSLd6C13qETUle-ZKqJ~4l8PxgpNKW5RIFwxjNNOEUo4gDT0dCs-iIv2-Q6z-FaAzICH2V3j~v0cBf5ILHjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236003bx.jpeg?Expires=1768857839&Signature=pcavjLP5jBntR1~A1oi-FpAn3BL1qMUW~KqfAp77vCApQUAG4RyhvaipEFp2ICoLBBAPNtLbxYbaVe0px3zdh61vGhnmehCtTvmiHyKWY1hcvnmCvK8ThQ~GfFjXYQ4r0Sghei3wGLqKdgoyIVoe~ZWhXF4XwJ3kpjxorUAfJHIbq9qCdaaSeTtcSL6jCIofX7Qu0SWxNNswvUuYb1mZTSxDeDoLbLldqtoCOyCLEaCqruOwPzFf8Hac5qRzdTJjy8KCDaftFjMuevjIds7bMxtKnznxRZ-rdJDD0r8llV7BKLBxezhrXgFA4AxTWQxuii9Uhgc4wF~Tua4GayhHMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236003cx.jpeg?Expires=1768857839&Signature=f74edfoqwobgoEgrpAPpcUnYYdYaD47CmRImG6J7uTdbaesahVVtwj3bm-I8rPG8v4ORBIqbrp7D3MlQ686QC9bneH5WgWsKLnychm0g-l4K1l2jNZj9HJXD3Z722mKkVVomuIkr1aWoS66~bcDB7v45N061d8qVuAGos7vAhXheNJ6IQp-lxxfbqGGM9WrShtqxf9guHNdWM8k5K1xzugcLSQ3Fri7x6ioIhZagCPuzPscO8teafSRTEdKrBo5u~WJQ8tDqk7qznwkhbayznwaJzTduw4PvS7u96LxEXG-6rNZyFZkiU5QrIjKe~lBMYih~XBvOQkv1ZrUzk9kz8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236003dx.jpeg?Expires=1768857839&Signature=l86SYPSPs96mwlfXMH55uJszqtCsW-iVt4Nc-kyUN2gVk-spArz1jDrkAcAgpEfZOKW0f3ZIbM-yd8YhaZMs3k5JXirNRpcg0lr9soSuFQ11bZV45w53Htzw5nmBFgVWYyG-8YqmqpJkOiRalRXBQOh246c-51Wv3RooKUZEiExkhdeI1IHbTHxh6IFNiwlvrFU~ALpSHoFdHBto402Csc8kLwc32IpXJOOiIPDKIdNUreFkrq~a5IQ-gTeM5cpYTPHHyKocDomeueOXkG~IvByxQGirI3RTB5BoqSxNL64QJv-KgxIkPVFDJY419sOnCYtLiGvJ-r0xAX-fdd92dw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236002bx.jpeg?Expires=1768857840&Signature=EWF18f6g6sf4Q1N8quNfSVsuuhgJC3tL1FeGFFldOGHRgVCnw47qUYHCtOVlJI5awudfqpSgYDHXjry0QLAXrPgMxuuly35GRC61h-9rTckQRftRr0e13r~KDCdifHU2iMMR7fLIKPWmGKl5zLgZsC2YCKjU7FI39VMPiKnhdBmBFlXDwOQS02robbK61stz76e9uKscVKXxOZtwWT6ZstK4Ud9kjKLXmnlgc09xRo5CTZ0KjARSW4acQePhdxR0hBeQy8s4PAVfImFaRKtH6fdpFXCc4jlFKV2dnBA660hbljyP0fTxKiJjRItFOAOYAUmQF6Uba3rVxL3Y6Aq5Cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236002cx.jpeg?Expires=1768857840&Signature=mBRLQCNisHkHzgV9IgZfRDPP2NK9eTqf9NIOBueY8aAzB6O~FYMNi15BmhkrNAqjl8G3tpxJjiHrI3M9e0um1xEjXgC2UKVrSrzmBIKwtCGg8JQqJygxLqlMcJvvJ8cXhAmS027sVpsqAuFc1gafF1Pt-Rs0eN2WQ6QsJXBx~3EC7v2K58AQXvqPbr8igiQu1IJ7h1TUsLx9ARrUeS5SdrXtZMv7W4jEFOnBQyVXaQaC3S01mVulGs5eME-ZAphplkQ4Z8K--COMrK1c3ThTNNwwBvJ2FzJj7HsN2K93Q44hM8VP77Rax7UJRxpq2eEuftCXy2hkGuqtXouMDPYYIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Detection of apoptosis in CLL cells (patient UPN9a) as detected by DNA strand breaks by in situ terminal deoxynucleotidyl transferase/Propidium iodine assay (TdT/PI or tunnel) after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Increasing uptake of biotinylated dUTP and avidin FITC as depicted in (B) and (D) correlates with the number of DNA fragments per cell. Cell cycle analysis is achieved by the intercalation of propidium iodine into cellular DNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236002dx.jpeg?Expires=1768857840&Signature=hOXUaxKPli3FcSju9ERHJqB~2Ys~HSinkT35bamyGIadB32oAaTM6fzJAv9z67rmAMqBT551p5jGJiiT2PgdYH53WoDgnBcu3K64E4XQEcKZ8mwnPI~Wcf8f4hufig~rAhSGOm7qxOOII07EA63G6Ztf9Lp4tstnDx7rGLgiYHgQR1AAo2s7ZhMznYUUtK9mdfBo1YDLvsBbLsPju-Gh0QurudVCEAqZkNs77oQT3VpO7eqgc4yTJBTvuDXIUKnGCZUhizVAWzpJpOp7e30ZbixHM-hHX3KyODKmPcVf3u8fZNLx77Af0Co2LbAXbQZGLhT5Slj0YOIw~iwhArM28Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236003ax.jpeg?Expires=1768857840&Signature=EToPA-pabNeOqWIFNkmdnd2t9vEpedOF-RBrZL5eNo1VY4ztixw~~ININu~x-pG3HjLTnWhvgjnzj7p~YYbWg9CxGY25yVA77iyacxL20ao7JkVC272-15~yizAhSK0tOTaCZ2EiVAaLwScy7YFc4Coczr2csuIc6EIYmnEiSUpargdANaQ8nSX2ZAVY6i7Z1bAFNQ~Fl62e2yhEF88eip6EpRB6EggppxDKR-AbxqD6c1v7Gnsvj9TQ4xXbLrRsl0mpO5LnxCOtCqw9XJUbJ51AKNXkWcpfth2zsTKdzeW0EI2tUEQ2-6-E3ViH7PlQi~uBKVAtZXzpK3VQLWkEpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236003bx.jpeg?Expires=1768857840&Signature=gxxW4TSOq9u9PE0f427qzl5bxifv5HnRy~IL8a2chP-SzBqPDNsi0Ovmy3IyE1H9mepfGN1-WYuUCgfZCogJMFsSLa9dK5IMm-JxP1s76W-cbseyHVoyfV7OIqpj1sr79DvVekUa2xY0WSPpB7NKXcGlTTOaKml~zBdMH87tIren-OolH8PAziXEkEiGb4ENhKeDelfKB9PTYBHNPIRCDBCTemckCEVhxao8-rdw2L81l10pHYye8PhcPsAwerNajOSyHJKGZ2Z0mTeY7nXRieCuzNdYz~axcnMU8GLs~tIBAuqJrwhvkrG19GPhlYsrD87t1wvcRwiDl5ZBgqcFGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236003cx.jpeg?Expires=1768857840&Signature=GuqR7eyEFhIE~ki~6k6-KNW7xbllTNFFz058W0F2hKGT7HKnmYuBc-s8RxaTbwALv9c4WMiQ5MR6iX6OfR3tCPoWazbjm1cpFQ~fGg3JIhyJow1xjLUkprDhpn2YFJiCG34DewvXn4dp~vgpnbGTIyvAzNY3dvE4rDF29MnYiMUOPwTzouSLf4bvd1hLI3vQ67EQXgE4GAlfd~YdTc3XRNnnWs4I5Jo8cFSR9qbBWMIFYzKJC0VmIP5mZkF5XtV0MM02t8RWkZUncZutZTVxSZFxr9U1w3vB8EheCEOVavfPW7JM6VlmD5YdYFssJXcIcmA7tK0oYwBQ5FCahWYpEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of apoptosis in CLL cells (patient UPN28a) as detected by annexin-V/PI assay after continuous exposure to medium ([A] 4 hours and [C] 24 hours) or 0.18 μmol/L flavopiridol ([B] 4 hours and [D] 24 hours). Phosphatidyl serine is restricted to the internal cell membrane but is externalized early during the process of apoptosis. Cellular uptake of propidium iodine indicates a disrupted cellular membrane generally observed during late apoptosis or with cell necrosis. Increased annexin binding to exposed phosphatidyl serine in quadrant 3 of (B) and (D) as compared with (A) and (C) is supportive of flavopiridol inducing cytotoxicity at least in part through the pathway of apoptosis in human CLL cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3804/4/m_blod42236003dx.jpeg?Expires=1768857840&Signature=FFL4~JO3sbrK-jLAuTWS1P5fMEOVuoSeyr3ypyGVVb-~3w6CNGtlZ1ltFoEC9W28EGl0Jn31R3ToMCO6A~GyijuwAg~AErd23CEya7wuaQn6GdI-85cHysaTuVNsllE1brrGBWfOLAM4tUeLesz4MacRdgFzzGGoFxrjbGxDesJu6lcPlk9YZFUHRGXj8L6IeSU1d8VGdl54Gzw~ogrgwYHoseBlH~N4PJae~j1IC6DSKZrpTkyjeVdb3vYJEoXsGr5-Reggc4YtrjsEtXKUjtANCjES7mr8lKb5nmKpPdvYvPOYa3tK7HjyMZO19op3QjBEcJ8kfEgKY7VUhgBeyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)