Infusion of complement fragments induces rapid sequestration of neutrophils within the pulmonary capillaries. This study examined the contributions of the bone marrow (BM) and the liver to the accumulation of neutrophils within the lungs. Complement fragments induced the release of neutrophils from the BM within 7 minutes of infusion, and these neutrophils sequestered in the lungs immediately upon reaching the pulmonary capillaries. Neutrophils expressing high levels of L-selectin were preferentially retained within the pulmonary microvasculature. By 30 minutes after the infusion was stopped, the circulating neutrophil counts had increased, primarily because of release from the BM. The number of neutrophils sequestered in the lung had decreased by only 27%, and the number of neutrophils in the liver increased by 223%. These studies indicate that complement fragments induce the release of neutrophils from the BM far more rapidly than previously described. These newly released neutrophils immediately sequester within the lung, increasing the number of neutrophils available to injure the lung many fold beyond the number that were circulating before infusion. The preferential retention of L-selectin–expressing neutrophils likely reflects the requirement for L-selectin–mediated adhesion in maintaining sequestered neutrophils within the pulmonary microvasculature. The number of circulating neutrophils reflects a balance between pulmonary sequestration, rapid release from the BM, and uptake by the liver and other organs.
INFLAMMATORY MEDIATORS produced at sites of infection or trauma often enter the bloodstream. Neutrophils and other leukocytes express receptors for many mediators and become activated when mediators bind to these receptors. Activated neutrophils are thought to sequester within the microvasculature of the lungs and other organs, contributing to tissue damage that results in vascular permeability changes and dysfunction.1-4 A complement fragment, particularly C5a, is one such mediator that induces neutrophil sequestration and neutropenia.1-5 Complement fragments including C5a aid clearance of organisms from the blood during sepsis, because both complement fragments and neutrophils are required for intravascular Pseudomonas to sequester within the lungs.6 Within the pulmonary microvasculature, the sequestered neutrophils are located primarily within the capillaries, and the majority are single rather than aggregated.5,7 8After the complement fragments are cleared from the blood, the circulating neutrophil counts recover, but the source of these neutrophils and the fate of neutrophils sequestered in the lungs has not been determined.
Previous studies have shown that the number of neutrophils sequestered within the pulmonary capillaries at the end of a 15-minute infusion of complement fragments is severalfold greater than the total number of neutrophils circulating in the blood before the infusion.5,7,8 These data suggest that complement fragments rapidly induce the release of neutrophils from a mobilizeable pool. While C5a, formyl-methionyl-leucyl-phenylalanine (fMLP), LTB4, granulocyte-macrophage colony-stimulating factor (GM-CSF), and other neutrophil activators can induce release of neutrophils from the bone marrow (BM),9 10 our studies suggested that large numbers of neutrophils could be released more rapidly than previously suspected.
Previous studies showed that the mechanism through which neutrophils sequester in the pulmonary capillaries involves at least two sequential steps.7,11,12 First, inflammatory mediators induce changes in the neutrophil's cytoskeleton that result in decreased ability to deform and pass through the narrow capillary bed, resulting in rapid sequestration and neutropenia. Second, engagement of adhesion molecules, both L-selectin and CD11/CD18, are required to maintain the sequestered neutrophils within the microvasculature and, presumably, to injure the endothelial cells. Van Eeden et al13 showed that young neutrophils newly released from the BM express higher levels of L-selectin than older circulating neutrophils. These observations led to the hypothesis that newly released neutrophils expressing high levels of L-selectin are preferentially retained within the pulmonary microvasculature.
This study tested the hypothesis that either complement fragments or fMLP induce a rapid and massive release of neutrophils from the BM that immediately sequester in the pulmonary microvasculature. The sequestration of neutrophils expressing low compared with high amounts of L-selectin was also determined. The release of neutrophils from the BM, as well as their expression of L-selectin, was quantitated by comparing neutrophils in simultaneously collected arterial and venous blood samples. Finally, these studies examined the recovery of circulating neutrophil counts after clearance of complement fragments and the contributions of the BM, lung, and liver to this recovery.
MATERIALS AND METHODS
Preparation of complement-activated plasma.
Zymosan-activated plasma was used as a source of complement fragments and was prepared as previously described.5,7 8 In brief, zymosan A yeast (Sigma Z-4250; Sigma, St Louis, MO) was boiled for 15 minutes and centrifuged twice. Heparinized blood obtained from donor rabbits was centrifuged. The plasma was incubated with the boiled zymosan yeast (5 mg/mL plasma) for 30 minutes at 37°C. The activated plasma was centrifuged to remove the yeast and was always used within 1 hour of preparation.
Protocol 1: Release of neutrophils from the BM.
Female New Zealand white rabbits (2.5 to 2.9 kg, n = 10) were anesthetized using ketamine hydrochloride (70 to 100 mg/kg intramuscular [im]) and acepromazine maleate (8 to 10 mg/kg im) with additional injections as needed to maintain anesthesia. A butterfly catheter was placed in a marginal ear vein, and the animals received heparin (100 U/kg intravenous). In 10 rabbits, an arterial catheter was placed in the aorta through the carotid artery to sample blood exiting the lungs. To sample blood entering the lungs, a venous catheter was passed through the right external jugular into the thoracic vena cava near the entrance to the right atrium, as estimated by the catheter length. In a second group of five rabbits, a second venous catheter was placed through the left external jugular vein into the inferior vena cava and positioned at the origin of this vessel just above the union of the common iliac veins and below the entrances of the renal, hepatic, splenic, pancreatic, and mesenteric veins. Catheter locations were confirmed at autopsy in all rabbits.
Rabbits with one central venous catheter near the right atrium received an infusion of complement fragments (0.3 mL/kg/min, n = 5) or the same volume of saline (n = 5) through the ear vein for 15 minutes. Blood samples were drawn simultaneously from the aortic and vena caval catheters before and at 0.5, 1, 2, 4, 7, 11, 15, 20, 25, 30, 35, 45, 60, 90, and 120 minutes after the start of the infusion for measurement of circulating leukocyte and platelet counts. The heart was stopped by injection of saturated potassium chloride at 120 minutes. The circulating neutrophil, mononuclear, and platelet counts in the arterial and venous blood samples were corrected for changes in hematocrit and were compared at each time point.
Rabbits (n = 5) with two central venous catheters, one at the right atrium and one at the origin of the inferior vena cava, received a similar infusion of complement fragments and blood was simultaneously sampled from the one arterial and two venous sites before and at 1, 4, 5.5, 7, 11, and 15 minutes of infusion.
A third group of rabbits (n = 5) was similarly anesthetized and prepared. Catheters were placed in the aorta and in the thoracic vena cava near the right atrium. These animals received an infusion of fMLP (Sigma; 0.01 mg/mL infused at a rate of 0.3 mL/kg/min) for 15 minutes. Arterial and venous blood samples were obtained before and at 0.5, 1, 2, 4, 7, 11, 15, 20, 25, 30, 35, 45, 60, 90, and 120 minutes after the start of the infusion for measurement of circulating neutrophil counts as described above.
Protocol 2: Expression of L-selectin on sequestering neutrophils.
Rabbits (n = 15) were similarly prepared as described in protocol 1. An arterial catheter was placed in the aorta through the carotid artery. A venous catheter was inserted through the external jugular vein into the vena cava near the right atrium. Three groups of animals were studied: Group 1: infusion of complement fragments for 15 minutes and studied immediately (n = 5); group 2: infusion of complement fragments for 15 minutes and studied at 45 minutes (ie, 30 minutes after the infusion was stopped) (n = 5); and group 3: infusion of unactivated plasma for 15 minutes and studied at 45 minutes (n = 5).
Blood was simultaneously sampled from the arterial and venous catheters before and 11 and 15 minutes after in all three groups and also at 30 and 45 minutes after the infusion of complement fragments was begun in groups 2 and 3. The circulating leukocyte, neutrophil, and lymphocyte counts were determined in each sample. At each time point, leukocyte-rich plasma was prepared by incubating 0.5 mL blood with 0.4 mL 4% dextran (100 to 200 kD; final concentration, 1.9%) to sediment the red blood cells (RBCs).5 Cytospins were immediately prepared and stored at −20°C for immunocytochemical studies.
L-selectin expression was evaluated in cytospins obtained from the arterial and venous blood samples using immunocytochemical techniques as previously described.14 In brief, after the cytospins were warmed to room temperature, they were fixed with acetone:methanol 1:1 for 90 seconds and transferred to 0.05 mol/L Tris-buffered saline (pH 7.6). The alkaline phosphatase–anti-alkaline phosphatase technique was used to localize the primary antibodies.14 In brief, the sections were incubated with either the anti–L-selectin antibody DREG 200, kindly provided by Dr Eugene C. Butcher (Stanford University), or nonspecific mouse IgG (Sigma) for 30 minutes. After washing with Tris-buffered saline, anti-mouse Ig (Dakopatts Z259; Dako Corp, Carpinteria, CA) was applied for 30 minutes, followed by the alkaline phosphatase–anti-alkaline phosphatase complex (D651; Dako Corp) for 30 minutes. After repeating the incubations with the secondary antibody and the complex, the alkaline phosphatase substrate containing new fuchsin was added for 20 minutes. After washing, the slides were counterstained with hematoxylin and mounted in aqueous mounting media.
Randomly sampled fields at 400× magnification in two cytospins from each experiment were examined by two observers (L.G. and C.M.D.). All the neutrophils in each field were evaluated and graded 0-3. Fields were selected until 100 to 150 neutrophils/cytospin were evaluated. In this grading system, a grade of 0 was assigned to neutrophils showing no staining, grade 1 corresponded to very sparse granular staining, grade 2 corresponded to moderate granular staining, and grade 3 corresponded to dense staining. The fraction of neutrophils showing each grade of staining were determined. The absolute number of neutrophils at each grade was calculated by multiplying the total number of neutrophils in the blood sample by the percent of neutrophils that showed that grade of staining. The number of neutrophils showing grades 0 and 1 (low expression) and grades 2 and 3 (high expression) were combined. The numbers of neutrophils expressing low and high amounts of L-selectin were compared in the arterial and venous blood at each time point. Intra- and inter-observer variability was less than 10% when all four grades were evaluated and was virtually zero when only low and high expression were compared.
Protocol 3: Changes in the accumulation of neutrophils in lungs, liver, and blood during and at 30 minutes after infusion of complement fragments.
The effect of infusion of complement fragments on the number of neutrophils sequestered in the lungs and liver was determined in the same animals as studied in protocol 2. At the end of the experiment, the animals were administered an intra-arterial injection of saturated potassium chloride, the chest was rapidly opened, the base of the heart ligated, and the lungs were fixed by instillation of 6% glutaraldehyde in phosphate buffer at 25 cm H2O with the vessels clamped for morphometric determination of neutrophil numbers. The vasculature of one lobe of liver was clamped and the lobe was fixed in formalin.
Tissue blocks were prepared from the lungs and the liver (n = 2 of each from each of five animals). The tissue was embedded in methacrylate and 1- to 2-μm sections were cut. In the lungs, the number of neutrophils and RBCs within the capillaries were counted in 20 400× fields of peripheral lung tissue for each rabbit, and the accumulation of neutrophils was expressed as both the number of neutrophils/1,000 RBCs and neutrophils/20 fields. In the liver, the number of neutrophils within the sinusoids was counted in 20 400× fields and expressed as neutrophils/20 fields.7 In the blood samples, the number of neutrophils was expressed as neutrophils per milliliter of circulating blood. The number of neutrophils in each location was compared after the 15-minute infusion of complement fragments and at 30 minutes after cessation of infusion of complement fragments or plasma.
To determine the absolute number of neutrophils sequestered within the liver, the volume of liver was determined by ligating all vessels to maintain the hepatic blood volume and measuring the volume of displacement when the excised liver was placed in a partially fluid-filled graduated cylinder. The volume fraction of the liver tissue occupied by neutrophils was calculated by multiplying the number of neutrophils/field of liver tissue (determined above) by the surface area of a rabbit neutrophil (32.16 μm2)15and dividing by the surface area of the microscopic field at 400× magnification (112,401 μm2). The total number of neutrophils in the liver was determined by multiplying the volume fraction occupied by neutrophils times the volume of the liver and dividing by the volume of a rabbit neutrophil (1.37 × 10−10 mL).15
Statistics.
Analyses of variance were used to compare the neutrophil counts over time, the accumulation of neutrophils in the lungs and the liver, and L-selectin expression.16 The modified Bonferroni correction was used to correct for multiple comparisons when significant overall differences were identified.17 A probability of less than 0.05 for the null hypothesis was accepted as indicating a statistically significant difference. Data are expressed as mean ± SEM unless otherwise noted.
RESULTS
Protocol 1: Release of neutrophils from the BM.
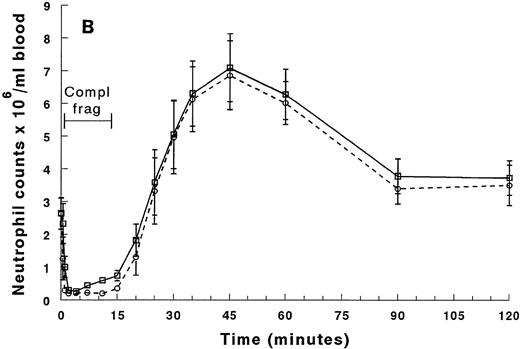
The neutrophil counts in the arterial and venous blood samples between 0 and 20 minutes from beginning the infusion of complements are shown in Fig 1A and for the entire 2-hour duration of the experiment in Fig 1B. The counts in the arterial and venous blood were similar before infusion of complement fragments (Fig1A). By 30 seconds after the start of the infusion, the neutrophil counts in the arterial blood samples, blood exiting the lungs, decreased rapidly and almost no neutrophils were present in these samples by 1 minute (Fig 1A). In contrast, the neutrophil counts in the venous blood entering the lungs did not decrease until 1 minute, and 2 minutes of infusion was required for neutropenia to occur (Fig 1A). This arterio-venous difference indicated that the majority of the neutrophils were sequestering within the lungs.
Circulating neutrophil counts in the arterial and venous blood during and after infusion of complement fragments. The counts between 0 and 20 minutes are shown in (A), and (B) shows the counts for the entire 2-hour duration of the experiment. The venous counts (□) decreased more slowly than the arterial counts (○) in the first 2 minutes of the infusion. Both arterial and venous counts were decreased at 2 to 4 minutes. The neutrophil counts in the arterial blood samples remained low throughout the entire infusion. However, the counts in the venous samples began to increase by 7 minutes, and continued to rise throughout the infusion. After the infusion was stopped, neutrophil counts in both arterial and venous blood increased similarly, reaching peak values that were twofold to threefold above baseline values by 45 minutes (30 minutes after the infusion was stopped). The counts returned to baseline values by 90 minutes. *Significantly greater than the neutrophil counts in the arterial blood, P < .05.
Circulating neutrophil counts in the arterial and venous blood during and after infusion of complement fragments. The counts between 0 and 20 minutes are shown in (A), and (B) shows the counts for the entire 2-hour duration of the experiment. The venous counts (□) decreased more slowly than the arterial counts (○) in the first 2 minutes of the infusion. Both arterial and venous counts were decreased at 2 to 4 minutes. The neutrophil counts in the arterial blood samples remained low throughout the entire infusion. However, the counts in the venous samples began to increase by 7 minutes, and continued to rise throughout the infusion. After the infusion was stopped, neutrophil counts in both arterial and venous blood increased similarly, reaching peak values that were twofold to threefold above baseline values by 45 minutes (30 minutes after the infusion was stopped). The counts returned to baseline values by 90 minutes. *Significantly greater than the neutrophil counts in the arterial blood, P < .05.
Both the venous and the arterial neutrophil counts remained decreased until 7 minutes when the neutrophil counts in the venous but not the arterial samples began to increase (Fig 1A). This arterio-venous difference continued until after the 15-minute infusion of complement fragments was discontinued. The increase in the venous counts together with the arterio-venous difference indicated that neutrophils were being released into the venous circulation and immediately sequestering within the pulmonary microvasculature.
By 20 minutes, when the infusion had been discontinued for 5 minutes, both the arterial and venous neutrophil counts increased similarly (Fig1A). At 45 minutes there was a significant rebound in the numbers of neutrophils in both samples that was greater than the baseline values (Fig 1B). Finally by 90 minutes this rebound recovered, and values similar to those obtained at baseline were observed. No change in neutrophil counts and no arterio-venous differences were observed at any time point when rabbits received infusion of saline instead of complement fragments.
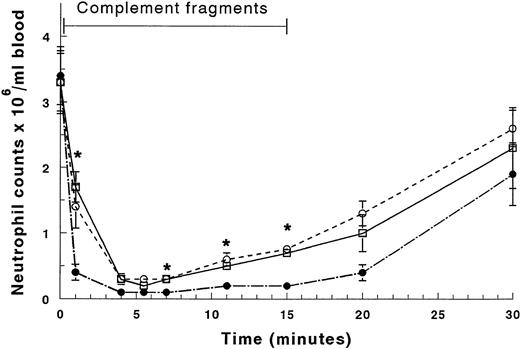
In an additional group of rabbits, blood was drawn simultaneously from catheters in the distal vena cava near its origin from the common iliac veins, the proximal vena cava near the right atrium above the entrance of renal and hepatic veins, and in the thoracic aorta as the blood leaves the lungs. The circulating neutrophil counts were similar at these three sites before infusion of complement fragments (Fig 2). By 4 minutes of infusion the counts at all three sites were significantly decreased. By 15 minutes the counts in the proximal venous catheter had increased, confirming the results described in Fig 1. Importantly, the circulating neutrophil counts in the distal venous blood had also increased by the same amount as those in the proximal venous blood (Fig2). These results indicate that neutrophils were not released from organs that directly enter the vena cava between the distal and proximal venous catheters, including the liver and kidneys, or from organs including the spleen, pancreas, and small intestines that drain into the portal vein, through the liver, and then into the inferior vena cava.
Circulating neutrophil counts in the arterial blood exiting the lungs sampled from the thoracic aorta (•), the proximal venous blood entering the lungs sampled near the right atrium (○), and the distal venous blood sampled from the origin of the inferior vena cava near the union of the common iliac veins (□). The neutrophil counts increased by a similar degree in the proximal and distal venous blood by 7 minutes of infusion, and this increase persisted throughout the infusion. *Significantly greater than the neutrophil counts in the arterial blood, P < .05.
Circulating neutrophil counts in the arterial blood exiting the lungs sampled from the thoracic aorta (•), the proximal venous blood entering the lungs sampled near the right atrium (○), and the distal venous blood sampled from the origin of the inferior vena cava near the union of the common iliac veins (□). The neutrophil counts increased by a similar degree in the proximal and distal venous blood by 7 minutes of infusion, and this increase persisted throughout the infusion. *Significantly greater than the neutrophil counts in the arterial blood, P < .05.
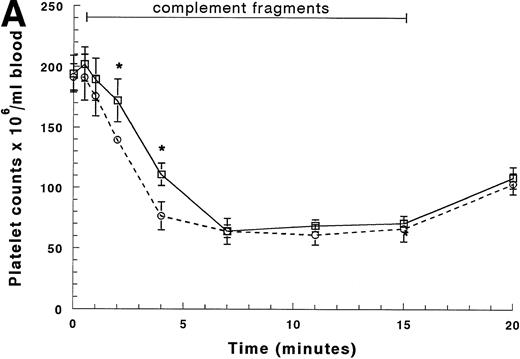
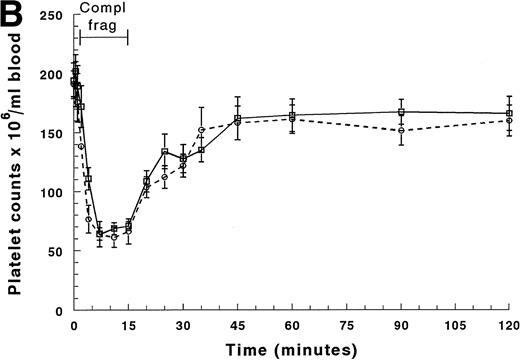
Circulating platelet counts decreased more slowly than the neutrophil counts (Figs 3A and B). The arterial counts began to decrease by 2 minutes of infusion and reached 30% to 35% of their baseline value by 7 minutes (Fig 3A). The arterial counts declined more quickly than the venous counts, indicating that platelets were sequestering within the lungs. No arterio-venous difference was observed after sequestration was complete (Fig 3A). The circulating platelet counts recovered by 45 minutes and no rebound was observed (Fig 3B). Circulating mononuclear cell counts decreased less, falling to 60% to 70% of their baseline value within 4 minutes of infusion, and there was no arterio-venous difference between 7 and 15 minutes (data not shown). Recovery occurred within 10 to 15 minutes after the infusion was discontinued, and no rebound was observed.
Circulating platelet counts in the arterial and venous blood during and after infusion of complement fragments. The counts between 0 and 20 minutes are shown in (A), and (B) shows the counts for the entire 2-hour duration of the experiment. The venous counts (□) decreased more slowly than the arterial counts (○) in the first 4 minutes of the infusion. The platelet counts in both the arterial and the venous blood samples remained low throughout the entire infusion. After the infusion was stopped, platelet counts in both arterial and venous blood increased similarly to baseline values by 45 minutes (30 minutes after the infusion was stopped). *Significantly greater than platelet counts in the arterial blood, P < .05.
Circulating platelet counts in the arterial and venous blood during and after infusion of complement fragments. The counts between 0 and 20 minutes are shown in (A), and (B) shows the counts for the entire 2-hour duration of the experiment. The venous counts (□) decreased more slowly than the arterial counts (○) in the first 4 minutes of the infusion. The platelet counts in both the arterial and the venous blood samples remained low throughout the entire infusion. After the infusion was stopped, platelet counts in both arterial and venous blood increased similarly to baseline values by 45 minutes (30 minutes after the infusion was stopped). *Significantly greater than platelet counts in the arterial blood, P < .05.
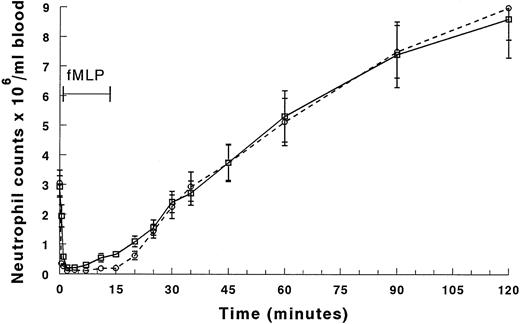
Infusion of fMLP induced neutropenia as rapidly and completely as complement fragments (Fig 4A and B). An initial arterio-venous difference was observed at 0.5 and 1 minute of infusion (Fig 4A). Arterial counts remained low throughout the infusion while venous counts increased by 7 minutes, and this arterio-venous difference persisted until the infusion was stopped (Fig 4A). Both arterial and venous counts recovered by 30 minutes after infusion (Fig4B). In contrast to complement fragments, the neutrophil counts continued to increase rather than returning to baseline values by 90 minutes.
Circulating neutrophil counts in the arterial and venous blood during and after infusion of fMLP. The counts between 0 and 20 minutes are shown in (A), and (B) shows the counts for the entire 2-hour duration of the experiment. The venous counts (□) decreased more slowly than the arterial counts (○) in the first 2 minutes of the infusion. Both arterial and venous counts were decreased at 2 to 4 minutes. The neutrophil counts in the arterial blood samples remained low throughout the entire infusion. However, the counts in the venous samples began to increase by 7 minutes, and continued to rise throughout the infusion. After the infusion was stopped, neutrophil counts in both arterial and venous blood increased similarly, reaching peak values more than threefold baseline values by 120 minutes (105 minutes after the infusion was stopped). *Significantly greater than the neutrophil counts in the arterial blood, P < .05.
Circulating neutrophil counts in the arterial and venous blood during and after infusion of fMLP. The counts between 0 and 20 minutes are shown in (A), and (B) shows the counts for the entire 2-hour duration of the experiment. The venous counts (□) decreased more slowly than the arterial counts (○) in the first 2 minutes of the infusion. Both arterial and venous counts were decreased at 2 to 4 minutes. The neutrophil counts in the arterial blood samples remained low throughout the entire infusion. However, the counts in the venous samples began to increase by 7 minutes, and continued to rise throughout the infusion. After the infusion was stopped, neutrophil counts in both arterial and venous blood increased similarly, reaching peak values more than threefold baseline values by 120 minutes (105 minutes after the infusion was stopped). *Significantly greater than the neutrophil counts in the arterial blood, P < .05.
Protocol 2: Expression of L-selectin on sequestering neutrophils.
Before infusion of complement fragments, about 20% to 25% of the neutrophils circulating in either the arterial or the venous blood expressed low levels of L-selectin while 75% to 80% expressed high levels, resulting in a ratio of high to low expressors of 3.8 (Table1). At both 11 and 15 minutes of complement fragments, the total number of neutrophils in either the venous or arterial blood decreased compared with baseline values (Table 1). However, the number of neutrophils in the 11- and 15-minute venous samples was significantly greater than in the arterial samples, as was also found in protocol 1. This increase in the venous samples was caused by an increase in the number of neutrophils that were expressing high levels of L-selectin, and this increase is reflected in the significantly higher ratio of high:low expressors in venous compared to arterial blood (Table 1).
The total number of neutrophils in the arterial and venous blood at 30 and 45 minutes was increased and there was no arterio-venous difference. However, the ratio of high:low L-selectin expressors was higher in both the venous and the arterial blood samples at 30 and 45 minutes than in blood obtained before infusion (Table 1).
Protocol 3: Changes in the accumulation of neutrophils in lungs, liver, and blood during and at 30 minutes after infusion of complement fragments.
Infusion of complement fragments for 15 minutes induced a significant increase in the number of neutrophils within the pulmonary capillaries and a decrease in the number of circulating neutrophils (Fig5), as shown previously in Fig 1. No change in the number of neutrophils was observed in the sinusoids of the liver at this time. However, at 45 minutes (30 minutes after the infusion was stopped), the number of neutrophils in the liver had increased by 223%, and the number of neutrophils in the lungs had decreased by 27% (Fig 5). The circulating counts had returned to baseline values.
Accumulation of neutrophils in the blood, pulmonary capillaries, and hepatic sinusoids. Complement fragments induced a significant decrease in the circulating neutrophil counts at 15 minutes and a significant increase at 30 minutes after the infusion was stopped. The neutropenia was accompanied by a significant increase in the number of neutrophils within the pulmonary capillaries. However, the number of sequestered neutrophils decreased only by 27% by 30 minutes after the infusion. In contrast, infusion of complement fragments did not induce an increase in the number of neutrophils within the hepatic sinusoids after 15 minutes, but caused a 2.3-fold increase within 30 minutes after the infusion was stopped. *Significantly different from the same organ in animals that did not receive complement fragments, P < .05. **Significantly different from the same organ in animals studied immediately after the 15 minutes of complement fragments, P < .05.
Accumulation of neutrophils in the blood, pulmonary capillaries, and hepatic sinusoids. Complement fragments induced a significant decrease in the circulating neutrophil counts at 15 minutes and a significant increase at 30 minutes after the infusion was stopped. The neutropenia was accompanied by a significant increase in the number of neutrophils within the pulmonary capillaries. However, the number of sequestered neutrophils decreased only by 27% by 30 minutes after the infusion. In contrast, infusion of complement fragments did not induce an increase in the number of neutrophils within the hepatic sinusoids after 15 minutes, but caused a 2.3-fold increase within 30 minutes after the infusion was stopped. *Significantly different from the same organ in animals that did not receive complement fragments, P < .05. **Significantly different from the same organ in animals studied immediately after the 15 minutes of complement fragments, P < .05.
The volume of liver was 86 ± 3 mL. The total number of neutrophils in the liver was 0.570 × 109 in the rabbits that did not receive infusions of plasma, 0.468 × 109 at the end of the 15-minute infusion of complement fragments, and 1.51 × 109 in rabbits given a 15-minute infusion of complement fragments and studied 30 minutes later. These data indicate that 1.04 × 109 neutrophils accumulated in the liver during the 30 minutes after the infusion was stopped.
DISCUSSION
Release of neutrophils from the BM or other organs was hypothesized to contribute to neutrophil accumulation in the lungs induced by complement fragments because the increase in the number of neutrophils accumulating within the pulmonary capillaries was much larger than the number circulating within the total blood volume before infusion.5,7,8 This new study shows that release of neutrophils begins to occur within 7 minutes of infusion, more rapidly than the previously reported 0.5 to 2.0 hours,9,10 where this early release was likely masked by the coincident neutropenia. This release is most likely from the BM for two reasons. First, the increase in the neutrophil counts was similar in blood sampled from the distal vena cava near its origin at the union of the common iliac veins and blood sampled from the proximal vena cava near the right atrium (Fig 2). This observation that the neutrophil counts in venous blood proximal to the entrance of venous drainage from the liver, spleen, intestines, kidneys, pancreas, and other abdominal organs were not greater than the counts in the distal venous blood indicates that there was no significant release of neutrophils by these organs during the 15-minute infusion of complement fragments. Second, neutrophil sequestration in muscle tissue, the major tissue other than BM that drains into the blood sampled by the distal catheter, is actually increased during infusion of complement fragments5 and, therefore, is unlikely to be the site of neutrophil release.
In fact, the number of neutrophils released into the distal venous circulation completely accounts for the observed numbers accumulating in the lungs, as described by the following calculations. The total number of neutrophils circulating in the blood immediately before infusion of complement fragments was calculated by multiplying the number of neutrophils per milliliter of blood at this time by the blood volume of the rabbit (60 mL/kg) and was equal to 6.1 × 108 neutrophils/rabbit. The number of neutrophils released into the venous circulation that immediately sequestered within the lungs was calculated by multiplying the difference in neutrophil counts per milliliter of blood between the venous and arterial blood samples from 4 to 15 minutes of infusion by the cardiac output, previously measured to be 496 mL/min,5 and was found to be 1.9 × 109 neutrophils/rabbit. Therefore, the predicted total number of neutrophils available to accumulate within the pulmonary capillaries was 6.1 × 108 + 1.9 × 109 = 2.5 × 109 neutrophils/rabbit. In control rabbits with no injury, the number of marginated neutrophils within the pulmonary capillaries was 2.1 × 109neutrophils/rabbit,5 so that the number of accumulated neutrophils after infusion of complement fragments was predicted to be the circulating + newly released + normally marginated neutrophils = 6.1 × 108 + 1.9 × 109 + 2.1 × 109 = 4.6 × 109 neutrophils/rabbit. This compares closely to the experimentally observed number of neutrophils accumulated within the lungs of complement-treated rabbits of 4.9 × 109.5 Therefore, the complement fragment-induced increase in the accumulation of neutrophils within the pulmonary circulation can be completely explained by sequestration of both previously circulating neutrophils and those newly released from the BM.
Infusion of fMLP, another potent neutrophil activator, induced a similar neutropenia due to sequestration within the lungs and a similar release of neutrophils from the BM. These data suggest that this rapid release of neutrophils is not induced solely by complement fragments but may be a response to a number of neutrophil activators and chemotactic factors.
This release from the BM was unique to neutrophils, as neither mononuclear cells nor platelets showed a similar behavior. The sequestration of platelets occurred after neutrophils had sequestered, suggesting that the formation of neutrophil-platelet aggregates were not the mechanism through which neutrophil sequestration occurred. In fact, these data suggest that platelets may actually be adhering to sequestered neutrophils.
The mechanism through which complement fragments and other inflammatory mediators induce this release of neutrophils is not clear. It is possible that neutrophils still in the BM bind to BM matrix by L-selectin–mediated interactions, and newly released neutrophils are known to express more L-selectin than the average circulating neutrophil.13 Complement fragments could induce activation of the protease that cleaves L-selectin on neutrophils within the BM, resulting in their release. However, this possibility seems unlikely in view of the observations that the number of neutrophils within the BM and the circulating blood in L-selectin–deficient mice are not altered.12,18,19 In addition, Jagels et al20showed that neither L-selectin nor CD11/CD18 was required for neutrophils to emigrate from the BM into the blood using antibodies against these molecules in rabbits. In fact, no known adhesion molecule appears to mediate this response, as mice deficient in P-selectin, E-selectin, L-selectin, CD18, or ICAM-1, either singly or in combination, have no apparent defect in the mobilization of neutrophils from the BM.12,18-29 Alternatively, complement fragments and other inflammatory mediators may stiffen neutrophils within the BM, similar to the changes in biomechanical properties observed in circulating neutrophils and in vitro.11,30-33 This stiffening of neutrophils that are adherent to the BM stroma may cause them to loosen and de-attach, resulting in release. In addition, inflammatory mediators may cause retraction of the reticular cells that line the adventitial surface of the venous sinusoids of the BM, widening the opening through which neutrophils are released.34-36
The pulmonary microvasculature preferentially retained neutrophils that were expressing high levels of L-selectin. This retention likely reflects the requirement for L-selectin–mediated adhesion to keep neutrophils within the capillaries and arterioles/venules once they are sequestered.12,37 Low L-selectin–expressing neutrophils may still stiffen in response to complement fragments, reducing their deformability and resulting in entrapment, but without L-selectin they eventually pass through the capillary bed within 2 to 5 minutes.12,37 Alternatively, the high expression of L-selectin may be an epiphenomenon, reflecting some other feature of neutrophils newly released from the BM. For example, these newly released neutrophils may be larger in volume or they may be less deformable than older neutrophils.38
After the infusion of complement fragments was stopped, the number of circulating neutrophil counts rapidly increased. By 30 minutes, the number of neutrophils in the lungs was reduced by 27%. In contrast, the number of neutrophils in the liver, which was not increased at the end of the infusion, had increased by 223% within 30 minutes, an increase of 1.04 × 109 neutrophils. These data support the hypothesis that the increase in circulating neutrophils is most likely caused by continued release from the BM without an inflammatory stimulus to sequester, while neutrophils released from the lungs do not circulate but are cleared by the liver, for the following reasons. First, Van Eeden et al13 have shown that the expression of L-selectin on neutrophils within the BM is higher than that on normally circulating neutrophils, while we have shown that neutrophils within the capillaries of rabbits given infusion of complement fragments for 15 minutes have an average reduction of 72% in the expression of L-selectin due to complement fragment-induced shedding using quantitative ultrastructural immunohistochemistry.39 The observation in the present study that the ratio of high to low L-selectin–expressing neutrophils was higher in blood samples obtained after the infusion was stopped than in samples obtained immediately before the infusion therefore support the hypothesis that the increase in circulating counts was caused by release of neutrophils from the BM. Second, a decrease of 27% in the number of sequestered neutrophils within the lungs corresponds to the release of 1.32 × 109 neutrophils (27% × 4.9 × 109 total sequestered neutrophils5). This value is very similar to the 1.04 × 109 neutrophils that sequestered in the liver within 30 minutes after cessation of complement fragments, suggesting that the neutrophils released from the lungs may not circulate well and are rapidly cleared by the liver. Taken together, these data suggest that the increase in circulating neutrophil counts is caused by continued release of neutrophils from the BM rather than from the lung. Because neutrophils sequestered in the lungs shed most of their L-selectin39 and L-selectin expression on most circulating neutrophils is high, the neutrophils released from the lungs appear to circulate poorly. Whether the neutrophils accumulating in the liver injure hepatocytes and contribute to multiorgan failure1-4remains to be determined.
In summary, infusion of complement fragments induces a rapid release of neutrophils, most likely from the BM, that immediately sequester on the first passage through the lungs. Neutrophils expressing high levels of L-selectin are preferentially sequestered within the lungs. After the infusion of complement fragments is stopped, the circulating neutrophil counts recover, most likely due to continued release of neutrophils from the BM. Neutrophils that were sequestered in the lungs appear to be released from the pulmonary microvasculature too slowly to account for the increase in the circulating neutrophils, to circulate poorly, and to rapidly accumulate in the liver. These studies show the complex nature of neutrophil kinetics during complement fragment–induced sequestration and the need to consider the balance between the many compartments that contribute to the distribution of neutrophils within the body.
ACKNOWLEDGMENT
The authors thank Taffy Hooser and Bonnie Meek for preparation of histologic sections.
Supported by Public Health Service Grants No. HL48160 and HL33009.
Address reprint requests to Claire M. Doerschuk, MD, Harvard School of Public Health, I-305, 665 Huntington Ave, Boston, MA 02115; e-mail:cdoersch@hsph.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.