Abstract
Extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP) activate multiple types of P2-nucleotide receptors expressed in platelets or leukocytes. Electrophysiological and biochemical studies have indicated expression of the P2X1 receptor, an ATP-gated cation channel, in human and rat platelets, rat basophilic leukemia (RBL) cells, and phorbol myristate acetate (PMA)-differentiated HL-60 myeloid cells. Although these findings suggest that P2X1 receptors are present in both blood leukocytes and blood platelets, the relative levels of P2X1receptor expression and function in human blood leukocytes and platelets have not been directly characterized. On the basis of both immunoblot analysis and functional assays of P2X1receptor-mediated ionic fluxes, we report that there is significant expression of P2X1 receptors in human platelets, but not in neutrophils, monocytes, or blood lymphocytes. Thus, unlike platelets and myeloid progenitor cell lines, fully differentiated human blood leukocytes do not express functionally significant numbers of P2X1 receptors, suggesting the downregulation of P2X1 receptor gene expression during the differentiation of phagocytic leukocytes. By contrast, P2X1 receptor expression is strongly maintained during megakaryocytic differentiation and platelet release. Immunoblot analysis indicated that the platelet P2X1 receptor migrates as an approximately 60-kD protein during SDS-electrophoresis under reducing or nonreducing conditions. Treatment of platelet membranes with endoglycosidase-F causes the P2X1 receptor band to migrate as a 46-kD protein, verifying the highly glycosylated nature of the mature receptor protein. Additional studies of nucleotide-induced changes in Ca2+influx/mobilization demonstrated that the platelet P2X1receptors are pharmacologically distinct from the well-characterized ADP receptors of these cells. This finding suggests a unique role for these ATP-gated ion channels during hemostasis or thrombosis.
EXTRACELLULAR adenosine triphosphate (ATP) affects the function of many tissues and cell types. ATP can be released by cytolysis or by the regulated exocytosis of vesicles and granules containing ATP.1 This released ATP can be rapidly metabolized to adenosine diphosphate (ADP) or adenosine monophosphate (AMP) or both, by ecto-ATPases and ecto-apyrases.2,3 Biologic responses to ATP and other extracellular nucleotides are mediated by P2-nucleotide receptors belonging to two major classes: G-protein–coupled P2Y receptors and nucleotide-gated ion-channel P2X receptors.4 Each class comprises a number of pharmacologically and genetically distinct subtypes.5
Multiple types of P2-nucleotide receptors are expressed in blood cells and the levels of certain P2 receptors can be rapidly modulated during activation of these cells or during differentiation of their hematopoietic progenitors. This suggests that released nucleotides are involved in multiple types of paracrine and autocrine signaling between blood cells and other cell types. ADP, which is copackaged with ATP in platelet dense granules, is a potent and well-characterized activator of many platelet responses including adherence, shape change, granule release, aggregation, and thromoboxane A2(TXA2) production.6-8 The platelet receptor that mediates these effects of ADP has been termed P2T (for “thrombocyte”) and exhibits a unique pharmacology in that ADP is agonistic, whereas ATP is antagonistic. Despite the large number of reports describing platelet responses to ADP, the molecular nature of the putative P2T receptor remains undefined. Some studies suggest that P2T receptor signaling may reflect the combined activation of two, or more, of the already cloned P2 receptor subtypes.9,10 Leon et al9 reported that cell lines expressing recombinant human P2Y1 receptor cDNA exhibit nucleotide-induced increases in intracellular Ca2+ with a pharmacological profile similar to that of P2T receptor responses in platelets. Moreover, since P2Y1 receptor mRNA was found in both human platelets and various megakaryocytic cell lines, these investigators suggested that the P2Y1 receptor may be the G-protein–coupled ADP receptor of platelets.9 The hypothesis that this ADP receptor is likely to be a conventional G-protein–coupled receptor is supported by recent studies of transgenic mice that lack expression of the α-subunit of Gq.11 Platelets from these Gq-deficient mice exhibit none of the conventional responses to ADP.
Human platelets and rat megakaryocytes additionally express ATP/ADP-gated ion channel receptors with properties similar to those of the cloned P2X1 ionotropic receptor.10,12,13These findings are consistent with recent molecular studies describing the presence of P2X1 receptor mRNA transcripts in human platelets and megakaryocytic cell lines.14 However, the role of these P2X1 receptor channels in the function of blood platelets remains unclear. Although P2X1 receptors have been studied most extensively in vascular and visceral smooth muscle cells,15 Northern blot analysis showed very high levels of P2X1 receptor mRNA in the total leukocyte fraction of human blood.16 Moreover, P2X1receptor protein and function have been characterized in phorbol myristate acetate (PMA)-differentiated HL-60 cells and rat basophilic leukemia (RBL) cells.17 The sequence of the P2X1 receptor initially cloned from rat vas deferens was found to be identical to an orphan mRNA (RP-2) upregulated in apoptotic rat thymocytes.18 These observations suggest that P2X1 receptors may be expressed not only in platelets, but also in the leukocytes from human peripheral blood. To test this, we have assayed expression of P2X1 receptor protein and P2X1 receptor function in various human blood cell types. We report that the P2X1 receptor is not significantly expressed in blood neutrophils, lymphocytes, or monocytes, but confirm and extend previous studies regarding the high expression of these ATP-gated ion channels in platelets.
MATERIALS AND METHODS
Isolation of platelets and leukocytes.
Venous blood (40 to 50 mL) from healthy volunteers was collected into 6 to 7 mL sterile acid-citrate-dextrose (ACD) 100 mmol/L disodium citrate, and 128 mmol/L d-glucose, pH 5. For isolation of platelets, the citrated whole blood was centrifuged in 50-mL polypropylene tubes at 200g for 15 minutes, and the platelet-rich plasma was removed and centrifuged at 2,500g for 15 minutes. The pelleted platelets were resuspended and washed twice in phosphate-buffered saline (PBS) supplemented with 13 mmol/L trisodium citrate (PBS-citrate) and 1 mg/mL bovine serum albumin (BSA). A similar protocol was used to obtain a mixed buffy-coat fraction containing all leukocytes and platelets by initially centrifuging the citrated whole blood at 2,500g for 15 minutes. The initial buffy coat was removed, resuspended in PBS-citrate, and recentrifuged at 2,500g. For isolation of neutrophils and mononuclear leukocytes, citrated whole blood was diluted 1:2 with PBS-citrate; 30-mL aliquots were layered over 12 mL Histopaque-1077 (Sigma, St Louis, MO) and centrifuged at 400g for 30 minutes. Mononuclear leukocytes banding on top of the Histopaque layer were collected into PBS-citrate and subjected to three cycles of resuspension and centrifugation (200g, 10 minutes). The neutrophil/erythrocyte pellet beneath the Histopaque was suspended in 3 vol of 155 mmol/L NH4Cl, 10 mmol/L KHCO3, 1 mmol/L EDTA, pH 7.4, and incubated on ice for 10 minutes to lyse the erythrocytes. This resulted in a cell fraction predominated by neutrophils (>95%), as judged by myeloperoxidase (MPO) staining.
Cell culture of hematopoietic cell lines.
HL-60 human promyelocytic leukemia cells, THP-1 human monocytic leukemia cells, and BAC1.2F5 murine macrophage cells were cultured using previously described protocols.19 20 HL-60 cells were differentiated into either macrophage-like cells by treatment for 2 days with 100 nmol/L PMA or into granulocyte-like cells by treatment for 3 days with .5 mmol/L dibutyryl cyclic AMP. THP-1 cells were differentiated toward an inflammatory macrophage phenotype by a 2-day treatment with either 100 nmol/L PMA or the combination of 100 ng/mL lipopolysaccharide (LPS), (List Biologicals, Campbell, CA) and 1,000 U/mL recombinant human interferon-γ (IFN-γ) (a gift from Genentech, San Francisco, CA).
Generation of HEK-293 cells stably expressing recombinant P2X1 receptors.
HEK-293 (human embryonic kidney) cells were maintained in Dulbecco's minimal essential medium (DMEM) supplemented with penicillin (100 U/mL) streptomycin (100 μg/mL), and 10% iron-supplemented newborn calf serum (Hyclone, Logan, UT). HEK-293 cells were stably transfected by electroporation (Bio-Rad Laboratories, Richmond, CA; 500 μF and 300 mV, time constant 7 to 10 ms) with 20 μg of the rat P2X1 receptor cDNA (in pcBK-CMV, a generous gift from G. Buell and A. Surprenant, Glaxo Institute for Molecular Biology, Geneva, Switzerland). Selection was accomplished with G418 (570 μg/mL) added 48 hours after transfection. After 3 weeks of selection, approximately 500 individual, resistant colonies were pooled, and these stably transfected cultures were maintained under continuous G418 selection. These cells (HEK-P2X1) and the nontransfected, parental HEK-293 line were used as positive and negative controls for Western blot analysis and electrophysiological studies of P2X1 receptors.
Membrane isolation and processing.
Blood cells or cultured cell lines were pretreated with PBS containing 4 mmol/L DIFP (diisopropyl fluorophosphate) for 20 minutes on ice. Cells were washed twice and resuspended with lysis buffer (137 mmol/L NaCl, 8.1 mmol/L Na2HPO4, 2.7 mmol/L KCl, 1.5 mmol/L KH2PO4, and 2.5 mmol/L EDTA) containing protease inhibitors (100 μmol/L phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL aprotinin, and 10 μg/mL leupeptin). Cells were lysed either by sonication (Branson model 185 sonifier, power setting 7) for 30 seconds or by nitrogen cavitation for 20 minutes at 400 psi (platelets at 1,200 psi). Total cell membranes were then pelleted in a Beckman TLOPTM tabletop ultracentrifuge (100,000g for 30 minutes at 4°C). Membranes were resuspended and homogenized in lysis buffer plus protease inhibitors and membrane protein was quantified using Bio-Rad Protein Assay solution. Membranes were stored at −80°C for subsequent analysis or were immediately processed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Membrane samples were routinely mixed with Laemmli sample buffer (final concentrations: 62.5 mmol/L Tris–HCl (pH 6.8), 2% SDS, 10% glycerol, 5% β-mercaptoethanol) and boiled for 5 minutes. However, for all Western blot analyses of CD61 and some P2X1 receptor analyses, membrane samples were processed under nonreducing conditions by deleting the mercaptoethanol from the sample buffer. As positive control sources of membranes containing large numbers of P2X1 receptors, rat vas deferens or urinary bladders were minced and Dounce homogenized in lysis buffer supplemented with protease inhibitors. The homogenate was centrifuged at 400g for 10 minutes, the supernatant was collected, and membranes were pelleted by ultracentrifugation, as described above.
Antibodies.
An antiserum against P2X1 receptor protein was generated in rabbits injected with two peptides corresponding to homologous sequences within the intracellular C-terminal domains of the cloned human16 and rat15 P2X1 receptors. These peptides (LPKRHYYKQKKFKYILYAED) and (TSSTLGLQENMRTS), respectively, correspond to residues 357 to 373 and residues 386 to 399 of the human P2X1 receptor sequence. Peptide injection, care, and bleeding of the rabbits were performed by a commercial service (Quality Controlled Biochemicals, Hopkintown, MA). Antisera were initially screened by Western blot analysis of rat urinary bladder and vas deferens membrane proteins.
A mouse MoAb against human CD61 (MoAb MCA728 from Serotec, Raleigh, NC) was used to assay membrane content of CD61, the GPIIIa β3 integrin expressed in platelets.21 This MoAb does not bind to CD61 treated with sulfhydryl reducing agents. Purified GPIIb/IIIa from human platelets (a generous gift from Dr S. D'Souza, Cleveland Clinic Research Foundation, Cleveland, OH) was used as a positive control source of GPIIIa. A mouse MoAb against the α-subunit of the Na+,K+-ATPase (MoAb 9A7, a generous gift from Dr Maureen McEnery, Case Western Reserve University, Cleveland, OH) was used to assay the relative content of plasma membrane in the total cell membrane fractions.22
Immunoblot analyses.
Membrane proteins were separated by SDS-PAGE (12%) and transferred electrophoretically to polyvinylidene fluoride (PVDF) membranes for 15 hours at 30 mV. PVDF membranes were rinsed in immunoblot buffer (10 mmol/L Tris, pH 7.4; 0.9% NaCl; 0.05% Tween-20; 1 mmol/L EDTA) and blocked with milk buffer (4% nonfat dried milk (Sigma Chemical Co, St Louis, MO) in immunoblot buffer). After washing (1 × 15 minutes 2 × 5 minutes) with immunoblot buffer, the PVDF membranes were incubated for 1 hour with primary antibodies dissolved in immunoblot milk buffer. Anti-P2X1 receptor antiserum was used at either a 1:500 dilution (bleed 2 antiserum) or a 1:3,000 dilution (bleed 3 antiserum); anti-CD61 was used at a 1:1,000 dilution; anti-Na+,K+-ATPase was used at a 1:10,000 dilution. Membranes were then washed and incubated for 1 hour at room temperature with 1:5,000 dilutions of horseradish peroxidase (HRP)-conjugated donkey anti-rabbit antibody (Amersham, Arlington Heights, IL) or HRP-linked sheep anti-mouse (Santa Cruz, Santa Cruz, CA) in milk buffer. Membranes were washed and developed with chemiluminescence reagents (SuperSignal from Pierce, Rockford, IL) for 1 to 5 min and exposed to Kodak x-ray film (Eastman-Kodak, Rochester, NY). In some cases, the immunoblot was stripped of bound antibodies using conventional methods and then reprobed with different antibodies.
Endoglycosidase F treatment of membrane proteins.
Membrane proteins were extracted and denatured by boiling for 5 minutes in 1% SDS. Duplicate aliquots of each denatured extract were diluted 1:5 in reaction buffer (50 mmol/L KH2PO4, 25 mmol/L EDTA, 1.3% N-octylglucoside, and 1.3% β-mercaptoethanol, pH 7.0), vortexed, and treated overnight at 37°C with, or without, 1 U/mL endoglycosidase F/N-glucosidase F (Boehringer, Mannheim, Germany). The extracts were then processed for electrophoresis and immunoblotting as described.
Fluorimetric analysis of cytosolic Ca2+.
Platelets, neutrophils, or mononuclear leukocytes were suspended (5 × 106/mL for white blood cells (WBCs) or 5 × 108/mL for platelets) in a Ca-free basal salt solution (BSS) containing 130 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 25 mmol/L Na-HEPES, pH 7.5, 5 mmol/Ld-glucose, 1 mg/mL BSA, and 0.5 U/mL apyrase (Type V, Sigma). The suspensions were supplemented with 1 μmol/L fura 2-AM (Molecular Probes, Eugene, OR) and incubated at room temperature for 45 minutes. The cells were pelleted, resuspended in fresh BSS containing apyrase, and stored at room temperature for up to 3 hours before assay. Cytosolic Ca2+ was fluorimetrically assayed using equipment and protocols previously described.19 20 Immediately before assay, the fura 2–loaded cell suspensions were diluted 1:3 with normal BSS or with reduced Na-BSS in which NaCl was iso-osmotically replaced with 130 mmol/L NMG-Cl (N-methylglucamine chloride) in thermostatted (37°C), stirred cuvettes. Each cuvette suspension was then supplemented with 1 mmol/L CaCl2 and an additional 2 U/mL apyrase. After thermal equilibration, cells were stimulated by addition of nucleotides (ATP, ADP, or αβ-methylene-ATP) or other Ca-mobilizing agonists (thrombin for platelets, FMLP for neutrophils, concanavalin A [ConA] for mononuclear leukocytes).
Electrophysiological analysis of ATP-gated cation channels in monocytes.
For electrophysiological studies of blood monocytes, washed mononuclear leukocytes were suspended at 106 cells/mL in Iscove's medium (supplemented with 0.1% BSA), plated in Teflon culture dishes, and incubated at 37°C in an atmosphere of 92.5% air/7.5% CO2. These isolated mononuclear cells were analyzed for P2X1 receptor activity within 5 to 6 hours after isolation. Aliquots of mononuclear leukocyte suspension were removed from the Teflon culture dish and placed in the patch clamp chamber. After about 5 minutes, the chamber was extensively washed with external solution, during which time the lymphocytes and platelets (most cells in the suspension) were washed away. Bath flow was constant during the experiments. Cells that were attached to the center of the patching chamber in the direct flow of solution and appeared flattened were considered to be monocytes and were used in patch-clamp experiments. As positive controls, HEK293 cells stably expressing recombinant rat P2X1 receptors were assayed using identical patch-clamp methods and solutions. Cells were sealed to patch electrodes with resistances of 4 to 20 mΩ, and the whole cell configuration was established by suction. Monocytes were perfused internally for several minutes (during which time they rounded up and detached) before lifting them off the chamber floor. All cells were held at −40 mV and perfused internally for 3 to 5 minutes before they were challenged with ATP. Voltages were commanded and the data recorded by computer using the pClamp series of programs and an Axopatch 1-D patch clamp with a TL-1 DMA interface. ATP, 100 μmol/L, was applied to cells suspended in the flowing bath solution by puffer pipette as previously described.23 External solution contained 140 mmol/L sodium chloride, 10 mmol/L HEPES, 10 mmol/L glucose, 5 mmol/L potassium chloride, 2.5 mmol/L calcium chloride, and 0.5 mmol/L magnesium chloride, brought to pH 7.4 with NaOH. Internal solution contained 120 mmol/L potassium chloride, 20 mmol/L tetraethyl ammonium chloride (TEA-Cl), 10 mmol/L EGTA, 10 mmol/L HEPES and 5 mmol/L magnesium chloride, brought to pH 7.3 with TEA-OH.
RESULTS
Immunologic characterization of P2X1 receptor expression and processing in human platelets.
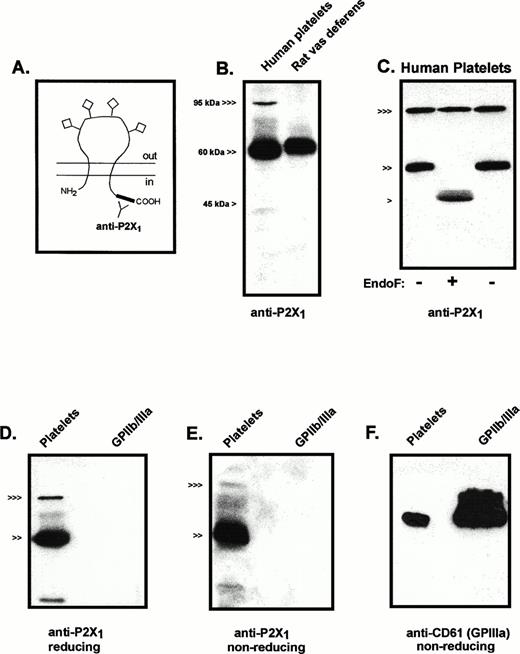
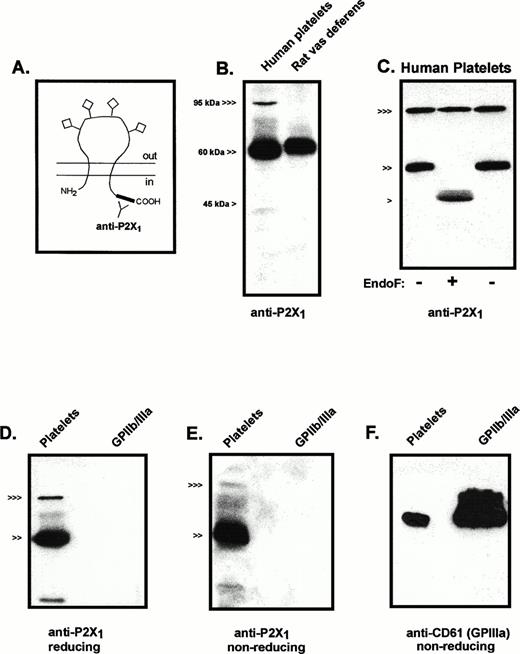
All seven cloned members of the P2X receptor family are predicted to share the basic membrane topography illustrated in Fig1A. This receptor topography includes intracellular N- and C-teminal segments, two transmembrane-spanning segments, and a large, extracellular loop that contains multiple cysteines for intramolecular disulfide bonding and sites for N-linked glycosylation.24 The C-terminal tails constitute the regions of least homology among the seven P2X receptor subtypes. Accordingly, a polyclonal antiserum against two peptides corresponding to C-terminal sequences of the human (and rat) P2X1 receptor was used to analyze the expression and processing of P2X1 receptor protein in human platelets (Fig1B). An intensely immunoreactive band of about 60 kD was observed in lanes containing total membrane protein from platelets; a band in the 95- to 100-kD region showed weaker but significant immunoreactivity. The 60-kD platelet protein migrated slightly faster than the major anti-P2X1 reactive protein in an adjacent lane containing total membrane protein from rat vas deferens, a tissue known to express very high levels of P2X1 receptor (Fig 1B). Although the amino acid sequence of the cloned human P2X1 receptor codes for a 45-kD protein, this sequence also predicts four glycosylation sites in the large extracellular loop.16 Previous photoaffinity labeling studies indicated that the molecular mass of processed P2X1 receptors in rat urinary bladder membranes was approximately 60 kD.25 To confirm that the 60-kD immunoreactive protein from human platelets corresponded to glycosylated P2X1 receptors, membrane extracts were subjected to endoglycosidase-F treatment. A 46-kD protein was the major immunoreactive species in these deglycosylated samples and the 60-kD product was eliminated (Fig 1C).
Immunologic characterization of P2X1 receptor expression and processing in human platelets. (A) The predicted membrane topography of the P2X1 receptor showing the extracellular glycosylation sites (◊) and the C-terminal tail location of the antigenic sites recognized by the anti-P2X1antiserum. (B) Anti-P2X1 receptor immunoblot of human platelet membranes (15 μg protein) versus rat vas deferens membranes (1 μg). (<<<, 95 kD; <<, 60 kD; < 45 kD). (C) Deglycosylation of P2X1R protein expressed in platelets. Parallel aliquots of platelet membranes were directly processed for electrophoresis (right lane, −) or were denatured in 1% SDS and then incubated in the absence (left lane, −) or presence (middle lane, +) of endoglycosidase-F, as described under Materials and Methods. Twenty-five micrograms of protein was loaded in each lane. (<<<, 95 kD; <<, 60 kD; < 45 kD). (D) Comparative anti-P2X1 receptor immunoblot of platelet membranes (30 μg) versus purified GPIIb/IIIa (400 ng) that were processed under reducing conditions. (<<<, 95 kD; <<, 60 kD). (E) Comparative anti-P2X1 receptor immunoblot of platelet membranes (30 μg) versus purified GPIIb/IIIa (400 ng) that were processed under nonreducing conditions. (<<<, 95 kD; <<, 60 kD). (F) Comparative anti-CD61 immunoblot of the same platelet membrane (30 μg) and GPIIb/IIIa (400 ng) samples used in (E) (nonreducing conditions).
Immunologic characterization of P2X1 receptor expression and processing in human platelets. (A) The predicted membrane topography of the P2X1 receptor showing the extracellular glycosylation sites (◊) and the C-terminal tail location of the antigenic sites recognized by the anti-P2X1antiserum. (B) Anti-P2X1 receptor immunoblot of human platelet membranes (15 μg protein) versus rat vas deferens membranes (1 μg). (<<<, 95 kD; <<, 60 kD; < 45 kD). (C) Deglycosylation of P2X1R protein expressed in platelets. Parallel aliquots of platelet membranes were directly processed for electrophoresis (right lane, −) or were denatured in 1% SDS and then incubated in the absence (left lane, −) or presence (middle lane, +) of endoglycosidase-F, as described under Materials and Methods. Twenty-five micrograms of protein was loaded in each lane. (<<<, 95 kD; <<, 60 kD; < 45 kD). (D) Comparative anti-P2X1 receptor immunoblot of platelet membranes (30 μg) versus purified GPIIb/IIIa (400 ng) that were processed under reducing conditions. (<<<, 95 kD; <<, 60 kD). (E) Comparative anti-P2X1 receptor immunoblot of platelet membranes (30 μg) versus purified GPIIb/IIIa (400 ng) that were processed under nonreducing conditions. (<<<, 95 kD; <<, 60 kD). (F) Comparative anti-CD61 immunoblot of the same platelet membrane (30 μg) and GPIIb/IIIa (400 ng) samples used in (E) (nonreducing conditions).
The 95- to 100-kD anti-P2X1 reactive protein is similar in size to the β subunits of certain integrins that are abundant proteins in the plasma membranes of platelets and leukocytes. Although GPIIIa (CD61) is the major β3 integrin in platelets, several observations indicated that GPIIIa is not the anti-P2X1reactive protein of approximately 100 kD. No P2X1-reactive bands were observed in lanes containing purified human GPIIb/IIIa that were prepared and electrophoresed under reducing or nonreducing conditions (Fig 1D and E). Parallel immunoblots using an anti-CD61 MoAb (under nonreducing conditions) verified the presence of immunoreactive GPIIIa in the lanes containing platelet membrane proteins or purified GPIIIb/IIa (Fig 1F). By contrast, the anti-P2X1 reactive band of approximately 100 kD was greatly diminished when these samples were processed under identical nonreducing conditions. Finally, endoglycosidase-F treatment caused no change in electrophoretic mobility of the 100-kD anti-P2X1 reactive protein (Fig 1C); similar treatments have been reported to decrease GPIIIa mass from 105 to 90 kD.21
Immunologic characterization of P2X1 receptor expression in myeloid cell lines and human blood leukocytes.
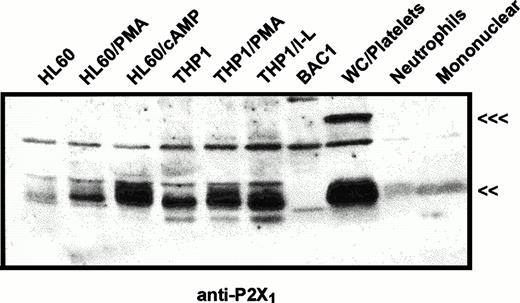
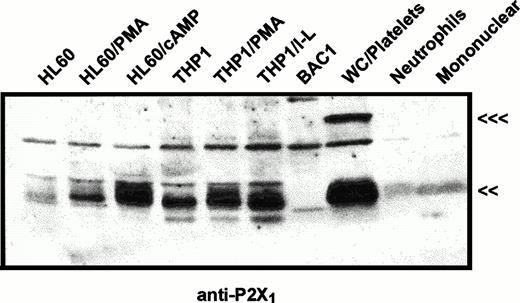
The same immunoblot protocol was used to compare relative levels of P2X1 receptor protein in membranes from human myeloid cell lines (Fig 2) and from human blood leukocytes (Fig 3). P2X1receptor expression was low in undifferentiated HL-60 promyelocytes but was upregulated following PMA treatment to induce a macrophage-like phenotype. P2X1 receptor expression was also markedly upregulated when HL-60 cells were treated with dibutyryl cAMP to induce a neutrophil-like phenotype. In contrast to the low expression of P2X1 receptors in uninduced HL-60 cells, there was significant expression in uninduced THP1 cells, a human leukemia line predominated by more mature monocyte-like cells. Although treatment of THP1 cells with PMA or LPS/interferon-γ (IFN-γ) induces expression of multiple inflammatory response genes, these latter agents did not significantly change P2X1 receptor expression from the levels observed in uninduced cells. These data confirmed and extended previous studies17 reporting P2X1 receptor expression in myeloid cell lines exhibiting either monocyte/macrophage (PMA-differentiated HL-60 cells) or granulocyte (RBL, rat basophilic leukemia) phenotypes.
Immunological characterization of P2X1receptor expression in myeloid cell lines versus human blood cells. HL60 cells or THP1 cells were untreated or were treated with 100 nmol/L PMA (HL-60 PMA or THP1 PMA), 0.5 mmol/L dibutyryl cAMP (HL-60 cAMP), or 1,000 U/mL IFN-γ plus 100 ng/mL LPS (THP1 I-L) as described under Materials and Methods. BAC1.2F5 murine macrophages (BAC1) were cultured without differentiating agents. Total cell membranes were isolated from these cell lines and from human peripheral blood cell fractions including total WBCs plus platelets (WC/platelets), neutrophils, and mononuclear leukocytes. Equal amounts of membrane protein (25 μg) were loaded in each lane and probed using the P2X1R antiserum (<<<, 95 kD; <<, 60 kD). The cross-reactive band at about 75 kD is a serum-derived contaminant, the intensity of which can be reduced by repeated washing of cells before lysis. Similar patterns of P2X1 receptor expression were observed in four other experiments.
Immunological characterization of P2X1receptor expression in myeloid cell lines versus human blood cells. HL60 cells or THP1 cells were untreated or were treated with 100 nmol/L PMA (HL-60 PMA or THP1 PMA), 0.5 mmol/L dibutyryl cAMP (HL-60 cAMP), or 1,000 U/mL IFN-γ plus 100 ng/mL LPS (THP1 I-L) as described under Materials and Methods. BAC1.2F5 murine macrophages (BAC1) were cultured without differentiating agents. Total cell membranes were isolated from these cell lines and from human peripheral blood cell fractions including total WBCs plus platelets (WC/platelets), neutrophils, and mononuclear leukocytes. Equal amounts of membrane protein (25 μg) were loaded in each lane and probed using the P2X1R antiserum (<<<, 95 kD; <<, 60 kD). The cross-reactive band at about 75 kD is a serum-derived contaminant, the intensity of which can be reduced by repeated washing of cells before lysis. Similar patterns of P2X1 receptor expression were observed in four other experiments.
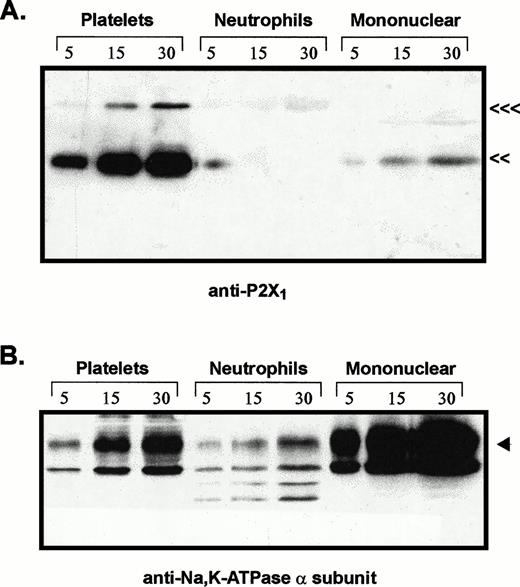
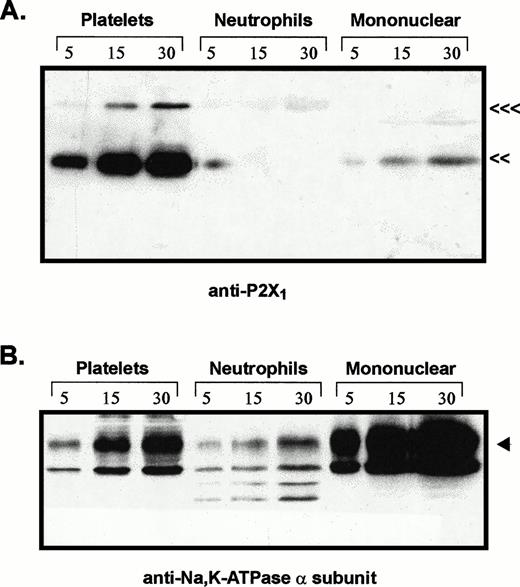
Comparative levels of P2X1 receptor protein and Na+,K+-ATPase α subunit in membranes from platelets, neutrophils, and mononuclear leukocytes. Adjacent lanes were loaded with 5, 15, or 30 μg of total membrane protein isolated from the indicated blood cell types (platelets, neutrophils, mononuclear). (A) The immunoblot initially probed with P2X1receptor antiserum (<<<, 95 kD; <<, 60 kD). (B) The same blot stripped and reprobed with MoAb 9A7 against the α subunit of the Na+,K+-ATPase. (◂), 110 kD α subunit.
Comparative levels of P2X1 receptor protein and Na+,K+-ATPase α subunit in membranes from platelets, neutrophils, and mononuclear leukocytes. Adjacent lanes were loaded with 5, 15, or 30 μg of total membrane protein isolated from the indicated blood cell types (platelets, neutrophils, mononuclear). (A) The immunoblot initially probed with P2X1receptor antiserum (<<<, 95 kD; <<, 60 kD). (B) The same blot stripped and reprobed with MoAb 9A7 against the α subunit of the Na+,K+-ATPase. (◂), 110 kD α subunit.
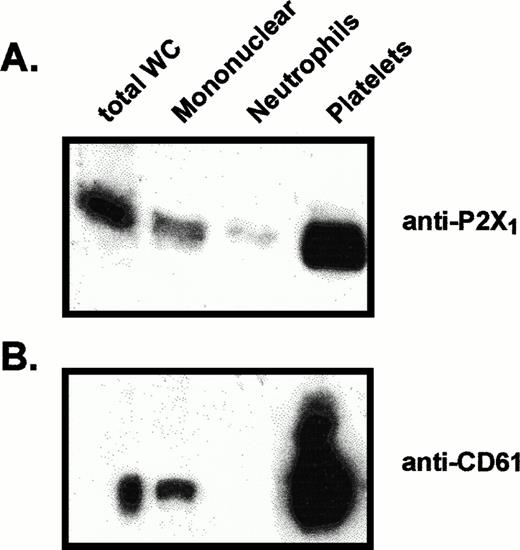
In contrast to the significant content of P2X1 receptors in human myeloid cell lines, no expression of these receptors was observed in BAC1.2F5 cells (Fig 2), a murine line that constitutively expresses many phenotypic characteristics of mature macrophages.20Control studies using membranes from mouse vas deferens verified that the antibody reacted with murine P2X1 receptor protein. Likewise, no expression was apparent in freshly isolated human neutrophils and only low expression was observed in the mononuclear leukocyte fraction of human blood (Figs 2 and 3). The absence of significant P2X1 receptor protein in samples of membranes from purified neutrophils and mononuclear leukocyte fractions contrasted with the strong signals observed in membranes derived from the mixed leukocyte/platelet fraction (buffy coat) of whole blood. This indicated that most P2X1 receptor protein was derived from platelet membranes.
The relative levels of P2X1 receptor protein in platelets versus blood leukocytes were examined by comparing intensities of the 60-kD immunoreactive bands among lanes loaded with equivalent amounts of total membrane protein from each cell fraction (Fig 3A). Total membranes from these blood cell types will contain differing amounts of plasma membrane because of the different contents of intracellular organelles and granules within these cells. Thus, it was important to normalize the P2X1 receptor signals relative to the plasma membrane content of each lane. Accordingly, the anti-P2X1immunoblot was stripped and re-probed with an antibody against the 110-kD α subunit of the Na+,K+-ATPase (Fig3B). The relative plasma membrane content (per microgram of total membrane protein) was highest in the mononuclear leukocyte samples, intermediate in platelets, and lowest in the neutrophil lanes. This is consistent with the high content of primary and secondary granules in neutrophils. Although 30 μg of total neutrophil membrane protein and 5 μg of total platelet membranes contained similar amounts of immunoreactive Na+,K+-ATPase, there was no appreciable P2X1 receptor signal in any lanes containing neutrophil membranes. Although 5 μg of total mononuclear leukocyte membranes and 30 μg of total platelet membranes contained roughly equivalent amounts of Na+,K+-ATPase, the P2X1 receptor signal was much stronger in the platelet membrane lane.
These observations indicate that the relative level of P2X1receptor expression in human platelets is at least 10-fold greater than in neutrophils or mononuclear leukocytes. Indeed, there was no measurable amount of anti-P2X1 reactive protein in any of five neutrophil preparations (from four different donors) that were assayed. Because the mononuclear leukocyte fraction is heterogeneous, the low (relative to platelets) amounts of P2X1 receptor protein in this cell population may reflect its predominant expression in only certain leukocytes or residual platelet contamination of the mononuclear leukocyte fractions. This fraction is usually composed of about 65% T lymphocytes, 15% B lymphocytes, 15% monocytes, 5% natural killer (NK) cells.26 Visual inspection of the washed, mononuclear cell fractions used in our experiments indicated approximately 20% monocytes (as assayed by rapid spreading on the glass hemocytometer surface), 80% lymphocytes, and relatively minor numbers of platelets (approximately 20 platelets per 100 mononuclear cells). Given this distribution, it is unlikely that T lymphocytes express P2X1 receptors to the level observed in platelets.
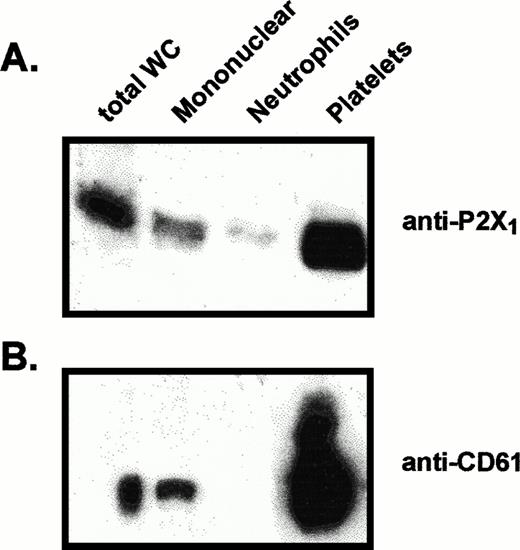
Equivalent amounts of platelet and mononuclear leukocyte membranes were assayed for their relative contents of P2X1 receptor and CD61 (Fig 4). Intense CD61 signals were detected in the platelet samples, whereas lower, but significant, CD61 signals were consistently observed in the mononuclear leukocyte samples. The relative intensities of the P2X1 receptor signals were strongly correlated with the intensities of the CD61 signals, suggesting that residual platelets are the source of P2X1 receptors observed in the mononuclear leukocyte samples. Because some previous reports have suggested that CD61 is expressed by monocytes, it is possible that monocytes, like platelets, coexpress P2X1 receptors and CD61. Mononuclear leukocyte samples were further fractionated (by adherence to plastic) into adherent monocytes and nonadherent lymphocytes. However, no significant differences were observed in the relative amounts of P2X1receptor, CD61, or residual platelets within these fractionated preparations of monocytes or lymphocytes (data not shown). Thus, contamination with platelets seems to be the most likely source of the P2X1 receptor protein observed in the mononuclear leukocyte fraction.
Comparative levels of P2X1 receptor protein and CD61 protein in membranes from different blood cell fractions. Lanes were loaded with 25 μg of total membrane protein from (left to right): total blood leukocytes (total WC), mononuclear leukocytes, neutrophils, platelets. (A) Anti-P2X1 receptor immunoblot of membrane proteins prepared for electrophoresis under standard reducing conditions. (B) Anti-CD61 immunoblot of samples from the same membrane preparations processed under nonreducing conditions.
Comparative levels of P2X1 receptor protein and CD61 protein in membranes from different blood cell fractions. Lanes were loaded with 25 μg of total membrane protein from (left to right): total blood leukocytes (total WC), mononuclear leukocytes, neutrophils, platelets. (A) Anti-P2X1 receptor immunoblot of membrane proteins prepared for electrophoresis under standard reducing conditions. (B) Anti-CD61 immunoblot of samples from the same membrane preparations processed under nonreducing conditions.
Functional characterization of P2X1 receptor expression in intact platelets and blood leukocytes.
The relative expression levels of functional P2X1 receptors in intact blood cell types were analyzed by assaying Ca2+influx responses to αβ-methylene ATP (Fig5). At micromolar concentrations, this synthetic nucleotide selectively activates only the P2X1and P2X3 subtypes of the P2X receptor family27and has no significant agonistic actions on any of the P2Y family receptors.1 4 Platelets responded to 30 μmol/L αβ-methylene ATP with rapid and transient twofold increases in cytosolic [Ca2+] over the basal level of 130 nmol/L (Fig5A). These same platelets did not respond to a second pulse of αβ-methylene ATP but did respond to 30 μmol/L ADP and, subsequently, to thrombin. The peak increase of the αβ-methylene ATP-induced Ca2+ transient was much smaller than the 5- and 15-fold increases stimulated by ADP and thrombin, respectively.
Comparative analysis of P2X1 receptor function in platelets, neutrophils, and mononuclear leukocytes. The indicated types of human blood cells were loaded with fura-2 and assayed for agonist-induced changes in cytosolic [Ca2+] as described under Materials and Methods. Each experiment was repeated twice using different preparations of blood cells and yielded similar results. For ease of graphic presentation, segments from certain continuous records have been deleted as indicated by braces { }; the duration of each deleted segment is noted. (A) Platelets were suspended in normal basal salt solution (BSS) containing 130 mmol/L NaCl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. The cells were first stimulated with 30 μmol/L αβ-methylene ATP (αβme-ATP). After about 3 minutes, the cells were rechallenged with additional αβ-methylene ATP (60 μmol/L final) and then serially stimulated with 30 μmol/L ADP, followed by 1 U/mL thrombin. (B) Platelets were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. The platelets were serially stimulated with 30 μmol/L αβ-methylene ATP (twice), 30 μmol/L ADP, and 1 U/mL thrombin. (C) Neutrophils were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. They were stimulated with 30 μmol/L αβ-methylene ATP, followed by 1 μmol/L FMLP. Another sample (suspended in the same medium) was stimulated by 30 μmol/L ATP. (D) Mononuclear leukocytes were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. They were stimulated with 30 μmol/L αβ-methylene ATP, followed by 5 μg/mL concanavalin A.
Comparative analysis of P2X1 receptor function in platelets, neutrophils, and mononuclear leukocytes. The indicated types of human blood cells were loaded with fura-2 and assayed for agonist-induced changes in cytosolic [Ca2+] as described under Materials and Methods. Each experiment was repeated twice using different preparations of blood cells and yielded similar results. For ease of graphic presentation, segments from certain continuous records have been deleted as indicated by braces { }; the duration of each deleted segment is noted. (A) Platelets were suspended in normal basal salt solution (BSS) containing 130 mmol/L NaCl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. The cells were first stimulated with 30 μmol/L αβ-methylene ATP (αβme-ATP). After about 3 minutes, the cells were rechallenged with additional αβ-methylene ATP (60 μmol/L final) and then serially stimulated with 30 μmol/L ADP, followed by 1 U/mL thrombin. (B) Platelets were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. The platelets were serially stimulated with 30 μmol/L αβ-methylene ATP (twice), 30 μmol/L ADP, and 1 U/mL thrombin. (C) Neutrophils were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. They were stimulated with 30 μmol/L αβ-methylene ATP, followed by 1 μmol/L FMLP. Another sample (suspended in the same medium) was stimulated by 30 μmol/L ATP. (D) Mononuclear leukocytes were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. They were stimulated with 30 μmol/L αβ-methylene ATP, followed by 5 μg/mL concanavalin A.
Moreover, removal of extracellular Ca2+ eliminated the response to αβ-methylene ATP but only marginally attenuated the responses to ADP or thrombin (data not shown). These differences are consistent with the ability of P2X1 receptors to trigger only Ca2+ influx in contrast to the abilities of ADP and thrombin to stimulate both Ca2+ mobilization from intracellular stores and Ca2+ influx. Since P2X1 receptors exhibit only a 5:1 selectivity for Ca2+ over Na+,15 ATP will primarily gate Na+, rather than Ca2+, influx when platelets are suspended in physiological medium containing 130 mmol/L NaCl and 1 mmol/L CaCl2. When extracellular Na+was partially replaced with NMG+, an impermeant cation, 30 μmol/L αβ-methylene ATP triggered a more pronounced 8-fold peak increase in cytosolic Ca2+ (Fig 5B).
Identical protocols were used to assay Ca2+ influx in neutrophils (Fig 5C) or mononuclear leukocytes (Fig 5D) stimulated with αβ-methylene ATP. No response to this nucleotide was observed in neutrophils. This contrasted with the robust Ca2+mobilization triggered by fMLP, an agonist for the myeloid-specific formyl peptide receptor, or ATP, an agonist for the G-protein–coupled P2Y2 nucleotide receptors that are expressed in neutrophils and monocytes.19 In mononuclear leukocytes, αβ-methylene ATP stimulated a very modest 15 nmol/L elevation in Ca2+ over the basal level of 170 nmol/L. By contrast, cross-linking of T-cell receptors with concanavalin A induced a sustained threefold increase in cytosolic Ca2+.
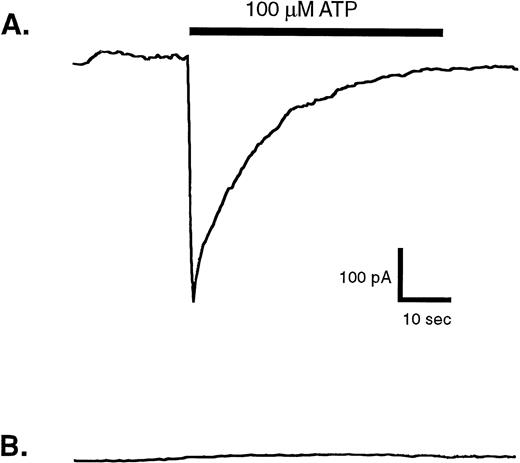
Owing to the high P2X1 receptor expression in platelets, minor numbers of residual platelets could account for the small response to αβ-methylene observed in the fura 2-loaded mononuclear leukocytes. Alternatively, this might reflect a low level of P2X1 receptor-mediated Ca2+ influx channel activity in the monocytes, which comprise only about 20% of the mononuclear suspension. Given the significant expression of P2X1 receptor protein and function in human monocytic cell lines17 (Fig 2) it was important to screen for P2X1 receptor activity in blood monocytes using sensitive electrophysiological measurements of ATP-gated inward Na+current. Patch-clamp assays were performed on single blood monocytes identified by their rapid adherence to, and spreading on, the plastic floor of the recording chamber. The monocytes were also incubated with medium containing apyrase (.5 U/mL) both before and during patch-clamp seal formation to minimize any autocrine desensitization of P2X1 receptors.17 However, none of the nine assayed monocytes (isolated from four donors) exhibited rapid ATP-induced inward currents (Fig 6B). As a positive control, identical recording conditions were used to assay P2X1 receptor function in HEK cells that stably express recombinant rat P2X1 receptors. In this particular experiment, four of eight patch-clamped HEK cells showed the rapid, transient inward currents characteristic of ATP-gated P2X1receptor channels (Fig 6A).
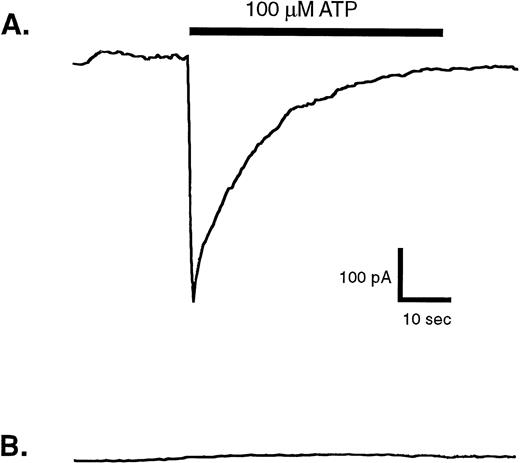
Membrane currents evoked by ATP in whole-cell patch-clamp recordings from human blood monocytes versus HEK cells expressing recombinant P2X1 receptors. Individual human monocytes or HEK cells expressing recombinant rat P2X1receptors (HEK-P2X1 cells) were patch-clamped as described under Materials and Methods. The membrane potential was held at −40 mV, while the cell was stimulated for indicated time with 100 μmol/L ATP. (A) Representative current record from an HEK–P2X1 cell. Similar responses were observed in four of eight patch-clamped HEK–P2X1 cells. Since this stably transfected cell line was not generated from a single cloned cell, the magnitude of P2X1 receptor expression and function varies considerably between individual cells. (B) Representative current record from a human monocyte. A similar absence of ATP-induced response was observed in nine other patch-clamped monocytes.
Membrane currents evoked by ATP in whole-cell patch-clamp recordings from human blood monocytes versus HEK cells expressing recombinant P2X1 receptors. Individual human monocytes or HEK cells expressing recombinant rat P2X1receptors (HEK-P2X1 cells) were patch-clamped as described under Materials and Methods. The membrane potential was held at −40 mV, while the cell was stimulated for indicated time with 100 μmol/L ATP. (A) Representative current record from an HEK–P2X1 cell. Similar responses were observed in four of eight patch-clamped HEK–P2X1 cells. Since this stably transfected cell line was not generated from a single cloned cell, the magnitude of P2X1 receptor expression and function varies considerably between individual cells. (B) Representative current record from a human monocyte. A similar absence of ATP-induced response was observed in nine other patch-clamped monocytes.
DISCUSSION
These experiments significantly extend recent reports10,12,14,16,17 regarding expression of P2X1 nucleotide receptors in various types of human blood cells. Although P2X1 receptor mRNA14 and ATP-gated cation channels10 have been previously described in human platelets, the studies illustrated in Figs 1 and 3 represent the first immunological demonstration that the human P2X1receptor is strongly expressed as a heavily glycosylated 60-kD protein in these cells. In addition, our studies of nucleotide-induced Ca2+ transients (Fig 5) show that these P2X1receptors are functionally and pharmacologically distinct from the well-characterized ADP receptors of platelets. An initial exposure of platelets to αβ-methylene ATP triggered a transient Ca2+ influx that was followed by a sustained desensitization to subsequent additions of this nucleotide. However, these αβ-methylene ATP-desensitized platelets continued to show strong Ca2+ mobilization responses to ADP. As noted previously, platelets from Gqα-deficient mice show no responses to ADP,11 whereas the findings of Leon et al9strongly suggest that the ADP receptor of platelets may be the already cloned P2Y1 receptor. Thus, platelets express at least two distinct types of P2 nucleotide receptors—the ionotropic P2X1 receptor and a Gq protein-coupled P2Y1-like receptor. It is significant to note that the P2Y1-like receptor of platelets shows an absolute selectivity for ADP as a physiological agonist and is antagonized by high concentrations of extracellular ATP.28 By contrast, ATP is the most potent physiological nucleotide agonist (EC50 ∼1 μmol/L) for the human P2X1receptor, with ADP a full but less potent (EC50 ∼70 μmol/L) agonist.29 These divergent nucleotide selectivities indicate that platelets may use ATP and ADP for distinct types of regulation. Although the roles for the P2Y1-like ADP receptor in platelet physiology are well established,6-8 the functions of the platelet P2X1 receptor are undefined.
Whether platelets express additional nucleotide receptors remains to be determined. Colman et al7,30 have used nucleotide affinity labeling methods to identify a 100-kD platelet protein termed aggregin, which may be part of an ADP receptor complex. The gene for this protein has not yet been cloned and its possible relationship to either the P2Y1-like ADP receptor or the P2X1 receptor of platelets is unclear. Our anti-P2X1 receptor antibody labeled a 100-kD platelet protein, in addition to the major 60-kD P2X1 receptor glycoprotein. We verified that this 100-kD protein was not CD61/GPIIIa, a major platelet membrane protein in this size range (Figs 1C and D). Because the amino acid sequence of aggregin is unknown, we cannot ascertain whether it contains either of the two peptide sequences recognized by the P2X1 receptor antiserum. However, the intensity of our 100-kD band was greatly decreased when platelet membranes were prepared in the absence of reducing agents (Fig 1F), suggesting that this protein is normally complexed with other platelet proteins by disulfide bonds. By contrast, affinity-labeled aggregin migrates as a 100-kD product when prepared with or without sulfhydryl reducing agents.7 The 100-kD immunoreactive protein was not apparent in other cell types expressing native (Figs 1-3) or recombinant (data not shown) P2X1 receptors. Therefore, it is unlikely to be essential for either the expression or function of these ATP-gated ion channels.
Previous studies of P2X1 receptor expression in other human hematopoietic cell types have reported P2X1 receptor mRNA in total human blood leukocytes16 and P2X1receptor protein and function in PMA-differentiated HL-60 myeloid cells.17 We also noted expression of P2X1receptor protein in a variety of leukemic human cell lines, including THP1 monocytes and dibutyryl cAMP-differentiated HL-60 granulocytes (Fig 2). Thus, we expected to observe expression of P2X1receptors in circulating human monocytes and granulocytes. However, we measured no significant amount of P2X1 receptor protein (Fig 3) or function (Figs 5 and 6) in gradient purified granulocytes (>95% neutrophils) and only low P2X1 levels in the total mononuclear leukocyte fraction (80% lymphocytes, 20% monocytes). The modest levels of P2X1 receptor protein in the mononuclear fraction were correlated with modest levels of CD61, a platelet marker protein. We also found that the relative amounts of P2X1receptor immunoreactivity in mononuclear leukocyte preparations varied with the isolation protocol. Although citrated blood was used for the experiments described in this study, our initial studies used heparinized blood and this resulted in stronger P2X1receptor signals and CD61 signals in the mononuclear fractions (data not shown). Significant platelet contamination may underlie the high levels of P2X1 receptor mRNA reported in previous Northern blot studies of total human blood leukocytes.16 Although we used CD61 as a marker for platelet contamination, this antigen has also been reported on monocytic and osteoclastic cells.31-34However, Krissansen et al34 showed that binding of platelets or platelet fragments to monocytes can generate false-positive CD61/CD41 signals in monocytes during fluorescence-activated cell sorter (FACS) analysis.
The absence of significant P2X1 receptor expression in circulating neutrophils and monocytes contrasts with the strong expression of this receptor in various human myeloid cell lines. This observation, coupled with the high P2X1 receptor levels noted in circulating platelets, suggests that the P2X1receptor gene is activated in the early colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM) cells that are the common marrow progenitors of both megakaryocytes and the committed granulocyte/monocyte precursors. If true, our data would suggest that P2X1 receptor expression is maintained during megakaryocyte development and platelet release but is repressed during the most distal phases of phagocyte differentiation. Current experiments are aimed at identifying the mechanisms and factors responsible for lineage- and stage-dependent expression of this receptor in the marrow-derived progenitor cells of the blood.
Supported by National Institutes of Health (NIH) Grant No. GM36387. E.E.C. was supported by NIH Training Grant HL07678.
Address reprint requests to George R. Dubyak, PhD, Department of Physiology and Biophysics, School of Medicine E565, Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106-4970.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. Comparative analysis of P2X1 receptor function in platelets, neutrophils, and mononuclear leukocytes. The indicated types of human blood cells were loaded with fura-2 and assayed for agonist-induced changes in cytosolic [Ca2+] as described under Materials and Methods. Each experiment was repeated twice using different preparations of blood cells and yielded similar results. For ease of graphic presentation, segments from certain continuous records have been deleted as indicated by braces { }; the duration of each deleted segment is noted. (A) Platelets were suspended in normal basal salt solution (BSS) containing 130 mmol/L NaCl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. The cells were first stimulated with 30 μmol/L αβ-methylene ATP (αβme-ATP). After about 3 minutes, the cells were rechallenged with additional αβ-methylene ATP (60 μmol/L final) and then serially stimulated with 30 μmol/L ADP, followed by 1 U/mL thrombin. (B) Platelets were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. The platelets were serially stimulated with 30 μmol/L αβ-methylene ATP (twice), 30 μmol/L ADP, and 1 U/mL thrombin. (C) Neutrophils were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. They were stimulated with 30 μmol/L αβ-methylene ATP, followed by 1 μmol/L FMLP. Another sample (suspended in the same medium) was stimulated by 30 μmol/L ATP. (D) Mononuclear leukocytes were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. They were stimulated with 30 μmol/L αβ-methylene ATP, followed by 5 μg/mL concanavalin A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3172/3/m_blod40919005x.jpeg?Expires=1767953204&Signature=Hot543WOjxdGlqK9Ignrb3MuHK38eDV9O0SOAP8cwm2gd8IeJo7VItgRqHok1DUkPeGy6q6QULaTwoks0mxm4QxlMRcgqI-C3XrorfRqFGzvKfp7QPZQwYn7Q7AaRMPArZaiDR3tJc1ttM-H~24-ETptz0m6cKA6aRSs6MrAch6igoQBpNY5CI8tfS9edVGCoKXgnBgbmXNyyszur3bOSD~MlCqv5XNRe~VvUu2dPFCq0GISKKZ5nf9VD0uZrepHeAzeJAnn1i2JmxqkfWKh3neTKzrH8t~wZV0ogapnNA8yaTXpRGxMiavfEWZqHIhaVCG5doqnPXjvSQGEVw~~cQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Comparative analysis of P2X1 receptor function in platelets, neutrophils, and mononuclear leukocytes. The indicated types of human blood cells were loaded with fura-2 and assayed for agonist-induced changes in cytosolic [Ca2+] as described under Materials and Methods. Each experiment was repeated twice using different preparations of blood cells and yielded similar results. For ease of graphic presentation, segments from certain continuous records have been deleted as indicated by braces { }; the duration of each deleted segment is noted. (A) Platelets were suspended in normal basal salt solution (BSS) containing 130 mmol/L NaCl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. The cells were first stimulated with 30 μmol/L αβ-methylene ATP (αβme-ATP). After about 3 minutes, the cells were rechallenged with additional αβ-methylene ATP (60 μmol/L final) and then serially stimulated with 30 μmol/L ADP, followed by 1 U/mL thrombin. (B) Platelets were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. The platelets were serially stimulated with 30 μmol/L αβ-methylene ATP (twice), 30 μmol/L ADP, and 1 U/mL thrombin. (C) Neutrophils were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. They were stimulated with 30 μmol/L αβ-methylene ATP, followed by 1 μmol/L FMLP. Another sample (suspended in the same medium) was stimulated by 30 μmol/L ATP. (D) Mononuclear leukocytes were suspended in reduced Na-BSS containing 40 mmol/L NaCl, 90 mmol/L NMG-Cl, 1 mmol/L CaCl2, and 2.5 U/mL apyrase. They were stimulated with 30 μmol/L αβ-methylene ATP, followed by 5 μg/mL concanavalin A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3172/3/m_blod40919005x.jpeg?Expires=1768318702&Signature=bypu~b3Nij2Tt9w220InBH6M2aIrSPyo4SF10wL52Mdd7B-KxZmcT~KnH7mdYYEjTX86zhdDmuo57d3abWEzoAp71YJUTCznmRzqj5Y3QPeQWLy2Bpta~M-fQRIwtfCEvABBdP99gWxXL-YuiUUcuUPtAhpcVXj5vY18SzAgnqhBETKKBST8fXvVHPZyD9J-mckLyMLpwuprxXYl3ouemUKO0-P4Udy3OKUO8eFaDTcxLsuJZrmut7tKN3OkCWNphBHH8c0CCM5MmhqUyRYFzWQ3~8QblgKBFlBwKmolFJf0rYi55mCgLQPpSr3pMbCApGtLA8DXG33HgeC0K12Omw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)